Abstract
Objective
To examine the effects of acute insulin-induced hypoglycemia on inflammation, endothelial dysfunction, and platelet activation in adults with and without type 1 diabetes.Research design and methods
We studied 16 nondiabetic adults and 16 subjects with type 1 diabetes during euglycemia (blood glucose 4.5 mmol/l) and hypoglycemia (blood glucose 2.5 mmol/l). Markers of inflammation, thrombosis, and endothelial dysfunction (soluble P-selectin, interleukin-6, von Willebrand factor [vWF], tissue plasminogen activator [tPA], high-sensitivity C-reactive protein [hsCRP], and soluble CD40 ligand [sCD40L]) were measured; platelet-monocyte aggregation and CD40 expression on monocytes were determined using flow cytometry.Results
In nondiabetic participants, platelet activation occurred after hypoglycemia, with increments in platelet-monocyte aggregation and P-selectin (P <or= 0.02). Inflammation was triggered with CD40 expression increasing maximally at 24 h (3.13 +/- 2.3% vs. 2.06 +/- 1.0%) after hypoglycemia (P = 0.009). Both sCD40L and hsCRP (P = 0.02) increased with a nonsignificant rise in vWF and tPA, indicating a possible endothelial effect. A reduction in sCD40L, tPA, and P-selectin occurred during euglycemia (P = 0.03, P <or= 0.006, and P = 0.006, respectively). In type 1 diabetes, both CD40 expression (5.54 +/- 4.4% vs. 3.65 +/- 1.8%; P = 0.006) and plasma sCD40L concentrations increased during hypoglycemia (peak 3.41 +/- 3.2 vs. 2.85 +/- 2.8 ng/ml; P = 0.03). Platelet-monocyte aggregation also increased significantly at 24 h after hypoglycemia (P = 0.03). A decline in vWF and P-selectin occurred during euglycemia (P <or= 0.04).Conclusions
Acute hypoglycemia may provoke upregulation and release of vasoactive substances in adults with and without type 1 diabetes. This may be a putative mechanism for hypoglycemia-induced vascular injury.Free full text

Effects of Acute Insulin-Induced Hypoglycemia on Indices of Inflammation
Abstract
OBJECTIVE
To examine the effects of acute insulin-induced hypoglycemia on inflammation, endothelial dysfunction, and platelet activation in adults with and without type 1 diabetes.
RESEARCH DESIGN AND METHODS
We studied 16 nondiabetic adults and 16 subjects with type 1 diabetes during euglycemia (blood glucose 4.5 mmol/l) and hypoglycemia (blood glucose 2.5 mmol/l). Markers of inflammation, thrombosis, and endothelial dysfunction (soluble P-selectin, interleukin-6, von Willebrand factor [vWF], tissue plasminogen activator [tPA], high-sensitivity C-reactive protein [hsCRP], and soluble CD40 ligand [sCD40L]) were measured; platelet-monocyte aggregation and CD40 expression on monocytes were determined using flow cytometry.
RESULTS
In nondiabetic participants, platelet activation occurred after hypoglycemia, with increments in platelet-monocyte aggregation and P-selectin (P ≤ 0.02). Inflammation was triggered with CD40 expression increasing maximally at 24 h (3.13 ± 2.3% vs. 2.06 ± 1.0%) after hypoglycemia (P = 0.009). Both sCD40L and hsCRP (P = 0.02) increased with a nonsignificant rise in vWF and tPA, indicating a possible endothelial effect. A reduction in sCD40L, tPA, and P-selectin occurred during euglycemia (P = 0.03, P ≤ 0.006, and P = 0.006, respectively). In type 1 diabetes, both CD40 expression (5.54 ± 4.4% vs. 3.65 ± 1.8%; P = 0.006) and plasma sCD40L concentrations increased during hypoglycemia (peak 3.41 ± 3.2 vs. 2.85 ± 2.8 ng/ml; P = 0.03). Platelet-monocyte aggregation also increased significantly at 24 h after hypoglycemia (P = 0.03). A decline in vWF and P-selectin occurred during euglycemia (P ≤ 0.04).
CONCLUSIONS
Acute hypoglycemia may provoke upregulation and release of vasoactive substances in adults with and without type 1 diabetes. This may be a putative mechanism for hypoglycemia-induced vascular injury.
In people with type 1 diabetes the rapid institution of strict glycemic control aggravates microvascular complications, particularly retinopathy (1). Although attributed to reduced capillary blood flow causing localized ischemia (1), greater exposure to hypoglycemia may have worsened microangiopathy through its putative effects on local vasculature (2). In addition, cardiovascular stress associated with hypoglycemia may precipitate acute macrovascular events in a diseased circulation. While supported by anecdotal reports (3), the increase in cardiovascular mortality in people with type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (4) (and possibly in the Veterans Affairs Diabetes Trial [5]), in which intensive treatment had tripled the frequency of severe hypoglycemia, has caused concern.
Possible mechanisms by which hypoglycemia may damage blood vessels include changes in regional blood flow, mobilization and activation of neutrophils, platelet activation, and enhanced coagulation and viscosity of the blood (3,6–8). Plasma concentrations of C-reactive protein, interleukin-6 (IL-6), and endothelin-1 increase during hypoglycemia (9–11) and may promote vascular disease (12).
Investigation of processes operating at a cellular level to cause atherosclerosis has focused on the potential influences of vascular inflammation, endothelial dysfunction, coagulation, and platelet activation. The present study sought to determine the effects of acute insulin-induced hypoglycemia on inflammation, coagulation, and platelet and monocyte function in adults with and without type 1 diabetes.
RESEARCH DESIGN AND METHODS
Participants in the study included 16 nondiabetic adult volunteers with no medical history and 16 healthy adults with type 1 diabetes (Table 1). Those with diabetes had no history of hypertension or macrovascular disease, and microvascular disease was excluded. Screening for retinopathy used digital retinal photography, absence of neuropathy was confirmed by clinical examination, and nephropathy was excluded by the absence of microalbuminuria. Subjects with a history of impaired awareness of hypoglycemia or a previous serious reaction to hypoglycemia were excluded. None had a history of head injury, seizure, blackouts, alcohol or drug abuse and psychiatric illness, and their only other medication was the contraceptive pill. Diabetes Control and Complications Trial–aligned A1C was measured using high performance liquid chromatography (nondiabetic reference range 5.0–6.05%; Bio-Rad Laboratories, Munich, Germany); the mean ± SD of the participants with diabetes was 7.91 ± 0.92%. All gave written informed consent before participation, and the study was approved by the Local Medical Research Ethics Committee.
Table 1
Baseline demographic characteristics
| Nondiabetic subjects | Subjects with diabetes | |
|---|---|---|
| n | 16 | 16 |
| Age (years) | 28 (26.7–35) | 28 (25–37.5) |
| BMI (kg/m2) | 22.86 ± 2.4 | 26.40 ± 4.0 |
| Male/female | 6/10 | 7/9 |
| Duration of diabetes (years) | N/A | 10 (4.2–19) |
| A1C (%) | N/A | 7.91 ± 0.9 |
Data are median (interquartile range) and means ± SD unless otherwise indicated.
A modified hyperinsulinemic glucose clamp (13) was used to maintain blood glucose at a predetermined level: euglycemia at 4.5 mmol/l and hypoglycemia at 2.5 mmol/l. Each subject underwent two laboratory sessions, separated by at least 2 weeks (mean 7.2 weeks), of a euglycemic study and a hypoglycemic study in a randomized, counterbalanced fashion.
The participants with type 1 diabetes monitored blood glucose intensively during the 48 h preceding each study, which was postponed if any blood glucose value was <3.5 mmol/l or if symptoms suggestive of hypoglycemia were experienced. After fasting overnight, morning insulin was withheld. A retrograde-intravenous cannula for blood-glucose sampling was inserted into the nondominant hand, which was heated to arterialize the venous blood (14). A cannula in the nondominant antecubital fossa was used to infuse 20% dextrose and soluble insulin (Human Actrapid; Novo Nordisk, Crawley, U.K.) at a constant rate of 1.5 mU/kg/min using a Gemini PCI pump (Alaris Medical Systems, San Diego, CA). The dextrose was infused at a variable rate depending on arterialized blood glucose concentrations, which were measured at 5 min intervals using the glucose oxidase method (2300 Stat; Yellow Springs Instruments, Yellow Springs, OH). A third cannula in the other antecubital fossa was dedicated to blood sampling for inflammatory markers.
On each study day, the arterialized blood glucose was stabilized initially at 4.5 mmol/l for 30 min and either maintained at that level (euglycemia) or lowered over 20 min to 2.5 mmol/l for 60 min (hypoglycemia), after which blood glucose was restored to 4.5 mmol/l. Subjects consumed a standardized meal after each study. Blood sample time points were: baseline, during the experimental session (+45 min), during recovery (+105 min), at +6 h, and at +24 h.
Flow cytometry
Whole blood samples were collected at the predetermined time points using d-Phenylalanyl-l-prolyl-l-arginine chloromethyl ketone, a selective thrombin inhibitor, as an anticoagulant. Samples (100 μl) of whole blood were immediately incubated with 10 μl of each monoclonal antibody (AbD Serotec, Kidlington, U.K.) for 30 min at room temperature, with subsequent red cell lysis by the addition of 1 ml of fluorescent-activated cell sorter (FACS) Lyse solution (Becton Dickinson, Oxford, U.K.). Flow cytometry using the FACS Calibur system (Becton Dickinson, Oxford, U.K.) was performed immediately after the experimental session to assess platelet-monocyte aggregation (CD14/CD42a) and CD40 expression on monocytes (CD14/CD40). Isotype controls were performed in addition to both mono- and dual-stain for each parameter assessed at each time point.
Soluble marker assays
Citrated plasma and serum samples were collected at the predetermined time points. These were separated immediately and frozen at −80°C until analysis for the soluble markers:
Von Willebrand factor (vWF) (enzyme-linked immunosorbent assay [ELISA]; coefficient of variation [CV] 7.3%), tissue plasminogen activator (tPA) antigen (Hyphen Biomed Zymutest; intra-assay CV 3.5%, inter-assay CV 4.4%), soluble CD40 ligand (sCD40L) (high sensitivity ELISA, Bender Medsystems; intra-assay CV 5.5%, inter-assay CV 7.2%), soluble P-selectin (ELISA, R&D Systems; intra-assay CV 5.1%, inter-assay CV 8.8%), IL-6 (High sensitivity ELISA, R&D Systems; intra-assay CV 5.9%, inter-assay CV 9.9%), and high sensitivity CRP (DRG Diagnostics; DRG Instruments, Marburg, Germany; intra-assay CV 4.2%, inter-assay CV 4.1%).
Catecholamine assays
Samples for epinephrine quantification were collected in EDTA tubes and immediately separated and frozen at −80°C until analysis by high-performance liquid chromatography and electrochemical detection (intra-assay CV 1.2%, inter-assay CV 3.9%).
Hypoglycemia symptom score
The Edinburgh Hypoglycemia Scale (15) was used to assess the symptoms experienced during each experimental session.
Statistical analyses
Results were analyzed using SPSS version 15.0 for Windows (SPSS, Chicago, IL). A general linear model (repeated-measures ANOVA) was used, with order of session (euglycemia-hypoglycemia or hypoglycemia-euglycemia) as a between-subjects factor, and condition (euglycemia or hypoglycemia) as a within-subjects factor, to compare hypoglycemia with euglycemia. Additional analysis using paired t tests was performed to assess the change in any given parameter from baseline. A P value <0.05 was considered to be significant. Results are reported as mean ± SD unless otherwise stated.
RESULTS
Hypoglycemia provoked a symptomatic response in all subjects with increased scores of autonomic (P ≤ 0.002), neuroglycopenic (P < 0.001), and malaise (P ≤ 0.008) symptoms compared with baseline. Comparison of baseline levels of inflammatory, endothelial and platelet markers in nondiabetic subjects and subjects with type 1 diabetes showed a significantly higher concentration of soluble P-selectin (P = 0.01) and of CD40 expression on monocytes (P = 0.006) in those with diabetes, demonstrating the chronic inflammatory response associated with diabetes.
Blood glucose
Target blood glucose concentrations were achieved (Fig. 1). In nondiabetic subjects, blood glucose concentrations were 2.58 ± 0.2 and 4.42 ± 0.5 mmol/l during hypoglycemia and euglycemia, respectively. In those with type 1 diabetes, blood glucose concentrations were 2.46 ± 0.22 and 4.53 ± 0.24 mmol/l, respectively. The blood glucose nadir was similar in both groups.
Counterregulatory response
Plasma epinephrine increased during hypoglycemia in participants with and without type 1 diabetes (P ≤ 0.001; Fig. 1). The epinephrine response occurred only during hypoglycemia and returned rapidly to baseline as anticipated (16).
Platelet activation
Platelet-monocyte aggregation.
In nondiabetic subjects, platelet-monocyte aggregation appeared to rise, from a baseline level of 0.72 ± 0.8% to 3.09 ± 8.1% during hypoglycemia, with a peak of 3.49 ± 10.4% at 24 h (Fig. 2). Platelet-monocyte aggregation remained unchanged throughout euglycemia. The difference between conditions, and from baseline, did not achieve statistical significance.
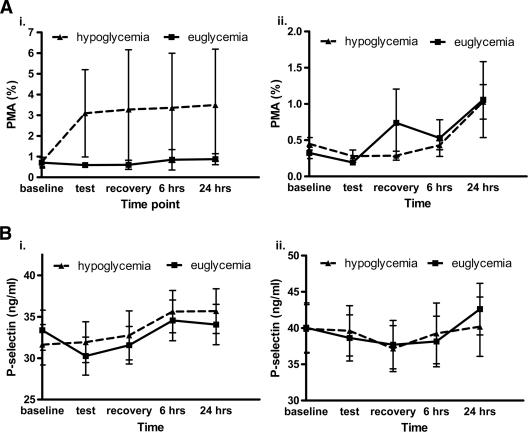
Platelet activation in response to experimental hypoglycemia and euglycemia. A: Platelet-monocyte aggregation. B: Soluble P-selectin, i. Nondiabetic subjects, ii. Subjects with type 1 diabetes.
In participants with diabetes, there was a late rise in platelet-monocyte aggregation after hypoglycemia at 24 h compared with baseline (P = 0.03).
Soluble P-selectin.
Soluble plasma P-selectin concentrations increased after hypoglycemia in nondiabetic subjects, exhibiting a late response at 6 h (P = 0.01) and 24 h (P = 0.02; Fig. 2) but decreasing during euglycemia (P = 0.006).
P-selectin also decreased during euglycemia in the diabetic group (P = 0.04), but did not change during hypoglycemia.
Endothelial markers
tPA.
In nondiabetic subjects, plasma tPA concentrations increased during hypoglycemia, with a higher peak tPA concentration (12.55 ± 16.7 compared with 6.80 ± 7.9 ng/ml) (NS between conditions). Plasma tPA decreased significantly between baseline and test phase (P = 0.004) and recovery phase (P = 0.006), with a paradoxical rise between baseline and 24 h (P = 0.06) after euglycemia (Table 2). However, a diurnal variation in tPA concentration is recognized to occur, which may account for the decline observed during euglycemia (17). No significant differences occurred in the diabetic group (Table 2).
Table 2
Endothelial function and inflammation in nondiabetic subjects and subjects with type 1 diabetes
| Euglycemia | Hypoglycemia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Test | Recovery | +6 h | +24 h | Baseline | Test | Recovery | +6 h | +24 h | |
| Nondiabetic subjects | ||||||||||
    tPA (ng/ml) tPA (ng/ml) | 7.37 ± 8.1 | 6.80 ± 7.9* | 6.44 ± 7.5* | 6.99 ± 9.3 | 8.51 ± 7.8† | 10.96 ± 11.8 | 12.55 ± 16.7 | 9.10 ± 10.2* | 9.83 ± 12.0 | 11.45 ± 11.6 |
    vWF (iU/ml) vWF (iU/ml) | 0.81 ± 0.3 | 0.76 ± 0.3 | 0.78 ± 0.2 | 0.80 ± 0.3 | 0.85 ± 0.3 | 0.82 ± 0.3 | 0.81 ± 0.3 | 0.89 ± 0.5 | 0.90 ± 0.3 | 0.89 ± 0.3 |
    CD40 (%) CD40 (%) | 1.51 ± 1.4 | 2.23 ± 3.2† | 2.40 ± 3.2† | 0.84 ± 0.7 | 2.06 ± 1.0 | 1.92 ± 2.2 | 1.47 ± 1.1 | 1.55 ± 1.5 | 1.98 ± 2.4‡ | 3.13 ± 2.3†‡ |
    sCD40L (ng/ml) sCD40L (ng/ml) | 2.68 ± 3.1 | 2.41 ± 2.8 | 2.40 ± 2.9* | 2.63 ± 2.9 | 3.08 ± 3.3 | 2.88 ± 3.3 | 2.80 ± 3.2 | 2.55 ± 3.2 | 2.72 ± 3.3 | 2.79 ± 3.2 |
    IL-6 (pg/ml) IL-6 (pg/ml) | 0.86 ± 0.5 | 1.06 ± 1.2 | 1.05 ± 1.0 | 5.98 ± 4.6† | 1.23 ± 0.9 | 0.72 ± 0.4 | 0.92 ± 0.5 | 1.62 ± 1.2† | 4.37 ± 4.3† | 1.00 ± 0.9 |
    hsCRP (ng/ml) hsCRP (ng/ml) | 1.04 ± 1.1 | 1.22 ± 1.9 | 1.18 ± 1.9 | 1.24 ± 1.6 | 1.31 ± 1.5 | 1.83 ± 1.5‡ | 1.81 ± 1.9‡ | 1.56 ± 1.3* | 1.69 ± 1.2 | 1.90 ± 1.6 |
| Subjects with type 1 diabetes | ||||||||||
    tPA (ng/ml) tPA (ng/ml) | 15.25 ± 30.2 | 17.70 ± 31.1 | 15.99 ± 27.5 | 22.13 ± 46.2 | 20.86 ± 34.8 | 18.12 ± 30.1 | 20.55 ± 36.1 | 17.69 ± 31.1 | 18.37 ± 32.6 | 22.98 ± 40.3 |
    vWF (iU/ml) vWF (iU/ml) | 0.91 ± 0.2 | 0.85 ± 0.2* | 0.91 ± 0.3 | 0.85 ± 0.2* | 0.99 ± 0.2 | 0.93 ± 0.2 | 0.95 ± 0.2 | 0.91 ± 0.2 | 0.90 ± 0.2 | 1.02 ± 0.2 |
    CD40 (%) CD40 (%) | 3.64 ± 2.0 | 3.65 ± 1.8 | 4.14 ± 2.5 | 3.97 ± 2.3 | 4.35 ± 2.0 | 3.69 ± 3.4 | 5.54 ± 4.4† | 3.36 ± 3.0 | 4.88 ± 2.4 | 4.70 ± 2.8 |
    sCD40L (ng/ml) sCD40L (ng/ml) | 2.86 ± 2.8 | 2.85 ± 2.8 | 2.84 ± 2.8 | 2.91 ± 2.9 | 3.25 ± 3.2† | 3.36 ± 2.9‡ | 3.41 ± 3.2‡ | 3.10 ± 2.9* | 3.05 ± 2.8* | 3.44 ± 2.9 |
    IL-6 (pg/ml) IL-6 (pg/ml) | 0.69 ± 0.5 | 1.38 ± 1.9 | 1.58 ± 1.8 | 2.25 ± 2.8† | 1.19 ± 1.2 | 1.21 ± 1.7 | 1.15 ± 1.5) | 1.76 ± 1.5 | 3.10 ± 4.9 | 1.96 ± 2.2 |
    hsCRP (ng/ml) hsCRP (ng/ml) | 2.52 ± 3.1 | 2.20 ± 2.9 | 2.32 ± 2.8 | 1.92 ± 1.8 | 3.40 ± 3.6 | 2.84 ± 3.2 | 2.72 ± 3.1 | 2.70 ± 3.2 | 2.89 ± 3.3 | 2.34 ± 2.8 |
Data are means ± SD.
*Significant decrease from baseline (P < 0.05);
†significant increase from baseline (P < 0.05);
‡significant difference between hypoglycemia and euglycemia (P < 0.05).
vWF.
A trend toward a difference in plasma vWF concentrations was observed between hypoglycemia and euglycemia at 6 h in the nondiabetic subjects (P = 0.07) (Table 2).
Plasma vWF concentrations decreased between baseline and test phase (P = 0.02) and recovery phase (P = 0.03) after euglycemia in the participants with diabetes. No such decrement was observed during hypoglycemia (Table 2).
Inflammation
CD40 expression.
CD40 expression on monocytes increased after hypoglycemia in nondiabetic subjects, from a baseline of 1.92 ± 2.2% to a maximum of 3.13 ± 2.3% at 24 h (P = 0.009). A significant difference between hypoglycemia and euglycemia conditions was present at 6 h (P = 0.05) and at 24 h (P = 0.04) (Table 2).
In participants with type 1 diabetes, monocyte CD40 expression increased from 3.69 ± 3.4% to 5.54 ± 4.4% during hypoglycemia (P = 0.006), compared with no change during euglycemia (3.64 ± 2.0% to 3.65 ± 1.8%, respectively; P = NS). The increment during hypoglycemia had dissipated by the time of the recovery phase and remained unchanged thereafter (Table 2).
sCD40L.
In nondiabetic subjects, plasma sCD40L concentrations were higher during hypoglycemia than during euglycemia (2.80 ± 3.2 vs. 2.41 ± 2.8 ng/ml), with a trend toward significance (P = 0.09). A significant reduction in sCD40L concentration occurred during euglycemia between baseline and recovery phase (P = 0.03) (Table 2).
In those with diabetes, a significant difference was observed between the baseline levels on each study day: 3.36 ± 2.9 ng/ml on the hypoglycemia day compared with 2.86 ± 2.8 ng/ml on euglycemia day (P = 0.03), rendering subsequent measurements difficult to compare. A significant difference was again observed between the experimental condition levels, with a level of 3.41 ± 3.2 ng/ml during hypoglycemia and 2.85 ± 2.8 ng/ml during euglycemia (P = 0.03) (Table 2). Changes from baseline did not achieve significance.
IL-6.
IL-6 levels rose in all experiments, maximally at 6 h, irrespective of condition, with no clear differences identifiable in either group between the study conditions (Table 2).
hsCRP.
Test phase hsCRP was higher in all subjects during hypoglycemia (1.81 ± 1.9 vs. 1.22 ± 1.9 ng/ml in nondiabetic participants [P = 0.02]; 2.72 ± 3.1 vs. 2.20 ± 2.9 ng/ml in subjects with diabetes [P = ns]) (Table 2). A significant difference was observed in the baseline concentrations in the nondiabetic participants (P = 0.01), frustrating interpretation of subsequent responses.
CONCLUSIONS
Previous studies have demonstrated that hypercoagulability, platelet and neutrophil activation, C-reactive protein, IL-6, and Endothelin-1 are upregulated after acute hypoglycemia (3,6–11), while a euglycemic insulin infusion (for at least 2 h) was shown to reduce inflammatory markers, consistent with an anti-inflammatory effect of insulin (18). The present study sought to replicate these effects, while investigating other underlying mechanisms of vascular disease, and tests were selected to investigate the effect of acute hypoglycemia on important cellular processes (platelet activation, endothelial dysfunction and inflammation) underlying the development of acute and chronic vascular complications in type 1 diabetes.
The present study showed that hypoglycemia generated a response in some of these markers, suggesting that hypoglycemia-induced metabolic stress may have adverse pathophysiological consequences while the euglycemic insulin infusion caused a potentially beneficial decrement in some parameters. However, the magnitude of most observed changes was small, and not all markers changed significantly.
The present study confirmed that platelet activation is promoted by hypoglycemia (8), with increments both in platelet-monocyte aggregation and soluble P-selectin. Conversely, P-selectin decreased during euglycemia. Endothelial function, using vWF and tPA Ag as surrogate markers, may have been disrupted, as shown by the increase in vWF after hypoglycemia in nondiabetic volunteers, but this change was not replicated in those with diabetes. However, a reduction in vWF occurred after euglycemia in diabetic participants, which should confer vascular benefit. tPA Ag also appeared to increase in nondiabetic subjects during hypoglycemia, while declining during euglycemia, whereas no significant changes occurred in the diabetic group. Soluble markers of inflammation, sCD40L and hsCRP, were higher during hypoglycemia, with an elevation of hsCRP being observed in all subjects. Unfortunately, baseline differences in hsCRP in nondiabetic subjects, and in sCD40L in the diabetic subjects, frustrated interpretation of subsequent responses. sCD40L was apparently reduced during euglycemia in nondiabetic participants. Surprisingly, IL-6 increased in all experiments regardless of glycemic status, with a maximal response at 6 h. This response is inexplicable, and contrasts with a previous report (10). Monocyte CD40 expression also increased, suggesting promotion of the interaction of the CD40-CD40 ligand dyad (from the tumor necrosis factor receptor family), thus affecting another process in the pathway leading to atherosclerotic plaque rupture (19,20). This change occurred much earlier in the diabetic than the nondiabetic subjects, in whom the response was delayed, prolonged, and still present at 24 h. The persistence of these vascular changes for 24 h after the hypoglycemic stimulus, or their later emergence, suggests that the period of risk after hypoglycemia may be present long after blood glucose recovery.
For some markers, a positive trend after hypoglycemia was evident, without achieving statistical significance, or the only measurable difference between conditions was a beneficial effect associated with euglycemia. The sample size may have been insufficient to achieve significance, particularly as the magnitude of responses was small. It was not feasible to study a larger number of subjects using a procedure that is labor-intensive and costly. In a previous study, larger increments in inflammatory markers were observed during an insulin tolerance test, where hypoglycemia of <39 mg/dl (<2.2 mmol/l) was induced (21). The more rapid reduction to a lower blood glucose causing a greater hypoglycemic stimulus may have heightened the magnitude of the responses, compared with the more modest changes that occurred during a controlled glucose clamp (blood glucose 2.5 mmol/l [45 mg/dl]), as observed in the present study. A further limitation of the present study was the need to examine the experimental conditions on two separate days in a counterbalanced fashion. Because the baseline levels of many inflammatory markers can differ on separate days, as was observed with sCD40L and hsCRP, this biological variability hinders the interpretation and comparison of subsequent results. However, the present study design was necessary to allow comparison of the euglycemia and hypoglycemia conditions in individual subjects, as both time and insulin infusion per se may exert effects on biomarker levels. This study design cannot control for other day-to-day factors that could influence baseline levels of inflammatory markers. However, the effects of hypoglycemia could be evaluated, as each participant acted as their own control. This produces less variability than a comparison of results among individuals, as more inter-individual variation in inflammatory marker levels is present than intra-individual variation. In addition, it was possible to analyze each study separately, by examining changes in parameters from baseline on that particular day, enabling the detection of significant effects exerted by hypoglycemia compared with euglycemia. Baseline levels of all markers (except IL-6) were higher in the diabetic group (significant for P-selectin and CD40 expression). This could affect the magnitude of response induced by the experimental procedures. However, an analysis of the percentage change from baseline was consistent with the trends identified in the absolute results (shown as in the online appendix available at http://care.diabetesjournals.org/cgi/content/full/dc10-0013/DC1).
As anticipated, epinephrine secretion was stimulated by hypoglycemia. It is likely that hormonal changes underlie the activation and upregulation of the vascular biomarkers. Catecholamines promote platelet activation (22), while adrenoceptor blockade attenuates these effects (23,24). The participants with type 1 diabetes exhibited attenuated plasma epinephrine responses to hypoglycemia compared with the nondiabetic subjects, who were naïve to such a hypoglycemic stimulus, this being consistent with the recognized decline in the magnitude of counterregulatory hormonal responses with increasing duration of type 1 diabetes (25). This attenuated epinephrine response may explain the lower responses of vascular biomarkers to hypoglycemia.
In summary, the effects of hypoglycemia on several vascular biomarkers that are implicated in the pathogenesis of vascular disease, would support the premise that acute hypoglycemia may be detrimental to an already diseased vasculature (2). Euglycemia may have a protective, anti-inflammatory effect. In the present study, the participants had no overt vascular disease and were unlikely to develop any demonstrable effects from a short period of exposure to hypoglycemia. However, in people with diabetes of long duration, who are likely to have underlying vascular disease, these responses may not be benign. The release of potent vasoactive substances could potentially aggravate chronic vasculopathy, and contribute to the precipitation of acute macrovascular events. This may aggravate established diabetic micro- and macrovascular disease in those who are exposed to recurrent hypoglycemia.
Acknowledgments
The cost of assays was supported by research grants from the Scottish Society of Physicians and the Edinburgh branch of Diabetes UK.
No potential conflicts of interest relevant to this article were reported.
Footnotes
References
Articles from Diabetes Care are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/dc10-0013
Read article for free, from open access legal sources, via Unpaywall:
https://diabetesjournals.org/care/article-pdf/33/7/1591/607020/zdc00710001591.pdf
Free to read at intl-care.diabetesjournals.org
http://intl-care.diabetesjournals.org/cgi/content/abstract/33/7/1591
Free after 6 months at intl-care.diabetesjournals.org
http://intl-care.diabetesjournals.org/cgi/reprint/33/7/1591.pdf
Free after 6 months at intl-care.diabetesjournals.org
http://intl-care.diabetesjournals.org/cgi/content/full/33/7/1591
Citations & impact
Impact metrics
Citations of article over time
Article citations
Estimating risk of consequences following hypoglycaemia exposure using the Hypo-RESOLVE cohort: a secondary analysis of pooled data from insulin clinical trials.
Diabetologia, 67(10):2210-2224, 22 Jul 2024
Cited by: 0 articles | PMID: 39037602 | PMCID: PMC11447089
Effect of Hypoglycemia and Rebound Hyperglycemia on Proteomic Cardiovascular Risk Biomarkers.
Biomedicines, 12(6):1137, 21 May 2024
Cited by: 0 articles | PMID: 38927344
Association of perioperative glucose profiles assessed by continuous glucose monitoring (CGM) with prognosis in Chinese patients with non-ST-elevation acute coronary syndrome: a cohort study protocol.
BMJ Open, 14(6):e079666, 12 Jun 2024
Cited by: 0 articles | PMID: 38866564 | PMCID: PMC11177667
Cardiometabolic Risk Factors among Women with Eating Disorders in Saudi Arabia.
J Nutr Metab, 2024:5953893, 05 Jun 2024
Cited by: 0 articles | PMID: 38867850
Assessing the impact of body composition, metabolic and oxidative stress parameters on insulin resistance as a prognostic marker for reactive hypoglycemia: a cross-sectional study in overweight, obese, and normal weight individuals.
Front Pharmacol, 15:1329802, 09 Apr 2024
Cited by: 0 articles | PMID: 38655176 | PMCID: PMC11035812
Go to all (117) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals.
Diabetes Care, 33(7):1529-1535, 01 Jul 2010
Cited by: 136 articles | PMID: 20587723 | PMCID: PMC2890354
Enhanced P-selectin expression and increased soluble CD40 Ligand in patients with Type 1 diabetes mellitus and microangiopathy: evidence for platelet hyperactivity and chronic inflammation.
Diabetologia, 47(3):537-540, 13 Feb 2004
Cited by: 56 articles | PMID: 14963650
Influence of glycaemic control on platelet bound CD40-CD40L system, P-selectin and soluble CD40 ligand in Type 2 diabetes.
Diabet Med, 27(4):384-390, 01 Apr 2010
Cited by: 23 articles | PMID: 20536508
Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies.
Cardiovasc Diabetol, 17(1):121, 31 Aug 2018
Cited by: 262 articles | PMID: 30170601 | PMCID: PMC6117983
Review Free full text in Europe PMC





