Abstract
Free full text

Optical activation of lateral amygdala pyramidal cells instructs associative fear learning
Associated Data
Abstract
Humans and animals can learn that specific sensory cues in the environment predict aversive events through a form of associative learning termed fear conditioning. This learning occurs when the sensory cues are paired with an aversive event occuring in close temporal proximity. Activation of lateral amygdala (LA) pyramidal neurons by aversive stimuli is thought to drive the formation of these associative fear memories; yet, there have been no direct tests of this hypothesis. Here we demonstrate that viral-targeted, tissue-specific expression of the light-activated channelrhodopsin (ChR2) in LA pyramidal cells permitted optical control of LA neuronal activity. Using this approach we then paired an auditory sensory cue with optical stimulation of LA pyramidal neurons instead of an aversive stimulus. Subsequently presentation of the tone alone produced behavioral fear responses. These results demonstrate in vivo optogenetic control of LA neurons and provide compelling support for the idea that fear learning is instructed by aversive stimulus-induced activation of LA pyramidal cells.
Fear conditioning is a simple form of associative learning that provides a powerful model system to study associative plasticity and memory formation (1–4). During fear conditioning, a neutral stimulus [termed the conditioned stimulus (CS)], often an auditory tone, is paired repeatedly with an aversive stimulus [termed the unconditioned stimulus (US)] and animals learn that the CS predicts the occurrence of the US. When the CS is encountered after learning, animals emit a stereotyped group of adaptive responses, including behavioral freezing and associated physiological adjustments, which together are termed the fear response.
The lateral nucleus of the amygdala (LA) is a site of associative plasticity, where US-evoked depolarization of LA pyramidal neurons is thought to instruct plasticity at synapses formed by CS inputs onto the same neurons (5–7). Several lines of indirect evidence support the idea that this plasticity occurs as a result of a Hebbian mechanism through which depolarization of LA pyramidal neurons by the shock US coincident with weaker activation of the same cells by auditory CS inputs results in fear learning (8–18). This hypothesis makes the strong prediction that pairing an auditory CS with direct activation of LA pyramidal neurons as an US should be sufficient, in the absence of a shock US, to support fear learning and memory formation. Here we tested this hypothesis by substituting the aversive US with optical stimulation (19, 20) of LA pyramidal neurons during learning, and we report that physiological activation of these cells results in fear conditioning.
Results
The light activated channelrhodopsin (ChR2) (19, 20) has been used in other neural systems to activate specific cell populations and produce learning (21–23). We took advantage of this technology and targeted ChR2 to pyramidal cells by in vivo viral-mediated gene transfer. We used an adeno-associated virus (AAV) to express a fusion protein of ChR2 and yellow fluorescent protein (YFP) from the CaMKII promoter in LA pyramidal neurons. Following injection of the virus into the LA (see example injection in Fig. 1A), the cell-type specificity of expression was assayed using immunocytochemistry. ChR2 expression was found to be largely restricted to CaMKII-expressing pyramidal neurons (92 ± 1%; Fig. 1 B and C) and expressed at very low levels in GABA-expressing cells (1.8 ± 0.0%; Fig. 1B and Fig. S1).

Tissue-specific expression of ChR2 in LA pyramidal neurons. (A) Example of ChR2/YFP expression in the LA. LA boundaries are outlined in yellow. (B) Percentage (y axis) of ChR2+ cells that were also CaMKII+ (black bar, n = 3) and percentage of ChR2+ cells that were also GABA+ (white bar, n = 2). (C) Immunolabeled ChR2+ cells and neuropil (green, Left), CaMKII+ cells (red, Right), and an overlay of the two (Bottom). Individual cell examples are indicated by yellow (ChR2/YFP+ cells) and white (CaMKII+ cells) arrows.
We next examined whether ChR2-expressing neurons were light responsive in vivo and could be driven to fire action potentials (APs). The ChR2/YFP-encoding virus, AAV-CaMKII-ChR2/YFP was injected into the LA during surgery using stereotactic coordinates. After 7–10 d, animals were anesthetized and a recording electrode, mounted to a fiber optic cable that was attached to a 473-nm laser, was advanced into the LA. Upon single-unit isolation, blue light was delivered at different frequencies (20 or 50 Hz) and pulse durations (2, 5, and 10 ms) and the light responsiveness of the cell was assessed. Although evoked firing rate was similar across different laser stimulation frequencies and laser pulse durations, evoked AP reliability was higher at lower frequencies (Fig. 2A and Fig. S2; total n = 15 cells from four animals). We also compared the firing rate evoked by 20-Hz laser stimulation to that evoked by both constant laser illumination and to that evoked by an actual eyelid shock, which has been used as an US for fear conditioning in other studies (24, 25) and by our laboratory (Discussion). Laser stimulation at 20 Hz produced significantly more action potential firing than either constant illumination or eyelid shock, whereas the latter two activated comparable levels of action potential firing (Fig. S3). Because 20-Hz laser stimulation with 10-ms pulse durations produced robust and reliable action potential firing (Fig. 2 A and B and Fig. S2), it was selected for use in the subsequent experiments.

Laser stimulation produces robust activation of LA neurons. (A) Example peristimulus time histogram (PSTH) plot (bin size = 50 ms) and spike rasters from a single LA neuron shows average firing rate (y axis, in Hz) in response to laser stimulation at 20-Hz stimulation frequency (10-ms laser pulse duration) for 1 s (thick blue lines denote each laser pulse during the 1-s stimulation period). (B) Population averaged firing rate in response to 20-Hz (Upper) and 50-Hz (Lower) laser stimulation at 10-ms (black lines), 5-ms (broken lines), and 2-ms (gray lines) laser pulse durations. Total n = 15 cells (from four animals) for 20-Hz stimulation, nine of which also received 50-Hz stimulation. Blue bar beneath graphs denotes the duration of the 1-s train at the different frequencies. (C and D) Examples of CFOS-immunolabeled cells (brown dot labeling) in the LA from the laser-stimulated (D) and nonstimulated (C) hemispheres.
To determine whether in vivo light stimulation in awake, behaving animals activated LA neurons, we next used immunocytochemical techniques to examine the expression of the immediate early gene CFOS in the LA following laser stimulation. For these experiments a chronic guide cannula was targeted just dorsal to the LA and microinjections of the virus encoding ChR2/YFP were made into the LA. Ten days postsurgery a fiber optic cable attached to a 473-nm laser was inserted into the guide cannula and laser stimulation was applied 3× for 2 s at 20 Hz with the 473-nm laser. Following tissue processing, cell counts were made through the LA on the stimulated and nonstimulated sides. On the laser-stimulated side, large numbers of CFOS+ cells were seen (average of 253 ± 46 cells/section, n = 3 animals) whereas there was minimal CFOS activation in the nonstimulated side (average of 29 ± 4 cells/section, n = 3 animals) (Fig. 2 C and D). Proportionally, this was a substantial number of activated neurons as the total number of CaMKII+ cells per LA section was 402 ± 37. Thus in vivo, 20-Hz laser stimulation resulted in activation of large numbers of LA cells, further validating the use of the 20-Hz stimulation protocol.
Behavioral conditioning experiments were then used to determine whether direct activation of LA pyramidal neurons as an US, when paired with an auditory CS, produces fear learning. For these experiments a chronic guide cannula was targeted just dorsal to the LA, as described above, and microinjections of the virus encoding either ChR2/YFP or GFP were made into the LA. Seven to 10 d after surgery, a fiber optic cable attached to a 473-nm laser was inserted into the guide cannula targeted to the LA. Subsequently, all of the GFP- (GFP/paired, n = 8) and half of the ChR2-treated animals (ChR2/paired, n = 8) underwent conditioning in which they received 16 pairings of an auditory CS and laser stimulation US (20-Hz, 10-ms pulse duration stimulation for 2 s). To test whether learning depended upon the temporal contiguity of the CS and laser US, and laser US presentation being contingent upon the CS (i.e., whether the learning was associative in nature) (26), another group of animals (ChR2/unpaired, n = 8) received random presentations of 16 CS and 16 laser-stimulation US, which were explicitly unpaired in time. During this “training” phase, behavioral freezing was measured. During training, ChR2/paired animals froze significantly more to the CS than the GFP/paired and ChR2/unpaired groups especially later in training (Fig. 3A). A repeated-measures ANOVA revealed a significant interaction (F6,63 = 4.7795, P = 0.00045) between trial block (1–4) and treatment (ChR2/paired, GFP/paired, and ChR2/unpaired, whereas post hoc comparisons demonstrated that CS-evoked freezing responses in ChR2/paired animals during trial blocks 3 and 4 were significantly greater than CS freezing responses in GFP/paired (P = 0002 and P = 0.0001 respectively) or ChR2/unpaired (P = 002 and P = 0.0001, respectively) groups. Laser activation itself produced a small, but statistically significant amount of unconditioned freezing as ChR2/unpaired animals froze more during laser stimulation periods compared with freezing during corresponding periods when the laser was off (paired t5 = 4.89, P = 0.005; Fig. S4).

Laser stimulation in the LA produces fear conditioning. (A) Percent freezing (of the total 18-s CS, y axis) across the four conditioning trial blocks (four trials/block, 16 total CS–US pairings or 16 CS presentations for the unpaired group) in the ChR2/paired (squares, n = 8), GFP/paired (triangles, n = 8), and ChR2/unpaired (open circles, n = 8) groups. (B) Percent freezing (y axis) during the LTM test in the different groups (x axis). For A and B, * and # indicate significant differences between the ChR2/paired group and the GFP/paired and ChR2/unpaired groups.
To determine whether this training procedure produced long-term fear memories in ChR2/paired animals, freezing was assessed in a separate context 24 h after training (Fig. 3B). A one-way ANOVA comparing CS-evoked freezing by group (ChR2/paired vs. GFP/paired vs. ChR2/unpaired) found a significant main effect (F2,21 = 5.41, P = 0.013) and post hoc analyses revealed that the ChR2/paired group froze significantly more than both the GFP/paired (P = 0.017) and the ChR2/unpaired (P = 0.017) groups. Because using constant illumination to stimulate LA neurons produced similar levels of action potential firing as that evoked by eyelid shocks (Fig. S3), we also used 2-s constant laser illumination as an US (instead of 10-ms laser pulses at 20 Hz described above) and found that this stimulation protocol produced similar levels of fear conditioning (Fig. S5) to that evoked by the 20-Hz laser US. Thus light activation of LA pyramidal cells as an US produced long-term fear memories.
Discussion
Our results show that specific activation of LA pyramidal cells as an US, in the absence of a peripheral shock US, produced fear conditioning. ChR2 was expressed preferentially in LA pyramidal neurons and laser stimulation of single cells evoked robust action potential firing in LA cells and activated large numbers of neurons in the LA. Importantly, we optically stimulated LA pyramidal cells as an US and this produced fear learning and memory formation. Finally, this process is associative (i.e., requires the close temporal contiguity of the CS and laser US and that the laser US occurrence is contingent upon the CS preceding it, ref. 26) as it does not occur when the CS and the light US are temporally unpaired during training in ChR2-treated animals. Although optical stimulation of LA pyramidal neurons produced some freezing behavior, these levels were considerably lower than fear responses evoked by stimulation of the central nucleus of the amygdala (CeA) (reviewed in ref. 27), suggesting that the CeA is more directly connected with the behavioral output pathways responsible for fear responses. Although stimulation of areas that project to the LA have been used as an US to produce fear conditioning (28–30), this demonstration that direct stimulation of LA pyramidal neurons can serve as a reinforcement signal for fear learning is novel.
A large number of studies have provided convincing evidence that associative plasticity in the LA contributes to fear memory formation (4, 6, 7). It is widely believed that LA plasticity underlying fear learning occurs as a result of a Hebbian mechanism whereby the shock US directly depolarizes LA pyramidal cells that are concurrently activated by weaker CS inputs, resulting in potentiation of the CS input synapses (5, 6, 9, 31). This model suggests that depolarization of LA pyramidal cells by the US provides an instructive signal for LA plasticity and fear learning. However, it is possible that LA plasticity underlying fear learning occurs through a non-Hebbian process, which is independent of US-evoked postsynaptic depolarization of LA neurons. For example, US-induced activation of neuromodulatory systems and subsequent intracellular signaling cascades could by itself serve to potentiate coactivated CS input synapses (a possibility, which is supported by some experimental evidence, refs 32, 33). Although various studies provide indirect evidence that fear learning occurs through a Hebbian mechanism (8, 9, 13–15, 34–36), other work (37) and interpretational issues make it difficult to determine whether this is in fact true. To adequately test whether depolarization of LA pyramidal neurons by the US instructs fear conditioning, it is necessary to manipulate neural activity (either increase or decrease depolarization) directly in this cell population specifically during the US period and examine the effects of these manipulations on fear memory formation. In the present study we produced strong depolarization and action potential firing in LA pyramidal neurons and showed that this stimulation, when used as an US, reinforces fear learning, offering compelling support for the idea that Hebbian mechanisms are involved in fear memory formation.
Although stimulation of LA neurons supported fear learning, freezing scores during the long-term memory (LTM) test were relatively low compared with results typically obtained with an electric shock US. One reason for this may have been that either a small number of neurons were activated by laser stimulation or because laser stimulation produced too little (or too much) depolarization of LA neurons compared with a shock US. However, the CFOS experiments reported here demonstrate that large numbers of LA neurons were activated by laser stimulation and the electrophysiology experiments provide evidence that 20-Hz laser stimulation caused more action potential firing in LA cells than a shock US, suggesting that these were not the reasons for lower levels of fear learning. Furthermore, the low levels of freezing were not the result of too much action potential firing (compared with a shock US) because using a constant illumination US, which produced similar amounts of action potential firing as a shock US (Fig. S3), resulted in comparable levels of fear learning (Fig. S5). Relatedly, the unilateral nature of the laser stimulation may have resulted in low fear learning. This is unlikely, however, as other studies have shown that robust fear memories are formed when LA plasticity is targeted to only one LA using unilateral eyelid shock (24, 25). In fact, using the same fear conditioning protocol, we also found substantial levels of fear conditioning induced by a unilateral eyelid shock US (see Fig. S6 for comparison of shock vs. laser stimulation US-induced learning). This occurred despite the fact that the eyelid shock US used in this conditioning protocol produced weaker or similar levels of action potential firing in LA neurons compared with the two laser stimulation US used in the current study. Together, the previous results and the present data suggest that the low freezing levels obtained using the laser-stimulation US were not the result of unilateral stimulation.
It is more likely that fear learning was limited because additional mechanisms (in addition to depolarization of LA pyramidal neurons) are required to modulate the strength of fear memory formation. One possibility is that plasticity in other parts of the fear circuit is required during fear conditioning (38–42). Although stimulation of LA pyramidal neurons may be sufficient to produce some fear learning by activating local LA plasticity mechanisms, plasticity in other parts of the circuit may be required as well for the full expression of fear conditioning. It is also possible that more specific cell targeting is necessary to direct plasticity to cells involved in generating the fear response (as opposed to other potentially competing responses). In addition to being essential for aversively motivated learning, plasticity in the LA is also required for several forms of appetitively motivated learning (43–45) and a number of studies have found that separate populations of amygdala neurons mediate aversive and appetitive processing (46–48). Thus separate, intermixed populations of amygdala neurons are likely to participate in producing different behavioral responses (i.e., aversively vs. appetitively motivated). The laser conditioning could have induced plasticity indiscriminately in both cell populations and this may have interfered, at least partially, with the expression of the conditional freezing response. A final possibility is that coactivation of neuromodulatory systems, in addition to depolarization of LA pyramidal neurons, may be required for maximal long-term memory formation (9, 49–51). In fact it has been suggested that coactivation of noradrenergic β receptors and subsequent G protein-coupled signaling pathways in conjunction with depolarization of LA neurons cooperatively regulates LA plasticity and fear conditioning (50). This multiprocess model may be required for maximal fear learning to occur. Although it is clear from the present results that activation of LA neurons can produce fear memories, it will be important in future studies to determine the additional factors that contribute to robust fear memory formation.
Materials and Methods
Subjects.
Male Sprague-Dawley rats (Hilltop) weighing 275–300 g on arrival were individually housed on a 12-h light/dark cycle and given food and water ad libitum. All procedures were approved by the New York University Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals.
Vector Construction.
The AAV construct carrying EGFP under the control of the CaMKII promoter (termed pAAV-CaMKII-GFP) was kindly provided by P. Osten (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). The backbone vector, pAAV-MCS (Stratagene) has been modified to contain the 1.3-kb mouse α-CaMKII promoter, a cDNA for EGFP, and woodchuck hepatitis posttranscriptional regulatory element (WPRE) from FCK(1.3)GW (52). pAAV-CaMKII-ChR2/EYFP construct was made by replacing EGFP in pAAV-CaMKII-GFP with a cDNA for ChR2/EYFP from pcDNA3.1/hChr2(H134R)-EYFP.
Virus Production and Purification.
To combine the advantages of AAV1 and AAV2 in relation to tissue tropism and ease of virus purification, we made the chimeric virus, which contains both AAV1 and AAV2 capsid proteins (53). Viruses were made by standard method of CaPO4 triple transfection of 293T cells with an expression vector, either of pAAV-CaMKII-ChR2/EYFP or pAAV-CaMKII-GFP and helper plasmids, pDP1and pDP2 (both provided by J. Kleinschmidt, German Cancer Research Centre, Heidelberg, Germany; ref. 54). Cells were harvested at 48–72 h after transfection, resuspended in 150 mM NaCl buffer containing 50 mM Tris, pH 8.4, subjected to three cycles of freeze/thaw, and treated with 50 U/mL of benzonase (Sigma) for 30 min at 37 °C. After brief centrifugation and filtration, clarified supernatants containing virus were subjected to HiTrap heparin column chromatography (GE Healthcare). Peak virus fractions were collected, concentrated by Amicon Ultra-15 (Millipore), and washed with PBS containing 1 mM MgCl2 and 2.5 mM KCl in the same filter unit. The virus genomic titer was quantified by real-time PCR using iCycler and iQ SYBR reagent (Bio-Rad Laboratories). Before real-time PCR, virus samples were pretreated with DNase I (Roche). The DNase I was then heat inactivated for 10 min at 70 °C. After the heat inactivation, the samples were treated with proteinase K (Invitrogen) at 50 °C for 60 min, and the proteinase K was subsequently heat inactivated for 20 min at 95 °C. A plasmid DNA standard curve was set up using 0.0001–100 ng of DNA. Primer sequences are EGFP-F: 5′-GGAGCGCACGATCTTCTTCA-3′ and EGFP-R: 5′-AGGGTGTCGCCCTCGAA-3′ (55). Final virus titers were 1.8 × 1012 genomic copies (GC)/mL (for AAV-CaMKII-ChR2/EYFP) and 5.2 × 1012 GC/mL (for AAV-CaMKII-GFP).
Stereotaxic Cannula Implantation and Virus Injection.
For electrophysiological and double-labeling immunocytochemistry experiments, animals were injected with a mixture of ketamine (100 mg/kg) and xylazine (6 mg/kg) intraperitoneally and placed in a stereotaxic apparatus (David Kopf Instruments). Supplemental doses were given to maintain a steady anesthetic state. The tip of a micropipette (tip diameter: ~12 μm) was targeted to the LA (stereotaxic coordinates from Bregma, anterior–posterior: −3.0 mm, dorsal–ventral: −8.0 mm, medial–lateral: 5.4 mm). Injections were made through the glass micropipette, which was attached to a 30-mL syringe by polyethylene tubing at a rate of ~0.1 μL/min (0.5 μL total volume) as described previously (56). Micropipettes were left in place for 20 min postinjection after which the incised skin was sutured and triple antibiotic ointment was applied.
For behavioral and CFOS immunocytochemistry experiments, animals were injected with a mixture of ketamine (100 mg/kg) and xylazine (6 mg/kg) intraperitoneally and placed in a stereotaxic apparatus (David Kopf Instruments). Supplemental doses were given to maintain a steady anesthetic state. Stainless steel guide cannulae (21 gauge; Plastics One) were targeted unilaterally to the LA (stereotaxic coordinates from Bregma, anterior–posterior: −3.0 mm, dorsal–ventral: −6.6 mm, medial–lateral: 5.4 mm). Guides were affixed to the skull using surgical screws and dental cement. Following cannula guide placement, 0.5-μL injections of virus were made through a stainless injection cannula (26 gauge; Plastics One), which protruded 1.4 mm beyond the tip of the guide cannula and was attached to a Hamilton syringe via polyethylene tubing. Experimenters were blind as to what virus (AAV-CaMKII-ChR2/EYFP or AAV-CaMKII-GFP) was being injected. Injections were made at a rate of 0.07 μL/min, which was controlled by an automatic pump (PHD 2000; Harvard Appartus) and the injector was left in place for 20 min postinjection.
Immunocytochemistry.
To determine the specificity of ChR2 targeting in CaMKII cells, rats were injected with an overdose of 25% chloral hydrate and perfused transcardially with PBS/heparin (40 U/mL) followed by 4% PFA/0.1% gluteraldehyde 7–10 d after virus injection. The brains were then removed and blocked (around the amygdala) and postfixed in 4% PFA overnight at 4 °C. Brains were then cut into 40-μm-thick sections on a vibratome and immersed in 1% sodium borohydride and then 1% hydrogen peroxide. For double labeling of ChR2 and CaMKII, brain sections (every fourth section) were labeled with mouse monoclonal anti-CaMKII (1:200; Millipore/Upstate) followed by the fluorescent secondary Alexa 555 goat anti-mouse (1:200; Invitrogen) and then reacted with rabbit polyclonal anti-GFP primary antibody (1:500 with 0.2% Triton X; Invitrogen) followed by Alexa 488 goat anti-rabbit (1:200; Invitrogen). For double labeling of ChR2 and GABA, brain sections were labeled with rabbit polyclonal anti-GABA (1:5,000; Sigma), followed by the fluorescent secondary Alexa 555 goat anti-rabbit (1:200; Invitrogen), and then reacted with mouse monoclonal anti-GFP primary antibody (1:500 with 0.2% Triton X; Invitrogen), followed by Alexa 488 goat anti-mouse (1:200; Invitrogen). All blocking steps were done in 1% BSA. Sections were then mounted and coverslipped with antifade (Invitrogen). Infection efficacy was quantified using a confocal microscope and counting the number of ChR2+ (EYFP) cells that were also CaMKII+ or GABA+ (i.e., number double labeled) as well as the total number of ChR2 positive cells within a counting frame (510 μm × 507 μm). This analysis was applied to each amygdala section in which ChR2 label was present. The proportion of double-labeled cells was calculated as the number of ChR2+/CaMKII+ or GABA+ double-labeled cells divided by the total number of ChR2+ cells. For behavioral experiments, sections were processed as described above and an experimenter blind as to animal and treatment group assessed whether ChR2 was expressed in the LA neurons and whether the tip of the guide cannula was dorsal and proximal to the LA. Three ChR2/paired and 2 ChR2/unpaired were not included in the final analysis as these criteria were not met.
For CFOS immunocytochemistry experiments, perfusion, postfixation brain slicing were identical to above. Brain sections (every fourth section) was then reacted with rabbit polyclonal anti CFOS primary antibody (1:20,000; Calbiochem) followed by goat anti-rabbit biotynilated secondary (1:200; Vector Labs) and then incubated in avidin–biotin complex (Vector Labs). This was followed by reaction with diaminobenzidine nickel reaction to reveal immunoreactivity. Individual CFOS-labeled puncta were counted on the stimulated and nonstimulated sides in sections throughout the amygdala. For total CaMKII+ cell counts, fluorescent immunocytochemistry was as described above (but for CaMKII alone) and the CaMKII+ cell counting procedure was identical to that used for CFOS counting. Cell counts were then expressed as counts/section by dividing the total number of cells counted by the number of sections analyzed.
In Vivo Electrophysiological Recording and Laser Stimulation.
Animals were anesthetized as described in Stereotaxic Cannula Implantation and Virus Injection and placed in a stereotaxic apparatus. A glass microelectrode (1 MΩ impedance filled with NaCl) was mounted alongside a fiber optic cable (200 μm core diameter, 0.37 numerical aperature), which extended slightly beyond the tip of the electrode (~0.3 mm). The fiber optic cable was attached to a 473-diode pumped solid state laser (Laserglow), which output 30–40 mW from the tip of the fiber optic cable. The electrode/cable apparatus was targeted to the amygdala and just dorsal to the LA, recordings began and periodic laser stimulation was given as the electrode was advanced in 1-μm steps until single laser responsive cells were isolated. Electrophysiological signals were amplified (×1,000) and filtered (300 Hz low/20 kHz high) using an AM Systems amplifier (model 1800) and then monitored and stored online using the Spike 2 acquisition system. Single neuron waveforms were then isolated offline and had to exhibit a refractory period of at least 1 ms and mean spike amplitude of at least 80 μV to be included in the study.
Stimulus-evoked responses were analyzed by plotting peristimulus time histograms (PSTHs) triggered by the stimulus onset using Neuralynx data analysis software. For each cell, raw spike counts in each bin of the PSTH were converted to firing rates using the equation Ri = Si/N(Δt), where Ri is the firing rate for the ith bin of the PSTH (in Hz), Si is the raw spike count in the bin, N is the number of trigger events for the PSTH, and Δt is the PSTH bin size in seconds. For population averaging of neural responses, each cell's PSTH was converted to a normalized scale. The response in the ith bin of the normalized PSTH was given by 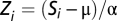 , where Si is the raw spike count in the ith bin, μ is the expected spike count in each bin at baseline, and α is the spike count SD at baseline. The expected spike count was for all analyses presented here, the PSTH bin size was 50 ms, and the expected spike count at baseline (μ) was obtained by concatenating all of the 50-ms bins within the 1.2-s prestimulus periods from every trial of the session.
, where Si is the raw spike count in the ith bin, μ is the expected spike count in each bin at baseline, and α is the spike count SD at baseline. The expected spike count was for all analyses presented here, the PSTH bin size was 50 ms, and the expected spike count at baseline (μ) was obtained by concatenating all of the 50-ms bins within the 1.2-s prestimulus periods from every trial of the session.
The number of spikes elicited per laser pulse (with a pulse defined as each single laser stimulation during a 1-s stimulation trial) was determined by summing the total number of spikes elicited following each laser pulse (from 0 to 50 ms for 20 Hz and 0–20 ms for 50 Hz) and dividing by the total number of laser pulses delivered across all of the 1-min stimulation periods for each cell. The number of spikes elicited per trial (with trial being defined as a 1-s stimulation period at 20 Hz, 50 Hz, or constant laser illumination) was determined by summing the total number of spikes during all 1-min stimulation periods for a given stimulation pattern for each cell and dividing by the number of 1-min stimulation trials for each cell.
To examine the reliability of spike firing (probability of spiking) in response to a laser stimulation at different frequencies and pulse durations, a perievent raster was constructed for each cell and the total number of spike occurrences (i.e., whether a spike occurred, not the number of spikes) after each laser pulse was determined for each stimulus condition (single laser pulses, 20 Hz and 50 Hz stimulation both with 10-, 5-, and 2-ms pulse durations). The total number of spike occurrences was then divided by the total number of laser pulses delivered for each condition to give spike probability.
Behavioral Conditioning Experiments.
A fiber optic cable, which had been threaded through a polyethylene tether (Plastics One) and then through the tip of a stainless steel tube (26 g, Plastics One), was inserted into the chronic guide cannula on the animal's head by an experimenter who was blind as to what treatment the animals would receive. The tether was then screwed onto the guide cannula, firmly affixing the fiber optic cable in place in the brain. The stainless steel tube was the same length as the guide cannula and the tip of the fiber optic cable protruded slightly (~0.3 mm) beyond the tip. The fiber optic cable was attached on the other end to a 473-nm laser (as described above). The animals were then placed into a sound isolating chamber and underwent one of two conditioning protocols. The “paired” groups received 16 CS–US pairing in which the CS was a series of 5-kHz tone pips (at 1 Hz with 250 ms on and 750 ms off) for 20 s and the US was laser stimulation at 20 Hz (10-ms pulse durations) for 2 s, which coterminated with the CS. The “unpaired” group received the same auditory CS and laser US, but they were temporally unpaired and presented randomly 16 times each. During the long-term memory testing phase 24 h later, animals were placed in a novel testing environment and presented with the identical CS 2 times. During the training and testing phases animals’ behavior was recorded on DVD. A rater who was blind with respect to the treatment group scored animals’ behavioral freezing during the first 18 s of the CS (during the training phase) and during all of the 20 CS (during “testing”) offline using a digital stopwatch. Freezing is defined as the cessation of all bodily movement with the exception of respiration-related movement. Freezing scores were statistically analyzed and compared using analysis of variance ANOVA statistical tests followed by post hoc analysis using the Newman-Keuls test. For training, freezing during the 16 conditioning trials (or CS presentations in the ChR2/unpaired group) was binned into 4 trials/bin (trial blocks 1–4) and a 4 × 3 repeated-measures ANOVA was performed in which trial block was the repeated measure and virus treatment (ChR2/paired, ChR2/unpaired and GFP/paired) was the between-subjects factor. For long-term memory testing, freezing during the two CS was averaged and compared in each group (ChR2/paired, ChR2/unpaired, and GFP/paired) using a one-way ANOVA. For CFOS immunocytochemistry experiments, procedures were identical to that described above for behavioral experiments, except that instead of receiving fear conditioning training, animals received three 2-s laser stimulations of the LA at 20 Hz over ~3 min. Animals were then removed and perfused 90 min after the last laser stimulation was administered to maximize CFOS expression. For all reported data, variance is expressed as SEM.
Acknowledgments
We thank Adam Carter for invaluable assistance and guidance in the use of lasers and experimental design, Moses Chao (New York University) for use of his laboratory and supplies for making viruses, Pavel Osten (Cold Spring Harbor Laboratory) for help in virus production and his gift of the CaMKII-GFP construct, and Jürgen Kleinschmidt (German Cancer Research Center) for his gift of the AAV helper vectors. We also thank Claudia Farb and Raquel Martinez for guidance and assistance on the immunocytochemistry and confocal analyses and Andreas Luthi for valuable comments on the manuscript. This work was supported by a F32-MH082505 to J.P.J. and R01-MH046516 to J.E.L.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1002418107/-/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.1002418107
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2906568?pdf=render
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/reprint/107/28/12692.pdf
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/full/107/28/12692
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/abstract/107/28/12692
Citations & impact
Impact metrics
Article citations
Differential neural activity predicts the long-term stability of the effects of positive and negative expectations on pain.
Sci Rep, 14(1):27874, 13 Nov 2024
Cited by: 1 article | PMID: 39537677 | PMCID: PMC11561249
EphrinB2 in excitatory neurons and astrocytes in the basolateral amygdala controls long-term fear memory formation.
Commun Biol, 7(1):1165, 17 Sep 2024
Cited by: 0 articles | PMID: 39289586 | PMCID: PMC11408618
Multi-Characteristic Opsin Therapy to Functionalize Retina, Attenuate Retinal Degeneration, and Restore Vision in Mouse Models of Retinitis Pigmentosa.
Transl Vis Sci Technol, 13(10):25, 01 Oct 2024
Cited by: 0 articles | PMID: 39412768 | PMCID: PMC11486081
Neuromodulator and neuropeptide sensors and probes for precise circuit interrogation in vivo.
Science, 385(6716):eadn6671, 27 Sep 2024
Cited by: 0 articles | PMID: 39325905 | PMCID: PMC11488521
Review Free full text in Europe PMC
Bidirectional fear modulation by discrete anterior insular circuits in male mice.
Elife, 13:RP95821, 01 Aug 2024
Cited by: 1 article | PMID: 39088250 | PMCID: PMC11293866
Go to all (185) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An organization of visual and auditory fear conditioning in the lateral amygdala.
Neurobiol Learn Mem, 116:1-13, 27 Jul 2014
Cited by: 17 articles | PMID: 25076183
Inhibition of Pyramidal Neurons in the Basal Amygdala Promotes Fear Learning.
eNeuro, 5(5):ENEURO.0272-18.2018, 01 Sep 2018
Cited by: 13 articles | PMID: 30406197 | PMCID: PMC6220591
Associative and plastic thalamic signaling to the lateral amygdala controls fear behavior.
Nat Neurosci, 23(5):625-637, 13 Apr 2020
Cited by: 34 articles | PMID: 32284608
Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning.
Learn Mem, 8(5):229-242, 01 Sep 2001
Cited by: 349 articles | PMID: 11584069
Review
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NIMH NIH HHS (4)
Grant ID: R01-MH046516
Grant ID: F32 MH082505
Grant ID: F32-MH082505
Grant ID: R01 MH046516





