Abstract
Free full text

A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma†
Abstract
The objectives of this study were to determine the safety and efficacy of polyinosinic-polycytidylic acid stabilized with poly-l-lysine and carboxymethylcellulose (poly-ICLC) when added to radiation and temozolomide (TMZ) in adults with newly diagnosed glioblastoma (GB). Patients received external beam radiation with concurrent TMZ (75 mg/m2/day) followed by adjuvant TMZ (150–200 mg/m2/day for 5 consecutive days once every 9 weeks) and intramuscular poly-ICLC (20 mg/kg/dose given 3× per week for weeks 2–8). An adjuvant cycle was operationally defined as 9 weeks and patients continued adjuvant therapy until toxicity or disease progression. Ninety-seven patients were enrolled (60 men) with a median age of 56 years (range 21–85) and Karnofsky performance status of 90% (range 60%–100%). Fourteen patients did not start adjuvant treatment. Common treatment-related Grade 3–4 toxicities included neutropenia (20.6%), leukopenia (16.5%), thrombocytopenia (9%), and rash (1%). The entire cohort had a median survival of 17.2 months (95% CI: 15.5–19.3 months) with survival at 12, 18, and 24 months of 73.2%, 47.4%, and 29.9%. For subjects 18–70 years old, median overall survival was 18.3 months (95% CI: 15.9–19.8 months), as compared with 14.6 (95% CI: 13.2–16.8) reported by the EORTC 26981/22981 trial. These results demonstrate that poly-ICLC can be added to standard radiation and TMZ in patients with newly diagnosed GB without additional significant toxicities. Survival data at 12 and 18 months suggest that this may improve the efficacy of chemoradiation and adjuvant TMZ in this patient population.
For a subset of patients with newly diagnosed glioblastoma (GB) the addition of temozolomide (TMZ) (both concurrent and adjuvant) to radiotherapy results in improved overall survival.1 Nonetheless, even with this therapy only 10% of patients survive 5 years and the majority of patients still succumb to disease progression within 2 years of diagnosis.2 A rational approach to improving current survival outcomes in patients with newly diagnosed GB is to coadminister novel agents that add to the efficacy of the standard of care without increasing toxicity.
Polyinosinic–polycytidylic acid stabilized with poly-l-lysine and carboxymethylcellulose (poly-ICLC, Hiltonol®, Oncovir) is a synthetic double-stranded ribonucleic acid (dsRNA) viral mimic or pathogen associated molecular pattern (PAMP) that activates multiple elements of innate and adaptive immunity including induction of a ‘natural mix’ of interferons, cytokines and chemokines, activation of NK cells, myeloid dendritic cells via TLR3 and MDA5, T4 cells, and cytotoxic lymphocytes.3,4
Poly-ICLC in addition has antiproliferative and antiviral effects mediated by the interferon inducible, dsRNA dependent 2′-5′-oligoadenylate synthetase, P68 protein kinase, and RIG-I helicase enzyme systems.5–7 Furthermore, poly-ICLC has been shown to independently up-regulate or down-regulate a broad variety of genes in glioma cell lines lacking the interferon response gene. Some of these genes play critical roles in controlling cellular functions, including protein synthesis, programmed (apoptotic) cell death, cell metabolism, and cellular growth.8–10
There is limited prior experience with poly-ICLC in glioma and none when combined with TMZ. A pilot study of 38 patients with newly diagnosed or recurrent anaplastic astrocytoma or GB evaluated the safety of poly-ICLC given 1–3 times weekly after completion of radiation.11 This was a mixed population and 20 of the 38 subjects also received 1 or more doses of lomustine. The study demonstrated the safety of poly-ICLC in these patients and suggested efficacy in that 66% of all subjects receiving poly-ICLC twice a week or more showed MRI regression or stabilization for at least 6 months. Median survival was 19 months and 6.5 years for newly diagnosed GB and anaplastic astrocytoma, respectively. Two recent phase II studies performed by the North American Brain Tumor Consortium (NABTC) confirmed the safety of poly-ICLC in both newly diagnosed GB and recurrent anaplastic astrocytoma.12,13 Although there was no improvement in 6 month progression free survival with poly-ICLC as a single agent in patients with recurrent anaplastic tumors, the median overall survival (mOS) of 15 months in newly diagnosed GB patients given poly-ICLC alone after radiation suggested an advantage over radiation alone.
The current standard of care for patients with newly diagnosed GB after optimal resection is radiation therapy with concurrent TMZ followed by 6 months of adjuvant TMZ based on the results from a phase III study carried out by the EORTC.1 On the basis of preclinical and the above mentioned clinical studies, we hypothesized that the combination of standard radiation and concurrent TMZ followed by adjuvant TMZ and poly-ICLC may prove more effective and improve overall survival in adults with newly diagnosed GB.
Patients and Methods
Patient Population
This was a noncomparative, open-label, multi-center, phase II clinical trial in patients with newly diagnosed GB conducted by the New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium. The study was reviewed and approved by the National Cancer Institute and the individual institutional review board of each participating institution and informed consent was obtained from each subject. Patients ≥18 years of age (no upper age limit) with histologically confirmed supratentorial GB diagnosed no more than 3 months prior to registration were eligible. Other required criteria included no prior tumor directed therapy other than surgery, Karnofsky performance status (KPS) ≥ 60%, normal hematologic, renal, and liver function (transaminases ≤4 times above the upper limits of the institutional normal was allowed), ability to provide written informed consent and Mini-Mental State Exam score of ≥15. Exclusion criteria included pregnancy or breast feeding, a concurrent or prior malignancy except curatively treated carcinoma in situ or basal cell carcinoma of the skin or free of disease for ≥5 years. Patients who received Gliadel wafers were not included in the study.
Treatment Plan
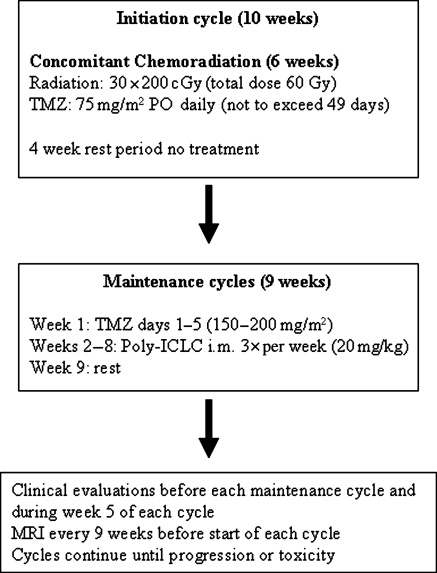
The overall treatment schema is shown in Fig. 1. All patients were enrolled after craniotomy and before the start of chemoradiation. Commencement of protocol therapy began as soon as medically appropriate but no later than 3 months after craniotomy or biopsy. All patients received conventional external beam radiation therapy as utilized by the Radiation Therapy Oncology Group (RTOG). This consists of treatment to the contrast-enhancing tumor volume plus the T2 weighted tumor volume and a 1‐cm margin for a tumor dose of 6000 cGy in 30 fractions delivered with megavoltage machines with energy ranging from 6 MV up to and including 18 MV photons. Electron, particle, implant, or stereotactic radiosurgery boosts were not allowed. Prophylaxis for Pneumocystis jiroveci was mandatory during chemoradiation.
Temozolomide was administered continuously at a daily dose of 75 mg/m2 orally 1 hour (±15 minutes) before each radiotherapy session or in the am on days without radiotherapy. Dose delays or discontinuation occurred for hematological and nonhematological toxicity; there were no dose reductions allowed during radiation. If TMZ administration was interrupted, radiotherapy proceeded with no catch-up days of TMZ given. Upon completion of chemoradiation there was a 4 week rest period. An MRI was performed at the end of the initiation phase (6 weeks of radiation + TMZ + 4 weeks off). This MRI was not used to assess tumor progression unless the progression was outside of the radiation port.
Maintenance cycles began immediately after the 4-week rest period and consisted of TMZ at a dose of 150 mg/m2/day for Days 1–5 of Week 1 (escalated in subsequent cycles to 200 mg/m2 in the absence of toxicity) followed by intramuscular injections of 20 mcg/kg poly-ICLC 3 times every 7 days during weeks 2–8. The days of poly-ICLC administration were at least 2 days apart with no more than 3 injections within a 7 day period. After 21 injections of poly-ICLC encompassing 7 weeks, there was a 1 week break from all therapies (week 9) prior to starting the subsequent maintenance cycle. There was no set number of maintenance cycles and patients could continue on therapy until tumor progression or toxicity requiring study discontinuation.
Patient Evaluations
Evaluations performed within 14 days of initiating therapy included a medical history, physical, and neurologic examinations, Mini-Mental Status Exam, KPS determination, electrocardiogram, chest X-ray, vital signs, blood count with differential and platelet counts (complete blood count), blood coagulation parameters, serum chemistry profile, urinalysis, and pregnancy test for women of childbearing potential. During the initiation cycle a complete blood count was performed weekly or more often if Grade 3 or 4 myelosuppression was observed. History, physical, and neurological examinations, Mini-Metal Status Exam, KPS determination, complete blood count, and serum chemistries were performed within 3 days before every maintenance cycle; a complete blood count was also done weekly during weeks 2–5 of each maintenance cycle and follow-up clinical and neurological examinations and KPS determination were performed during week 5 of each maintenance cycle.
Response to therapy was determined by MRI or CT imaging and neurologic examinations. The use of CT was restricted to patients who were unable to undergo MRI for physical or medical reasons. Imaging studies to provide tumor measurements were performed within 14 days of beginning treatment and after each maintenance cycle until relapse. A confirmatory scan was obtained 1 month after the initial detection of a complete or partial response. Tumor progression was defined by new lesions on imaging, a 25% increase in contrast-enhancing dimensions, or neurologic deterioration on stable or increasing corticosteroids without another explanation.
Statistical Considerations and Data Analysis
The trial was a single-arm unblinded study to estimate the safety and efficacy of chemoradiation followed by adjuvant therapy with TMZ and poly-ICLC in newly diagnosed GB with results compared with that of the EORTC phase III trial.1 The primary endpoint of the study was the efficacy endpoint of overall survival and the secondary endpoint was frequency of toxicity associated with the regimen. The trial was designed to have a total of 64 death events to detect a hazard ratio of 0.75, a 25% reduction in hazard of death compared with the EORTC phase III trial, with 85% power at an alpha level of 0.1 to be statistically significant.1 Central review of pathology and neuroimaging was mandated for all patients with a documented complete or partial response.
Ninety-seven patients were accrued into the trial from January 6, 2006 to January 29, 2007. The trial database was closed on April 2009 for the final analysis with 75 death events. All subjects were included in the analysis (intent to treat principle). One patient was lost to follow-up at 28 months. This data was censored on the last day that the patient was known to be alive. Survival time was calculated from the date of initial histological diagnosis to the date of death or censored at the time of analysis if patients were alive. Overall survival was estimated using the Kaplan–Meier method.14 As we did not have access to the original trial data set from the phase III EORTC trial, the published survival results from that trial were compared with the current study.1 For patients who were in the same age group as the EORTC trial (18–70 years old), Cochran–Mantel–Haenszel statistics were used to assess the relative risk of death between the NABTT poly-ICLC trial and the EORTC phase III trial at times of 0, 6, 12, 18, and 24 months of follow-up. All P values reported are two-sided. All analyses were performed with the use of SAS software (version 9.1; SAS Institute).
Evaluation of Safety
Subjects were monitored for adverse events and toxicities were graded according to CTCAE version 3. There was a planned safety interim analysis after the first 30 patients had received treatment for at least 1 maintenance cycle. This stipulated that the study would be considered for early termination if excessive Grade 3 or 4 toxicities were observed. The safety rule would be invoked if any of the following occurred: lower 95% confidence bounds on the role of hematological toxicities ≥Grade 3 with an attribution of possible, probably, or definite, the occurrence of radionecrosis, or early death (individually) that exceeded 10% of such events. Furthermore, the composite of these 3 safety signals were reviewed and accrual would be suspended if the lower 95% confidence bound exceeded 10%.
Results
Patient Characteristics
A total of 97 patients were enrolled (60 men) between January 2006 and January 2007. The median age was 56.6 years (range 21–85) with a median KPS of 90 (range 70–100). Other baseline characteristics and demographics are shown in Table 1. Table 1 also provides a comparison of only those subjects ages 18–70 from the study cohort to those of the EORTC phase III trial.
Table 1.
Baseline characteristics of all study subjects; subjects 18–70 and of EORTC phase III cohort
| Characteristics | RT + TMZ + Poly-ICLC (all subjects) (n = 97) | RT + TMZ + Poly-ICLC (ages 18–70) only) (n = 83) | EORTC phase III (ages 18–70) (n = 287) |
|---|---|---|---|
| Age, yr | |||
 Median Median | 56.6 | 55 | 56 |
 Range Range | 21–85 | 21–69 | 19–70 |
| Sex, n (%) | |||
 Male Male | 60 (62) | 51 (61) | 185 (64) |
 Female Female | 37 (38) | 32 (39) | 102 (36) |
| Karnofsky performance | WHO PS (n, [%]) | ||
 100 100 | 34 (35) | 33 (40) | 0, 113 (39) |
 90 90 | 45 (46) | 36 (43) | 1, 136 (47) |
 80 80 | 12 (12) | 9 (11) | |
 70 70 | 3 (3) | 3 (4) | 2, 38 (13) |
 60 60 | 3 (3) | 2 (2) | |
| Extent of surgery, n (%) | |||
 Biopsy Biopsy | 18 (19) | 17 (20) | 48 (17) |
 Craniotomy Craniotomy | 79 (81) | 66 (80) | 239 (83) |
| Time from diagnosis to radiotherapy (weeks) | |||
 Median Median | 4.4 | 4.4 | 5 |
 Range Range | 2–12.6 | 2–12.6 | 1.7–10.7 |
| Baseline MMSE score, n (%) | |||
 30 30 | 56 (57) | 54 (65) | 100 (35) |
 27–29 27–29 | 30 (31) | 22 (27) | 96 (33) |
 ≤26 ≤26 | 11 (11) | 7 (8) | 81 (28) |
 Data missing Data missing | 10 (3) | ||
| Corticosteroid therapy, n (%) | |||
 Yes Yes | 70 (72) | 61 (74) | 193 (67) |
 No No | 26 (27) | 21 (26) | 94 (33) |
 Data missing Data missing | 1 (1) | ||
| Histological diagnosis, n (%) | |||
 Glioblastoma Glioblastoma | 94 (97) | 80 (96) | 221 (92) |
 AA AA | 1 (1) | 1 (1.2) | 7 (3) |
 Inconclusive Inconclusive | 3 (1) | ||
 Other Other | 2 (2) | 2 (2) | 8 (3) |
Abbreviations: RT, radiation therapy; TMZ, temozolomide; MMSE, Mini-Mental Status Examination; AA, anaplastic astrocytoma.
Efficacy
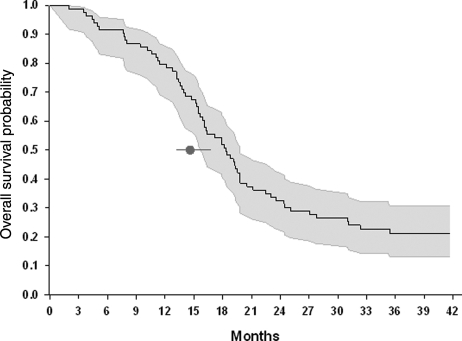
As of April 2009, the time of final analyses, 75 patients had died and 22 were still alive. Nine patients remained on study treatment. Median onset of radiotherapy and TMZ from initial surgery was 4.4 weeks and the average time on treatment was 32 weeks ± 23 weeks. Eighty-eight patients (91%) are off treatment, 60% due to disease progression or death (60%), 12% due to toxicity, 7% due to patient refusal of further treatment, or 4% due to investigator withdrawal. The mOS for the entire cohort was 17.2 months (95% CI: 15.4–19.3 months). For patients age 18–70 years old, mOS was 18.3 months (95% CI: 15.9–19.8 months) when compared with 14.6 (95% CI: 13.2–16.8) reported by the EORTC 26981/22981 trial (Fig. 2). Overall, 23% of patients who were treated in the current trial were alive at 24 months after initial diagnosis, and for those aged 18–70, 32.5% lived beyond 24 months. For patients between 18 and 70 years of age the percent overall survival at 12 months and 18 months were statistically significantly higher compared with the EORTC trial (79.5% vs 61.1%; 51.8% vs 39.4%; Table 2). At the time of the final analysis (November 24, 2009), 18 patients were still alive. Fourteen out of these 18 patients had lived beyond 36 months. The other 4 patients were 1 month (n = 2), 2 months (n = 1), or 3 months (n = 1) shy of completing the 36 month follow-up.
Table 2.
Comparison of overall survival for all subjects, those age 18–70 only, and EORTC phase III cohort
| Trial | Median time of survival (month, 95% CI) | Overall survival at 6 months % (95% CI) | Overall survival Overall survival at 24 months % (95% CI) | Overall survival at 18 months % (95% CI) | Overall survival at 24 months % (95% CI) |
|---|---|---|---|---|---|
| RT + TMZ + Poly-ICLC | |||||
 Age ≥ 18 (n = 97) Age ≥ 18 (n = 97) | 17.2 (16–19) | 91.8 (84–96) | 73.2 (63–82) | 47.4 (37–58) | 29.9 (21–40) |
 Age 18–70 (n = 83) Age 18–70 (n = 83) | 18.3 (16–20) | 91.6 (82–96) | 79.5 (69–88)* | 51.8 (41–63)* | 32.5 (23–44) |
| EORTC Phase III | |||||
 Age 18–70 (n = 287) Age 18–70 (n = 287) | 14.6 (13–17) | 86.3 (82–90) | 61.1 (55–67) | 39.4 (34–45) | 26.5 (21–32) |
*P < .05.
Abbreviations: RT, radiation therapy; TMZ, temozolomide.
Safety
The regimen was well tolerated and the planned interim safety analysis concluded that accrual should continue. The most frequent Grade 3–4 toxicities occurring in >5% of subjects at least possibly related to TMZ or poly-ICLC were neutropenia (20.6%), leukopenia (16.5%), and thrombocytopenia (9%) (Table 3). There were no Grade 3 or 4 adverse events with attributes of possibly, probably, or definitely related to TMZ and poly-ICLC in >5% of subjects (Table 4). One Grade 3 rash was attributed to poly-ICLC alone. Two deaths during adjuvant treatment were considered not related to poly-ICLC.
Table 3.
Adverse events Grade 3 or 4 with attributes of possibly, probably, or definitely related to temozolomide or poly-ICLC
| Grade 3 (#) | Grade 4 (#) | Total (%) | |
|---|---|---|---|
| Diarrhea | 1 | 1 (1%) | |
| Fatigue (asthenia, lethargy, malaise) | 3 | 3 (3%) | |
| Muscle weakness, generalized or specific (not due to neuropathy) | 1 | 1 (1%) | |
| Nausea | 1 | 1 (1%) | |
| Vomiting | 1 | 1 (1%) | |
| Rash/desquamation | 2 | 2 (2%) | |
| SGOT (ALT) | 1 | 1 (1%) | |
| Febrile neutropenia | 2 | 2 (2%) | |
| Hemoglobin | 2 | 2 (2%) | |
| Infection with unknown ANC—Lung (pneumonia) | 1 | 1 (1%) | |
| Leukopenia | 11 | 5 | 16 (16.5%) |
| Neutrophils/granulocytes (ANC/AGC) | 13 | 7 | 20 (20.6%) |
| Platelets | 6 | 3 | 9 (9%) |
Abbreviations: SGOT, serum glutamic-oxaloacetic transaminase; ANC, absolute neutrophil count; AGC, absolute granulocyte count.
Table 4.
Adverse events Grade 3 or 4 with attributes of possibly, probably, or definitely related to TMZ and poly-ICLC
| Grade 3 (#) | Grade 4 (#) | Total (%) | |
|---|---|---|---|
| Fatigue (asthenia, lethargy, malaise) | 1 | 1 (1%) | |
| Rash/desquamationa | 2 | 2 (2%) | |
| SGOT (ALT) | 1 | 1 (1%) | |
| Leukopenia | 4 | 4 (4%) | |
| Neutrophils/granulocytes (ANC/AGC) | 2 | 2 (2%) | |
| Platelets | 2 | 2 (2%) |
Abbreviations: TMZ, temozolomide; SGOT, serum glutamic-oxaloacetic transaminase; ANC, absolute neutrophil count; AGC, absolute granulocyte count.
aOnly one Grade 3 rash/desquamation was attributed to poly-ICLC alone.
Discussion
This study suggests that poly-ICLC provides a survival advantage to patients with newly diagnosed GB when given in the adjuvant phase of treatment after TMZ chemoradiation. Median survival was 17.2 months for the entire cohort and 18.3 months for patients aged 17–70. This compares favorably with the 14.6 month mOS for a large similarly aged cohort receiving TMZ-based chemoradiation alone in the EORTC trial. Likewise, the failure (death) hazard ratio of 0.46 compares favorably with the reported ratio of 0.63 for the EORTC trial. Finally, of the 22 subjects (23%) alive at 24 months, 14 had reached 36 months survival at the time of this analysis and follow-up continues in the remainder.
There are several confounding factors to be considered. The comparison to the EORTC data is based on the available published data but is not a formal comparison of the primary data. Furthermore, the current study was carried out by 6 institutions with highly similar treatment approaches, including management of intercurrent issues such as seizures, tumor-associated edema, venous thrombosis, and concurrent medications, all of which may impact outcome. The EORTC study was performed at over 80 institutions ranging from smaller community hospitals to large academic centers, and treatment of recurrences may have been more aggressive in our patients. Another confounding issue noted since the completion of the EORTC trial and relevant to the present study, is a favorable shift in mOS in patients treated with radiation and concurrent and adjuvant TMZ, suggesting improved care and recognition of pseudoprogression may further increase mOS.15,16 Increasingly bevacizumab is used to manage GB recurrence and the use of this agent as salvage therapy may have favorably altered mOS.17,18 Finally, the current study was initiated before the impact of MGMT promoter methylation on outcome in GB was known and these data were not collected in the present study.19 An evaluation of outcome with respect to the presence or absence of methylated MGMT promoter could have potentially shifted our outcomes, albeit in either direction.
The results of the current study are, however, intriguing. Poly-ICLC appears safe and can be combined with adjuvant TMZ chemotherapy in this patient group. The apparent utility of adjuvant poly-ICLC may mechanistically relate to its immunomodulatory and potent vaccine adjuvant activity, and the design of the current trial was based on the hypothesis that cycles of chemotherapy-induced antigen release alternating with a PAMP-adjuvant such as poly-ICLC could result in the generation of a tumor-specific immune response. To avoid a theoretical suppression of this response by TMZ, the interval between TMZ dosing was increased, although recent data from vaccine trials suggest this may not be necessary.20 However, other than the possible subgroup of long-term survivors in this trial, there is no evidence to support this hypothesis at this time. Nevertheless, therapeutic vaccination with a more specifically designed combination of tumor antigen and poly-ICLC PAMP-adjuvant still holds much promise for the generation of effective, tumor-specific responses in humans.3,21 Several ongoing pilot clinical trials are investigating the adjuvant actions of poly-ICLC with a variety of cancer vaccine platforms, including both peptide-based and dendritic cell vaccine trials for gliomas.22
Malignant gliomas remain one of the most challenging cancers. The current standard of care of radiation with concurrent and adjuvant TMZ does not provide the majority of patients with prolonged survival. Several recent phase II studies have suggested that the addition of other novel agents to this standard regimen may offer small survival advantages as well.23,24 The current study clearly demonstrates that it is safe to combine poly-ICLC with standard radiation and chemotherapy and that the overall survival of patients with GB treated with this combination provides results superior to the EORTC trial. These observations strongly support further investigations of poly-ICLC combined with other cytotoxic, cytostatic, and immunomodulatory therapies for malignant gliomas which will ultimately lead to definitive phase III studies utilizing this agent.
Conflict of interest statement. A.M.S. is employed by and owns stock in Oncovir.
Funding
This study was supported in part by National Cancer Institute grant UO1CA137443.
References
Articles from Neuro-Oncology are provided here courtesy of Society for Neuro-Oncology and Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/neuonc/noq071
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/neuro-oncology/article-pdf/12/10/1071/6328716/noq071.pdf
Subscription required at ingentaconnect
http://openurl.ingenta.com/content?genre=article&issn=1522-8517&volume=12&issue=10&spage=1071
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/neuonc/noq071
Article citations
Harnessing innate immune pathways for therapeutic advancement in cancer.
Signal Transduct Target Ther, 9(1):68, 25 Mar 2024
Cited by: 4 articles | PMID: 38523155 | PMCID: PMC10961329
Review Free full text in Europe PMC
Harnessing the innate immune system by revolutionizing macrophage-mediated cancer immunotherapy.
J Biosci, 49:63, 01 Jan 2024
Cited by: 1 article | PMID: 38864238 | PMCID: PMC11286319
Review Free full text in Europe PMC
Radiation dose, schedule, and novel systemic targets for radio-immunotherapy combinations.
J Natl Cancer Inst, 115(11):1278-1293, 01 Nov 2023
Cited by: 3 articles | PMID: 37348864 | PMCID: PMC10637035
Review Free full text in Europe PMC
Neurofibromatosis Type 1-Associated Optic Pathway Gliomas: Current Challenges and Future Prospects.
Cancer Manag Res, 15:667-681, 13 Jul 2023
Cited by: 4 articles | PMID: 37465080 | PMCID: PMC10351533
Review Free full text in Europe PMC
ASP-2/Trans-sialidase chimeric protein induces robust protective immunity in experimental models of Chagas' disease.
NPJ Vaccines, 8(1):81, 31 May 2023
Cited by: 2 articles | PMID: 37258518 | PMCID: PMC10231858
Go to all (75) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States.
Clin Cancer Res, 16(8):2443-2449, 06 Apr 2010
Cited by: 290 articles | PMID: 20371685 | PMCID: PMC2861898
A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05).
J Neurooncol, 91(2):175-182, 17 Sep 2008
Cited by: 76 articles | PMID: 18797818 | PMCID: PMC4779120
Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study.
Neurosurgery, 38(6):1096-103; discussion 1103-4, 01 Jun 1996
Cited by: 75 articles | PMID: 8727138
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
Health Technol Assess, 11(45):iii-iv, ix-221, 01 Jan 2007
Cited by: 43 articles | PMID: 17999840
ReviewBooks & documents Free full text in Europe PMC