Abstract
Objectives
We investigated whether coronary microvascular dysfunction predicts major adverse outcomes during follow-up among women with signs and symptoms of ischemia.Background
Altered coronary reactivity occurs frequently in women evaluated for suspected ischemia, and the endothelium-dependent component is linked with adverse outcomes. Possible links between endothelium-independent microvascular coronary reactivity and adverse outcomes remain uncertain.Methods
As part of the National Heart, Lung and Blood Institute-sponsored WISE (Women's Ischemia Syndrome Evaluation), we investigated relationships between major adverse outcomes and baseline coronary flow reserve (CFR) after intracoronary adenosine in 189 women referred to evaluate suspected ischemia.Results
At a mean of 5.4 years, we observed significant associations between CFR and major adverse outcomes (death, nonfatal myocardial infarction, nonfatal stroke, or hospital stay for heart failure). An exploratory receiver-operator characteristic analysis identified CFR <2.32 as the best discriminating threshold for adverse outcomes (event rate 26.7%; and >or=2.32 event rate 12.2%; p = 0.01). Lower CFR was associated with increased risk for major adverse outcomes (hazard ratio: 1.16, 95% confidence interval: 1.04 to 1.30; p = 0.009). This held true among the 152 women without obstructive coronary artery disease (CAD) (hazard ratio: 1.20, 95% confidence interval: 1.05 to 1.38; p = 0.008). The CFR significantly improved prediction of adverse outcomes over angiographic CAD severity and other risk conditions.Conclusions
Among women with suspected ischemia and atherosclerosis risk factors, coronary microvascular reactivity to adenosine significantly improves prediction of major adverse outcomes over angiographic CAD severity and CAD risk factors. These findings suggest that coronary microvessels represent novel targets for diagnostic and therapeutic strategies to predict and limit adverse outcomes in women. (Women's Ischemia Syndrome Evaluation [WISE]; NCT00000554).Free full text

Coronary Microvascular Reactivity to Adenosine Predicts Adverse Outcome in Women Evaluated for Suspected Ischemia: Results from the NHLBI Women's Ischemia Syndrome Evaluation (WISE)
Abstract
Objective
We investigated whether coronary microvascular dysfunction predicts major adverse outcomes during follow-up among women with signs and symptoms of ischemia.
Background
Altered coronary reactivity occurs frequently in women evaluated for suspected ischemia and the endothelium-dependent component is linked with adverse outcomes. Possible links between endothelium-independent microvascular coronary reactivity and adverse outcomes remain uncertain.
Methods
As part of the National Heart, Lung and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE), we investigated relationships between major adverse outcomes and baseline coronary flow reserve (CFR) following intracoronary adenosine in 189 women referred to evaluate suspected ischemia.
Results
At 5.4 (mean) years, we observed significant associations between CFR and major adverse outcomes (death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure). An exploratory ROC analysis identified CFR <2.32 as the best discriminating threshold for adverse outcomes (event rate 26.7% and ≥2.32 event rate 12.2%; p = 0.01). Lower CFR was associated with increased risk for major adverse outcomes (HR 1.16, 95% CI 1.04 to 1.30; p = 0.009). This held true among the 152 women without obstructive coronary artery disease (CAD) (HR 1.20, 95% CI 1.05 to 1.38; p = 0.008). CFR significantly improved prediction of adverse outcomes over angiographic CAD severity and other risk conditions.
Conclusions
Among women with suspected ischemia and atherosclerosis risk factors, coronary microvascular reactivity to adenosine significantly improves prediction of major adverse outcomes over angiographic CAD severity and CAD risk factors. These findings suggest coronary microvessels represent novel targets for diagnostic and therapeutic strategies to predict and limit adverse outcomes in women.
Introduction
Women with chest discomfort and other findings suggesting myocardial ischemia are diagnostic and therapeutic challenges due, in part, to low likelihood for obstructive coronary artery disease (CAD) and costs of care related to repeated testing, hospitalization, and disability(1). Although knowledge of mechanisms explaining these findings is limited, impaired coronary reactivity (endothelium- and non endothelium-dependent) has been proposed to contribute(2–10). The endothelium-dependent component has been linked to risk factors and proinflammatory processes promoting atherosclerosis(8,9) as well as adverse clinical outcomes(5,7,10). Although the non endothelium-dependent component has received less attention, the concept that patients with risk factors may have evidence for reduced coronary flow reserve is not new(11–17). There is increasing interest in this microvascular response as recently reviewed elsewhere(18), and in particular the response among women(8). For example, hypercholesterolemia abolishes the voltage-dependent K+ channel contribution to adenosine-mediated smooth muscle relaxation, in both endothelium-intact and -denuded coronary arterioles, in a sex-specific manner(19,20). Vascular smooth muscle cells undergo alterations in phenotype in response to physiological and pathophysiological stimuli like hypertension and diabetes, which are highly prevalent in postmenopausal women, as well as estrogen receptor alpha expression(21,22).
Myocardial perfusion alterations during adenosine-induced vasodilation are not infrequent in the absence of significant epicardial CAD(23,24). While there has been long interest in microvascular ischemia, most work has focused on the endothelium-dependent component(25–28) but adenosine-related vascular smooth muscle alterations do not necessarily correlate with dysfunctional endothelium(8,20,29). Thus additional information on adenosine-related coronary microvascular reactivity would facilitate an improved understanding of processes underlying these vascular alterations in women. If these alterations contribute to adverse outcomes they potentially offer a target important for risk stratification and evaluation of preventive treatments among these women, particularly now that coronary microvascular reactivity can be readily assessed non-invasively(30–32).
Accordingly, we investigated the relationship between adenosine-coronary reactivity at baseline and adverse outcomes during follow-up in women referred for coronary angiography.
Methods
The Women's Ischemia Syndrome Evaluation (WISE) is a National Heart, Lung and Blood Institute-sponsored study aimed at improving diagnostic evaluation and understanding of pathological mechanisms of ischemic heart disease in women, and protocol details, including selection criteria, have been previously published(33). Site institutional review boards approved the study and each participant provided written informed consent. Women aged 18–84 years undergoing clinically indicated angiograms were enrolled, underwent a variety of testing, and were followed for clinical outcomes. A subgroup of 189 women from the Universities of Florida and Pittsburgh sites also had evaluation of coronary reactivity to adenosine. Their selection criteria also included informed consent for this additional testing, absence of stenosis warranting coronary revascularization and an appropriate coronary segment for Doppler flow testing.
Baseline evaluation included physical examination and collection of clinical and laboratory data (Table 1). Inflammatory markers were measured in a subgroup of 134 women from blood frozen on site at −70 °C and analyzed at a core lab using validated techniques. Qualitative and quantitative coronary angiographic analyses were done by core lab masked to patient data(34). Any ≥50% diameter stenosis was defined as obstructive CAD, 20–49% as mild CAD, and <20% as no CAD. A CAD severity score was defined as an aggregate of percent stenosis, extent and location of stenosis, and degree of collateral vessels(34).
Table 1
Baseline characteristics
| Characteristic | All women N=189 | CFR <2.32 N=74 | CFR≥2.32 N=115 | P |
|---|---|---|---|---|
| Age, years | 55 ± 10 | 58 ± 10 | 54 ± 10 | 0.02 |
| Years since last menses | 16 ± 11 | 19 ± 12 | 14 ± 10 | 0.004 |
| White/ Caucasian (%) | 83 | 88 | 80 | 0.16 |
| Body Mass Index (kg/m2) | 31.2 ± 7.4 | 31.2 ± 6.3 | 31.2 ± 8.0 | 0.67 |
| SBP (mmHg) | 136 ± 20 | 139 ± 23 | 134 ± 18 | 0.08 |
| DBP (mmHg) | 77 ± 10 | 77 ± 9 | 76 ± 11 | 0.93 |
| Family history of premature CAD (%) | 69 | 71 | 67 | 0.61 |
| History of: | ||||
 Diabetes (%) Diabetes (%) | 21 | 26 | 18 | 0.24 |
 Hypertension (%) Hypertension (%) | 57 | 58 | 56 | 0.79 |
 Dyslipidemia (%) Dyslipidemia (%) | 50 | 54 | 48 | 0.51 |
 Smoking currently (%) Smoking currently (%) | 19 | 16 | 20 | 0.50 |
 Smoking in past (%) Smoking in past (%) | 37 | 39 | 35 | 0.57 |
 Menopause (%) Menopause (%) | 76 | 81 | 72 | 0.18 |
| Blood assays: | ||||
 Total cholesterol (mg/dL) Total cholesterol (mg/dL) | 185 ± 44 | 188 ± 45 | 183 ± 42 | 0.39 |
 HDL cholesterol (mg/dL) HDL cholesterol (mg/dL) | 52 ± 12 | 53 ± 12 | 52 ± 13 | 0.84 |
 LDL cholesterol (mg/dL) LDL cholesterol (mg/dL) | 107 ± 37 | 111 ± 40 | 104 ± 36 | 0.16 |
 Triglycerides (mg/dL) Triglycerides (mg/dL) | 141 ± 141 | 148 ± 118 | 136 ± 155 | 0.72 |
 Plasma glucose (mg/dL) Plasma glucose (mg/dL) | 105 ± 48 | 108 ± 52 | 102 ± 44 | 0.34 |
 Hemoglobin (g/dL) Hemoglobin (g/dL) | 13.0 ± 1.4 | 13.0 ± 1.6 | 13.0 ± 1.3 | 0.35 |
 Serum creatinine (mg/dL) Serum creatinine (mg/dL) | 0.8 ± 0.5 | 0.8 ± 0.3 | 0.8 ± 0.6 | 0.35 |
 Serum amyloid A (mg/dL) Serum amyloid A (mg/dL) | 1.0 ± 2.3 | 1.1 ± 2.6 | 1.0 ± 2.1 | 0.06 |
 C-reactive protein (mg/L) C-reactive protein (mg/L) | 7.8 ± 11.6 | 8.8 ± 13.7 | 7.2 ± 10.2 | 0.22 |
 Interleukin-6 (pg/mL) Interleukin-6 (pg/mL) | 3.7 ± 2.8 | 3.9 ± 3.0 | 3.6 ± 2.6 | 0.72 |
| Medication use, currently: | ||||
 Aspirin (%) Aspirin (%) | 50 | 57 | 46 | 0.14 |
 Statin (%) Statin (%) | 16 | 19 | 15 | 0.47 |
 ACE-inhibitor (%) ACE-inhibitor (%) | 24 | 27 | 23 | 0.51 |
 Beta-blocker (%) Beta-blocker (%) | 29 | 36 | 24 | 0.06 |
| Hormone use, ever: | ||||
 Hormone replacement (%) Hormone replacement (%) | 56 | 53 | 58 | 0.53 |
 Oral contraceptives (%) Oral contraceptives (%) | 60 | 60 | 59 | 0.88 |
| Ejection Fraction (%) | 64.7 ± 9.6 | 64.5 ± 10.9 | 64.9 ± 8.8 | 0.71 |
| Coronary artery disease: | ||||
 None (<20% stenosis) (%) None (<20% stenosis) (%) | 51 | 46 | 54 | |
 Mild (20–49% stenosis) (%) Mild (20–49% stenosis) (%) | 30 | 28 | 32 | |
 Obstructive (≥50% stenosis) (%) Obstructive (≥50% stenosis) (%) | 19 | 26 | 15 | 0.11 |
 CAD severity score (medians [IQR]) CAD severity score (medians [IQR]) | 5.0(5.0, 9.2) | 7.5(5.0, 11.2) | 5.0(5.0,8.0) | 0.036 |
CFR=coronary flow reserve; SBP=systolic blood pressure; DBP=diastolic blood pressure; HDL=high-density lipoprotein; LDL=low-density lipoprotein; ACE=angiotensin-converting enzyme; CAD=coronary artery disease; IQR=interquartile range.
Data expressed as mean ± SD unless otherwise noted.
Coronary reactivity testing was performed in a stenosis-free area of the left anterior descending coronary artery (n = 138) when possible, with the left circumflex artery as a secondary choice. A Doppler-tipped guidewire (0.014 inch FloWire; JOMED/Cardiometrics, Mountain View, CA) was advanced through the diagnostic catheter. Once a stable velocity signal was obtained baseline recordings were made. Intracoronary bolus injections of 18 μg of adenosine (Adenocard; Fujisawa USA, Deerfield, IL), a predominantly non-endothelium-dependent microvascular dilator, were administered into the left main coronary artery(35). At least three injections were done to assure that a stable average peak coronary flow velocity was obtained after adenosine, with return to baseline flow velocity documented before each bolus. Pulsed-wave Doppler flow spectra were used to calculate time-averaged peak velocity. Recordings were analyzed at a core lab (University of Florida) masked to all other data, and coronary flow reserve was defined as the ratio of average peak velocity after adenosine to average baseline velocity just prior to adenosine. Since this measure correlated closely (r= 0.87, p< 0.001) with volumetric flow(35) it was used to represent coronary flow reserve(CFR).
To access the possible influence of left ventricular (LV) hypertrophy on CFR, 39 of these women without coronary stenosis had quantitative analysis of echocardiograms performed using a standardized protocol within several days of CFR measurements according to American Society of Echocardiography recommendations. These analyses included measurement of LV mass (2D echo) determined by an anatomically validated short axis area length method(36), mass index, and an index of LV concentric remodeling [end diastolic ratio of short axis myocardial to cavity area (MA/CA)]. All echo measurements were done at a core lab masked to patient data.
Adverse Outcomes during Follow-up
During protocol-directed yearly follow-up, records of women reporting an event were reviewed by an events committee and tabulated as death, nonfatal MI, nonfatal stroke, and hospitalization for CHF, angina, and other vascular events. Women sustaining multiple events were counted only once and by the initial event. Those with death, nonfatal MI, nonfatal stroke, or CHF hospitalization were categorized as having major adverse outcomes. Categorization of deaths as cardiovascular (CV) was made only when documentation definitely confirmed that death was due to CV causes.
Statistical Analyses
Values are expressed as means ± standard deviations or percentages as indicated, and t-test or Chi square analysis, where appropriate, was used to evaluate differences among groups. The CAD angiographic severity score was non-normally distributed and was expressed as medians and interquartile ranges with differences among groups evaluated by the Kruskal-Wallis test. To determine the CFR value best predictive of major adverse outcomes (death, MI, stroke, or hospitalization for CHF), we generated a receiver operating characteristic (ROC) curve from the PROC LOGISTICS function in SAS which calculates area under the curve (AUC), indicated by the c-statistic, using the trapezoidal rule. The CFR value corresponding to the point on the curve closest to 100% sensitivity and specificity was matched, first by visual means and then by verification runs of incremental cut-points near this value. The discriminating threshold by ROC analysis was then used to categorize women into low versus high CFR groups. Kaplan-Meier analysis was used to compare time to adverse event by CFR group in all women and in women without obstructive CAD. Multivariate Cox proportional hazards regression was used to examine the role of baseline characteristics including the natural logs of both the CAD severity score and CFR (logCFR ×10) on adverse outcomes. Baseline characteristics (Table 1) were chosen for entry into multivariable Cox models based upon their discrimination between low and high CFR as well as on univariate associations with adverse outcomes of p < 0.20. A combination of forward and backward selection procedures was used to aid in determining the best model of independent predictors. This was followed by forcing potential confounders, including drugs used at baseline and during follow-up, into the models and determining their effect on the relationship of interest. The likelihood ratio test was used to compare the incremental goodness of fit of nested models. All tests were two-sided, and p ≤ 0.05 was considered statistically significant. All analyses were performed using SAS 9.1 software (SAS Institute; Cary, NC).
Results
Baseline Characteristics
Pertinent characteristics of the women are summarized in Table 1. Their mean age was 55 years (standard deviation ±10 years), most were white, about three-quarters were post-menopausal, and more than half were obese (body mass index ≥30), had a history of hypertension or dyslipidemia, had a family history of premature heart disease, or used hormone replacement or oral contraceptive pills. History of diabetes was present in about a fifth. Approximately one-half were currently taking aspirin; about one-quarter, angiotensin-converting enzyme (ACE) inhibitors; less than one-third, beta-blockers; and about one-fifth, statins.
Angiographic CAD, echo LV mass, and CFR
Most (152 or 81%) of the women had either no or <50% obstructive CAD (Table 1) and lower CFR was weakly associated with higher CAD severity score (rs = −0.15, p = 0.04). Comparing groups with obstructive, mild, or no angiographic CAD, the CFRs were 2.3 ± 0.7, 2.5 ± 0.7, and 2.6 ± 0.7, respectively (p = 0.09 for trend). Regression analyses and comparisons of subgroups with normal vs. elevated LV mass, mass index, and MA/CA showed no significant relationships between LV mass variables and CFR (data not shown).
Adverse Outcomes by Risk Factors and CFR
During follow-up (mean 5.4 years), 79 women (42%) had an adverse outcome, and in total there were 138 events including 11 deaths (Table 2). Thirty-four of these 79 women had major adverse outcomes (death, MI, stroke, or hospitalization for CHF). Note that 25 of the women without obstructive CAD had major adverse outcomes.
Table 2
Index (first) and total adverse events during follow-up
| Events | Number of index (first) events | Total number of events |
|---|---|---|
| Major events | ||
 Death Death | 8 | 11 |
 Hospitalization for nonfatal event: Hospitalization for nonfatal event: | ||
  MI MI | 3 | 7 |
  CHF CHF | 6 | 18 |
  Stroke Stroke | 8 | 10 |
| Other events | ||
 PCI PCI | 12 | 23 |
 CABG CABG | 1 | 4 |
 Angina Angina | 36 | 52 |
 Other vascular event Other vascular event | 5 | 13 |
| Total (%)s | 79 (42%) | 138* |
All analyses summarized below were done with only major adverse outcomes. Substituting definite CV deaths for all-cause deaths produced very similar results.
Among all women in this cohort, those with major adverse events during follow up had at baseline higher SBP (p = 0.01), CRP (p = 0.038), and IL-6 (p = 0.02) levels and CAD severity scores (p = 0.0009) and were more frequently postmenopausal (p = 0.02) compared with those without events. Similar trends were found among the subgroup without obstructive CAD but only postmenopausal status (p = 0.025) and higher CAD severity score (p = 0.0005) reached statistical significance due to smaller sample size with fewer events.
By ROC analysis, a CFR <2.32 provided the best prediction, with a sensitivity of 62% and specificity of 65%, for major adverse outcomes (CFR <2.32 outcomes rate 27.0% and ≥2.32 outcomes rate 12.2%, X2 = 6.73, p = 0.010). The AUC (95% confidence interval) was 0.63 (CI 0.53, 0.73). Similar results were obtained when restricting the analysis to women without obstructive CAD: women with low CFRs experienced significantly more major adverse outcomes (CFR < 2.32 outcome rate 26.6% vs. 9.3%, X2 = 5.84; p = 0.016). Women with low CFR were older (p=0.02) and had a higher CAD severity (p=0.036) versus those with high CFR. There was a significant decline over time in freedom from major events for women with lower (<2.32) CFR compared with those with higher CFR (p = 0.003) (Figure 1), which remained consistent adjusting for risk factors (SBP, CAD severity, age, diabetes, and smoking). The Cox PH-adjusted time-to-event curves are similar to the unadjusted KM curves shown in Figure 1, and the p values remain significant.
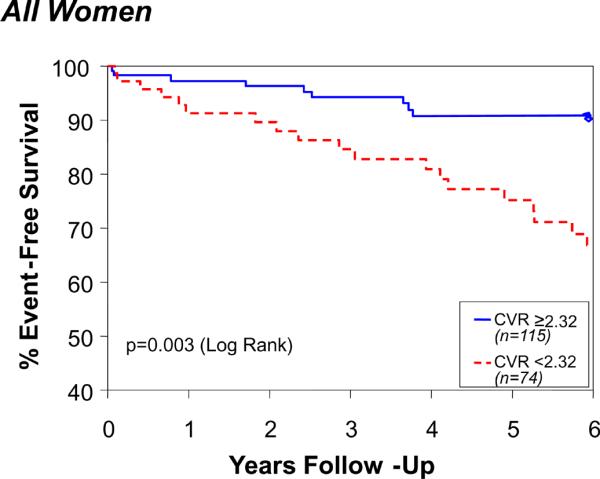
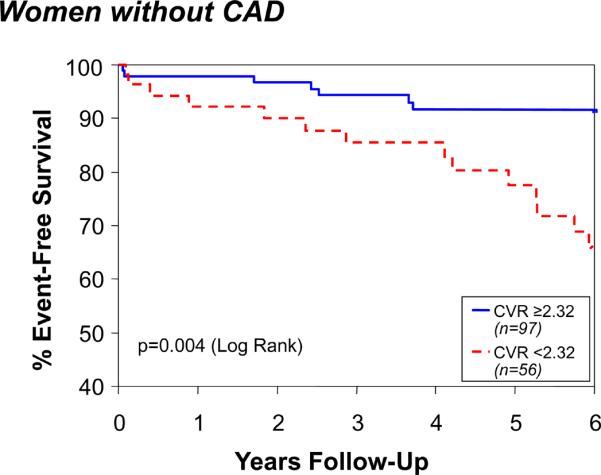
Coronary flow reserve (CFR) and event-free survival among all women (Top) and those without CAD (Bottom). Data represent unadjusted Kaplan-Meier curves for absence of death, nonfatal MI, nonfatal stroke, or hospitalization for CHF during follow-up.
When used as a continuous variable, low CFR also significantly predicted increased risk for major adverse outcomes (Table 3). For each 0.1 unit decrease in logCFR the risk for major adverse outcomes increased by 20% among women without obstructive CAD.
Table 3
Decreasing LogCFR and risk for adverse outcomes (unadjusted Cox regression analyses)
| All women | |||
|---|---|---|---|
| Outcome | Hazard Ratio | 95% CI | p |
| Major adverse outcome (death, nonfatal MI, nonfatal stroke, or hospitalization for CHF) | 1.16 | 1.04 – 1.30 | 0.009 |
| CV death, nonfatal MI, nonfatal stroke, or hospitalization for CHF | 1.15 | 1.02 – 1.30 | 0.019 |
| CV death, nonfatal MI, or hospitalization for CHF | 1.18 | 1.03 – 1.36 | 0.018 |
| Women without obstructive CAD | |||
|---|---|---|---|
| Outcome | Hazard Ratio | 95% CI | p |
| Major adverse outcome (death, nonfatal MI, nonfatal stroke, or hospitalization for CHF) | 1.20 | 1.05 – 1.38 | 0.008 |
| CV death, nonfatal MI, nonfatal stroke, or hospitalization for CHF | 1.19 | 1.03 – 1.37 | 0.020 |
| CV death, nonfatal MI, or hospitalization for CHF | 1.23 | 1.03 – 1.47 | 0.021 |
MI=myocardial infarction; CHF=congestive heart failure; CV=cardiovascular; CI=confidence interval.
Multivariate Cox regression modeling (Table 4) identified only logCFR (p = 0.043), log CAD severity score (p = 0.058), and systolic blood pressure (p = 0.012) as independent predictors of major adverse outcome. When multiple CAD risk conditions (e.g., age, history of diabetes, and smoking) were forced into the model, potentially overfitting the model, CFR remained a significant predictor (p = 0.038) while the effect of log CAD severity was attenuated. No other risk factors contributed (data not shown). When forcing any statin, ACE inhibitor, and beta-blocker use into the models, use of these drugs was neither a significant predictor of adverse outcome nor did use influence the relationship between CFR and adverse outcome. In the combined model, the likelihood ratio test determined that adding CFR significantly improved prediction of major adverse outcomes over the other variables (X2 = 4.37; p = 0.036).
Table 4
Multivariate modeling of major events (Cox regression analysis)
| Predictor | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Log CFR | 1.15 (1.02–1.30) | 0.018 | 1.13 (1.004–1.27) | 0.043 | 1.14 (1.01–1.29) | 0.038 |
| SBP | 1.02 (1.005–1.04) | 0.011 | 1.02 (1.004–1.04) | 0.012 | 1.02 (1.001–1.04) | 0.035 |
| Log CAD Severity | - | 1.68 (0.98–2.88) | 0.058 | 1.61 (0.92–2.81) | 0.10 | |
| Age | - | - | - | - | 1.00 (0.96–1.04) | 0.90 |
| Hx Diabetes | - | - | - | - | 1.44 (0.65–3.20) | 0.37 |
| Ever Smoked | - | - | - | - | 1.22 (0.60–2.46) | 0.58 |
CFR=coronary flow reserve; CAD=coronary artery disease; SBP=systolic blood pressure; Hx=history of; HR=hazard ratio; CI=confidence interval.
Notes: When interpreting these tables, the hazard ratios reflect the % change in events for every 0.1 unit increment in the log CFR. Adjusting for one covariate at a time among other variables in Table 1 did not substantially change the relationship between CFR and major adverse outcome.
Discussion
Many women presenting with ischemic-type symptoms and signs do not have obstructive CAD but have impaired coronary reactivity to adenosine, which has the potential to limit myocardial flow, but an association with adverse outcome is unclear. Most reports did not examine endothelium-independent microvascular responses, few patients had major serious events (most were revascularization), relatively few women were included, and/or gender specific data were not reported.
Nitemberg et al published CV outcome in hypertensive and diabetes patients without CAD, using cold-pressor testing with epicardial coronary diameter measurements, which is related both to endothelial and non endothelial-dependent mechanisms, but no gender stratification or flow responses were reported(3). Halcox et al found a significant association between acetylcholine responses and outcomes(2). Although trends were noted for nitroprusside and adenosine they concluded in this underpowered study that endothelium-independent responses were not predictive of outcome(2). Al Suwaidi et al reported the prognostic value of the response to adenosine and nitroprusside but simply stated that CFR response to adenosine was significantly lower in those with endothelial dysfunction, implying that endothelial-dependent and independent mechanisms show similar responses(6).
Other studies indicate that positron emission tomography (PET) measures of absolute myocardial blood flow (MBF) and CFR or myocardial perfusion reserve(1,2,5,7,10,14) may be abnormal in individuals with risk factors without apparent CAD. Overall these reports document reduced coronary reactivity in individuals with a greater coronary risk factor burden. Except for a report(5) suggesting impaired MBF responses to cold-pressor testing were associated with increased risk of events, the prognostic value of traditional clinical risk assessment versus myocardial perfusion reserve by PET cannot be compared because of lack of follow-up data. Additionally, other studies(37) have demonstrated that maximum MBF and CFR may be impaired in myocardial territories supplied by arteries that do not appear obstructed in patients with obstructive CAD elsewhere. Finally, reduced MBF reserve in patients with hypertrophic(9) or dilated cardiomyopathy(29) has predictive value for prognosis. Thus the overall topic of coronary microvascular dysfunction and its clinical implications has increasing interest(11–13,15–18,38–43).
A better understanding of adverse outcomes associated with dysfunctional microvessels could help to clarify the pathophysiology of ischemic heart disease in women and perhaps identify new targets for both diagnostic testing and therapeutic intervention.
Our findings indicate that endothelium-independent CFR is a predictor of major adverse outcomes in the women studied. The link between CFR and major adverse outcomes remained regardless of presence or absence of obstructive CAD or multiple risk conditions. The low but significant association of this component of coronary reactivity with CAD severity is intriguing, as atherosclerotic plaque is usually localized to conduit arteries and those selected for flow measurement did not have flow-limiting stenoses. Furthermore, among the 152 women without any obstructive stenoses, the link with adverse outcomes remained significant. Although adenosine receptors are present in both endothelial and smooth muscle cells, it is unlikely that dilation of conduit arteries, through either an endothelial or smooth muscle mechanism, could explain our findings. Thus the location of the defect exposed by adenosine in these women is most likely at the microvascular level. This may represent an early manifestation of vascular defects underlying ischemic heart disease in these women, with the potential to contribute to subsequent major adverse outcomes even in the absence of conduit vessel obstruction. Indeed, women in the WISE cohort, including those without obstructive CAD, had a surprisingly high risk for major adverse events during follow-up(44,45). A possible conclusion from this analysis is that non-obstructive CAD (≤49% diameter stenosis) in women is perhaps of greater importance than can be surmised merely from speculation about conduit artery hemodynamic impairment (or lack thereof). Such findings appear to be associated with evidence of microvascular disease and a poorer outcome. Presence of such findings in women might warrant CFR measurements to refine estimates of prognosis. Alternatively, a lower percent narrowing threshold (or CAD score) may be identified in such women that might be useful for prognostic purposes (even if not useful for intervention purposes).
It would be highly desirable to have a simple reliable physiologic measure to identify women at highest risk for adverse outcomes, and this measure could be altered coronary microvascular reactivity. If proven so in other studies, this could lead to more targeted treatment and the reactivity measurement could be non-invasively performed and followed to assess response to treatment and prevention strategies.
Other evidence implicates the coronary microcirculation to explain some findings in ischemic heart disease that are observed in women(46). In the absence of conduit vessel obstruction this includes myocardial metabolic, electrocardiographic, and scintigraphic evidence for ischemia(47); histologic evidence for small vessel disease(48); the predictive value of brain natriuretic peptide and C-reactive protein for adverse outcomes(49); and findings of microvascular obstruction in women dying with acute coronary syndromes (ACS)(50). Even more important is the question, could microvascular dysfunction deteriorate into, or perhaps promote macrovascular disease?
Study Limitations
Although this represents the largest group of women with microvascular reactivity and follow-up data reported, we evaluated a relatively select cohort with ischemic-type symptoms and multiple risk conditions prompting referral for angiography. This indication bias limits generalization of results. Unknown factors, including individual variability in dose responses or in vascular smooth muscle effects of similar CAD risk conditions, could potentially affect CFR. Although adenosine-induced CFR increases are mediated largely via smooth muscle relaxation, endothelium-dependent mechanisms could contribute(51). It is likely that the 18 μg intracoronary adenosine dose may not have achieved near maximal hyperemia in every patient. Similar adenosine doses provide near maximal increase in flow in 90–92% of cases(52), and larger doses used for fractional flow reserve measurement to assess coronary stenosis severity may provide different results. Left ventricular hypertrophy (LVH) may influence coronary microvascular reactivity; however, quantitative echocardiographic analysis from a subgroup of these women without CAD suggested that low CFR could not be explained by LVH. We used only blood flow velocity measurements, but we have previously shown in an analysis from WISE that this measure agrees closely with volumetric flow reserve(35). Finally, despite the relatively large sample of women in this cohort, the low number of major events limits statistical power and creates the risk of over-fitting the models when adding covariates. However, despite this potential problem, the addition of such covariates in no case affected the relationship between CFR and adverse outcomes.
Conclusions
In women undergoing coronary angiography to further evaluate suspected ischemia, a limited coronary microvascular response to adenosine is associated with increased risk for major adverse outcomes even in the absence of significant obstructive CAD. This finding supports the need for more investigation of altered coronary smooth muscle reactivity and the smaller vessels in women with suspected ischemia. Long-term follow-up of new cohorts of women should help to determine if coronary microvascular dysfunction and its link with adverse outcomes can be confirmed and modified.
Acknowledgments
Grant Support: This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, 1R01 HL090957-01A1, T32HL69751, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women's Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Abbreviations
| ACS | acute coronary syndromes |
| CV | cardiovascular |
| CI | confidence intervals |
| CAD | coronary artery disease |
| CFR | coronary flow reserve |
| CHF | congestive heart failure |
| HR | hazard ratios |
| LV | left ventricular |
| MI | myocardial infarction |
| ROC | receiver operating characteristic |
| WISE | Women's Ischemia Syndrome Evaluation |
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jacc.2010.01.054
Free to read at content.onlinejacc.org
http://content.onlinejacc.org/cgi/content/abstract/55/25/2825
Free after 12 months at content.onlinejacc.org
http://content.onlinejacc.org/cgi/reprint/55/25/2825.pdf
Free after 12 months at content.onlinejacc.org
http://content.onlinejacc.org/cgi/content/full/55/25/2825
Citations & impact
Impact metrics
Article citations
The ANOCA/INOCA Dilemma Considering the 2024 ESC Guidelines on Chronic Coronary Syndromes.
J Cardiovasc Dev Dis, 11(10):302, 01 Oct 2024
Cited by: 0 articles | PMID: 39452273 | PMCID: PMC11508505
Coronary microvascular dysfunction in autoimmune rheumatic diseases: beyond coronary flow velocity reserve.
Front Cardiovasc Med, 11:1372703, 21 Aug 2024
Cited by: 0 articles | PMID: 39234606 | PMCID: PMC11371758
Review Free full text in Europe PMC
Evaluation of coronary microvascular dysfunction using magnetocardiography: A new application to an old technology.
Am Heart J Plus, 44:100424, 10 Jul 2024
Cited by: 0 articles | PMID: 39108843 | PMCID: PMC11301233
Heterogeneity of coronary vascular function and myocardial oxygenation in women with angina and non-obstructive coronary artery disease.
Eur Heart J Cardiovasc Imaging, 25(8):1136-1143, 01 Jul 2024
Cited by: 0 articles | PMID: 38546135 | PMCID: PMC11288741
The role of cardiac PET in diagnosis and prognosis of patients with ischemia with no obstructive coronary arteries (INOCA).
Am Heart J Plus, 43:100399, 18 May 2024
Cited by: 1 article | PMID: 38828445 | PMCID: PMC11141139
Go to all (448) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study.
JACC Cardiovasc Interv, 5(6):646-653, 01 Jun 2012
Cited by: 121 articles | PMID: 22721660 | PMCID: PMC3417766
Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study.
J Am Coll Cardiol, 33(6):1469-1475, 01 May 1999
Cited by: 111 articles | PMID: 10334410
Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women.
J Am Coll Cardiol, 73(6):684-693, 01 Feb 2019
Cited by: 100 articles | PMID: 30765035 | PMCID: PMC6383781
Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management.
Curr Probl Cardiol, 36(8):291-318, 01 Aug 2011
Cited by: 66 articles | PMID: 21723447 | PMCID: PMC3132073
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Barbra Streisand Women's Cardiovascular Research and Education Program
Edythe L. Broad Women's Heart Research Fellowship
GCRC (4)
Grant ID: T32HL69751
Grant ID: U01 HL649141
Grant ID: U0164829
Grant ID: U01 HL649241
Gustavus and Louis Pfeiffer Research Foundation
NCRR NIH HHS (2)
Grant ID: M01 RR000425
Grant ID: MO1-RR00425
NHLBI (4)
Grant ID: N01-HV-68161
Grant ID: N01-HV-68162
Grant ID: N01-HV-68164
Grant ID: N01-HV-68163
NHLBI NIH HHS (23)
Grant ID: N01-HV-68162
Grant ID: U01 HL064924
Grant ID: U01 HL064924-04
Grant ID: U01 HL064924-05
Grant ID: U01HL649241
Grant ID: N01HV68162
Grant ID: T32 HL069751
Grant ID: U01 HL064829-02
Grant ID: U01 HL064924-03
Grant ID: U01 HL649141
Grant ID: N01-HV-68163
Grant ID: R01 HL090957
Grant ID: T32HL69751
Grant ID: U01 HL064829-01A1
Grant ID: U01 HL064924-02
Grant ID: N01 HV068161
Grant ID: N01 HV068164
Grant ID: N01HV68163
Grant ID: U01 HL064829
Grant ID: U01 HL064829-04
Grant ID: U01 HL064829-05
Grant ID: U01 HL064924-01A1
Grant ID: U01 HL064829-03
National Center for Research Resources (1)
Grant ID: MO1-RR00425
PHS HHS (1)
Grant ID: U0164829





