Abstract
Free full text

Functionally-Detected Cognitive Impairment in High School Football Players without Clinically-Diagnosed Concussion
Abstract
Head trauma and concussion in football players have recently received considerable media attention. Postmortem evidence suggests that accrual of damage to the brain may occur with repeated blows to the head, even when the individual blows fail to produce clinical symptoms. There is an urgent need for improved detection and characterization of head trauma to reduce future injury risk and promote development of new therapies. In this study we examined neurological performance and health in the presence of head collision events in high school football players, using longitudinal measures of collision events (the HIT™ System), neurocognitive testing (ImPACT™), and functional magnetic resonance imaging MRI (fMRI). Longitudinal assessment (including baseline) was conducted in 11 young men (ages 15–19 years) participating on the varsity and junior varsity football teams at a single high school. We expected and observed subjects in two previously described categories: (1) no clinically-diagnosed concussion and no changes in neurological behavior, and (2) clinically-diagnosed concussion with changes in neurological behavior. Additionally, we observed players in a previously undiscovered third category, who exhibited no clinically-observed symptoms associated with concussion, but who demonstrated measurable neurocognitive (primarily visual working memory) and neurophysiological (altered activation in the dorsolateral prefrontal cortex [DLPFC]) impairments. This new category was associated with significantly higher numbers of head collision events to the top-front of the head, directly above the DLPFC. The discovery of this new category suggests that more players are suffering neurological injury than are currently being detected using traditional concussion-assessment tools. These individuals are unlikely to undergo clinical evaluation, and thus may continue to participate in football-related activities, even when changes in brain physiology (and potential brain damage) are present, which will increase the risk of future neurological injury.
 : behavioral assessment, cognitive function, human studies, magnetic resonance imaging, traumatic brain injury
: behavioral assessment, cognitive function, human studies, magnetic resonance imaging, traumatic brain injuryIntroduction
The popular press has recently reported growing concern over the effects of concussions sustained while playing football.1–3 A recent congressional hearing on sports-related concussions brought particular attention to high school football players,2 a group annually comprising over one million young men (http://www.nfhs.org), of which approximately 67,000 per year are clinically diagnosed with a concussion.3,4 More significantly, it is estimated that a similar number of concussed players go undiagnosed.5 Failure to diagnose concussions is a concern for two reasons. First, players with neurological damage who are not removed from play are at higher risk for additional concussions.6 Second, biomechanics research has suggested that injury may be accumulated,7,8 a finding supported by histological evaluation of deceased athletes.9–11 Players who are not removed from play could thus accumulate injury in the form of multiple sub-concussive insults. Such a mechanism has been suggested by McKee and associates,12 based on observations made in autopsies of individuals with long-term neurodegeneration that did not strictly correlate with a clinical history of concussion.
The effects of concussion—defined herein as a closed-head injury to the brain induced by mechanical insult—are part of a broader public concern about brain health. Concussion falls within the larger category of traumatic brain injury (TBI), which represents a significant component of brain health in the United States, with as many as 3.8 million sports-related incidents occurring every year,13 with approximately 50,000 deaths and 235,000 hospitalizations from all causes of TBI.14 Previous TBI has been shown to be a significant risk factor for repeat concussions,15 and other neurological conditions, including early-onset Alzheimer's disease,16,17 chronic depression,18 epilepsy,13 and chronic traumatic encephalopathy (CTE).9,19 At least 17% of individuals who experience multiple concussions develop CTE,20 with McKee and associates12 proposing that the incidence is likely higher. Athletes participating in sports involving a significant probability of head collisions, such as American football, represent a group that is at particularly high-risk for concussion and other forms of TBI.
Currently, on-site healthcare professionals evaluate athletes for the presence of concussion by examining them for symptoms such as loss of consciousness, amnesia, headaches, dizziness, and an inability to respond correctly to specific, direct questions.15 Drawbacks to this process include the fact that symptoms often do not become manifest until several hours after trauma,21 that symptoms may not clearly indicate a specific, treatable neurological disorder,22 and that damage may accumulate over time as a result of injuries that do not produce symptoms that meet the clinical criteria for concussion.12
While concussion is inherently a mechanically-induced injury, efforts to determine its underlying biomechanical mechanisms have been inconclusive.7,23–25 Attempts to correlate injury with kinematic input variables such as peak acceleration or the Head Injury Criterion have proven inadequate in their ability to accurately predict the occurrence of concussion.7,26,27 Similarly, efforts to identify metabolic factors that predispose an individual to concussion have also remained elusive.18
In order to model the onset and development of cognitive impairment associated with head trauma in high school football players, we monitored head collision events (with the HIT System) experienced throughout the course of a single season, including both practices and games, by 21 members of a high school varsity football team. Based on the number and nature of the collision events, we longitudinally evaluated 11 of these athletes (eight varsity starters and three reserves) for changes in neurocognitive function and neurophysiology. By linking these findings we identified a group of high school football players without clinically-observable signs of concussion who exhibited neurocognitive and neurophysiological impairments comparable to or exceeding those exhibited by teammates who were diagnosed as concussed.
Methods
Subjects
Twenty-four male high school football players (age range 15–18 years, mean age 17.0 years) were enrolled. Twenty-one participated in each aspect of the study throughout the 2009 season (Table 1). Of the three players who did not complete the study, two quit participation in football prior to the end of the season, and the third suffered a season-ending knee injury during the first game and did not participate in team activities thereafter.
Table 1.
Dates and Results of ImPACT Tests, and Dates of fMRI Assessments for All Players Enrolled in the Study
| Assessment | ImPACT test dates | ImPACT memory composite (verbal) | ImPACT memory composite (visual) | fMRI session dates |
|---|---|---|---|---|
| Player 1001 | ||||
| Pre-season | 5 Augusta | 85 | 93 | 1 August |
| In-season | 29 Augustb | 75†,‡ | 57†,‡ | 29 August |
| Post-season | 29 Novemberb | 93 | 68†,‡ | 29 November |
| Player 101 | ||||
| Pre-season | 5 Augusta | 93 | 81 | 1 August |
| Player 102 | ||||
| Pre-season | 5 Augusta | 93 | 59 | 2 August |
| In-season | 19 September 1 Octobera 7 Octoberb | 96 97 83†,‡ | 56† 75 79 | 19 September 7 October |
| Post-season | 23 Novemberb | 91†,‡ | 79 | 23 November |
| Player 103 | ||||
| Pre-season | 5 Augusta | 98 | 70 | 2 August |
| In-season | 6 Septemberb | 82†,‡ | 76 | 6 September |
| 7 Octoberb | 78†,‡ | 61†,‡ | 7 OctoberA | |
| Post-season | 29 Novemberb | 84†,‡ | 84 | 29 November |
| Player 1042 | ||||
| Pre-season | 5 Augusta | 77 | 86 | 2 August |
| Player 105 | ||||
| Pre-season | 5 Augusta | 87 | 67 | 2 August |
| In-season | 18 Octoberb | 99 | 78 | 18 October |
| Post-season | 21 Novemberb | 95 | 72 | 21 November |
| Player 106 | ||||
| Pre-season | 5 Augusta | 90 | 81 | 2 August |
| Player 107 | ||||
| Pre-season | 5 Augusta | 94 | 75 | 2 August |
| In-season | 20 Septemberb | 99 | 83 | 20 September |
| Player 108 | ||||
| Pre-season | 5 Augusta | 63 | 80 | 2 August |
| Player 1092 | ||||
| Pre-season | 5 Augusta | 80 | 59 | 2 August |
| Player 1103 | ||||
| Pre-season | — | — | — | 2 August |
| Player 111 | ||||
| Pre-season | 5 Augusta | 84 | 70 | 2 August |
| Player 112 | ||||
| Pre-season | 5 Augusta | 92 | 78 | 2 August |
| In-season | 6 Septemberb | 97 | 80 | 6 September |
| Post-season | 5 Januarya,c | 86 | 77 | 18 November |
| Player 1131,2 | ||||
| Pre-season | 14 Augusta | 67 | 85 | 2 August |
| Player 114 | ||||
| Pre-season | 7 Augusta,d | 61 | 49 | 2 August |
| Player 115 | ||||
| Pre-season | 5 Augusta | 94 | 73 | 3 August |
| In-season | 5 Septemberb | 94 | 66† | 5 September |
| Post-season | 18 Novemberb | 100 | 65† | 18 November |
| Player 116 | ||||
| Pre-season | 6 Augusta | 98 | 95 | 3 August |
| Player 117 | ||||
| Pre-season | 5 Augusta | 84 | 66 | 4 August |
| Player 1181 | ||||
| Pre-season | 5 Augusta | 91 | 75 | 4 August |
| In-season | 18 Octoberb | 88†,‡ | 61† | 18 October |
| Post-season | 23 Novemberb | 96 | 84 | 23 November |
| Player 119 | ||||
| Pre-season | 5 Augusta | 100 | 78 | 5 August |
| Player 120 | ||||
| Pre-season | 5 Augusta | 88 | 96 | 5 August |
| In-season | 29 Augustb 10 Octoberb | 98 100 | 76†,‡ 73†,‡ | 29 August 10 October |
| Post-season | 19 Novemberb | 93 | 75†,‡ | 19 November |
| Player 121 | ||||
| Pre-season | 5 Augusta | 77 | 91 | 6 August |
| In-season | 26 Septemberb 25 Octoberb | 76 88 | 79† 70†,‡ | 26 September 25 October |
| Post-season | 23 Novemberb | 93 | 75†,‡ | 23 November |
| Player 122 | ||||
| Pre-season | 6 Augusta | 78 | 52 | 7 August |
| In-season | 16 Septemberb | 91 | 68 | 16 September |
| Post-season | 23 Januaryb | 89 | 81 | 23 January |
| Player 123 | ||||
| Pre-season | 11 Augusta | 93 | 59 | 10 August |
Player footnotes
ImPACT assessment footnotes
ImPACT score footnotes
fMRI assessment footnote
fMRI, functional magnetic resonance imaging.
Head collision event monitoring
Participants in this study had Head Impact Telemetry (HIT™) System (Simbex, New Lebanon, NH) sensors installed in their helmets. This system utilizes six accelerometers that provide three components each of linear and angular acceleration, providing measurement of direction and intensity of collision events experienced by the head.29 Each set of sensors is equipped with a wireless transmitter that provides real-time telemetry to a nearby laptop computer, which records the linear accelerations and impact location for each event.
Pre-season assessment
Prior to the beginning of contact drills, 23 of the enrollees completed both pre-season neurocognitive (ImPACT™) and neurophysiological (fMRI) assessments to quantify individual and group baselines. Neurocognitive testing was conducted at the high school, either in groups of up to 10 players in the library (19/23), or individually at the desk of the athletic trainer (4/23).
Neurocognitive testing
The on-line version of ImPACT (http://www.impacttestonline.com) was used as the gold standard of neurocognitive assessment, based on its widespread use for evaluating athletes. All participants selected the “high school” group for evaluation relative to an age-appropriate norm. Note that ImPACT was not used by the high school athletic training or medical staff in the return-to-play evaluation process.
Neurophysiological testing
Functional MRI was performed at the Purdue MRI Facility (West Lafayette, IN) on a 3-T General Electric Signa HDx (Waukesha, WI). This system is equipped with real-time monitoring, permitting excessive (greater than 0.5 mm) within-acquisition motion to be identified on-site, and acquisitions repeated as necessary until subject compliance is achieved. All 30-min imaging sessions used a 16-channel brain array (Nova Medical; Wilmington, MA). For registration, whole-brain high-resolution images (3D-FSPGR; 1
mm) within-acquisition motion to be identified on-site, and acquisitions repeated as necessary until subject compliance is achieved. All 30-min imaging sessions used a 16-channel brain array (Nova Medical; Wilmington, MA). For registration, whole-brain high-resolution images (3D-FSPGR; 1 mm isotropic resolution) were acquired, including the cerebellum.
mm isotropic resolution) were acquired, including the cerebellum.
Three functional runs were conducted of a visual working memory (N-back) paradigm, using gradient-echo echo planar imaging (TR/TE=1500/26 msec; matrix=64×64; FOV=20
msec; matrix=64×64; FOV=20 cm; 34 slices; 3.8
cm; 34 slices; 3.8 mm thickness; 117 volumes). In each run subjects performed one block (15 presentations, 3-sec interval, 5 targets per block) each of 0-, 1-, and 2-back tasks for single letters.30,31 Visual presentation was via fiber-optic goggles (NordicNeuroLab, Bergen, Norway). Subjects responded by dominant index finger via fiber-optic button box (Current Designs, Philadelphia, PA). Presentations and responses were implemented using E-Prime (Psychology Software Tools, Sharpsburg, PA). The order of the task blocks in the three runs was counter-balanced within each session, and across assessments.
mm thickness; 117 volumes). In each run subjects performed one block (15 presentations, 3-sec interval, 5 targets per block) each of 0-, 1-, and 2-back tasks for single letters.30,31 Visual presentation was via fiber-optic goggles (NordicNeuroLab, Bergen, Norway). Subjects responded by dominant index finger via fiber-optic button box (Current Designs, Philadelphia, PA). Presentations and responses were implemented using E-Prime (Psychology Software Tools, Sharpsburg, PA). The order of the task blocks in the three runs was counter-balanced within each session, and across assessments.
In-season assessment
During each of the 10 weeks in the season, 1–3 players were invited to undergo in-season assessment. Players were invited if: (1) they were diagnosed by the team physician as having experienced a concussion; (2) they were not identified by the physician as being concussed, but their HIT System data indicated that they had accrued unusually large numbers of collision events, or at least one high-magnitude (i.e., >100g) acceleration during that week's practices and games; or (3) athletes who participated in both practices and games, but did not experience either a large number of collision events or a high-magnitude acceleration. Participant compliance with these invitations was 75%, and 15 in-season assessments were initiated. All 15 ImPACT assessments were completed. Due to a network malfunction, only 14 fMRI sessions were performed in all, and were included in our analysis.
In-season assessments were conducted within 48 h of a game or 72
h of a game or 72 h of a diagnosis of concussion. ImPACT testing was conducted at the MRI facility with the player isolated in an office, and fMRI was conducted as above. The 11 players undergoing in-season assessment included eight who were invited on the basis of criterion (2) or (3), above. Players invited under criterion (2) were primarily recruited from among those who had accrued large numbers (i.e., the top 25%) of head-collision events, as assessed by the HIT System. The three remaining players undergoing in-season assessment represented three of the four players who were diagnosed by the team physician as having experienced a concussion. Note that one of the eight players receiving an in-season assessment while exhibiting no symptoms associated with concussion later received a diagnosed concussion, but declined to participate in further assessments. Because this player had not yet experienced a concussion at the time of pre-season and in-season assessment, his data have been included with the group of players who exhibited no symptoms of concussion.
h of a diagnosis of concussion. ImPACT testing was conducted at the MRI facility with the player isolated in an office, and fMRI was conducted as above. The 11 players undergoing in-season assessment included eight who were invited on the basis of criterion (2) or (3), above. Players invited under criterion (2) were primarily recruited from among those who had accrued large numbers (i.e., the top 25%) of head-collision events, as assessed by the HIT System. The three remaining players undergoing in-season assessment represented three of the four players who were diagnosed by the team physician as having experienced a concussion. Note that one of the eight players receiving an in-season assessment while exhibiting no symptoms associated with concussion later received a diagnosed concussion, but declined to participate in further assessments. Because this player had not yet experienced a concussion at the time of pre-season and in-season assessment, his data have been included with the group of players who exhibited no symptoms of concussion.
Post-season assessment
Ten of the 11 players (excluding the player noted above) who underwent in-season assessment returned 1–3 months after the end of the season for post-season assessment. ImPACT testing was conducted at the MRI Facility (nine players), or in the high school athletic training room (one player). fMRI was conducted as above.
Player categorization
Observed changes in neurological health were subsequently examined in the context of a clinical history of diagnosis or non-diagnosis of concussion during the course of the season, and by detection or non-detection of abnormal re-test behavior using ImPACT. To this end a 2×2 categorization matrix was defined for group evaluation (Fig. 1). Players who were diagnosed by the team physician with a concussion were deemed to be positive for clinically-observed impairment, and are labeled COI+. Players who were not diagnosed with a concussion were negative for this feature, and are labeled COI−. Players who exhibited deviant ImPACT re-tests were said to be positive for a functionally-observed impairment (FOI+), while those whose ImPACT scores fell within the 99% confidence intervals were negative for this feature (FOI−).
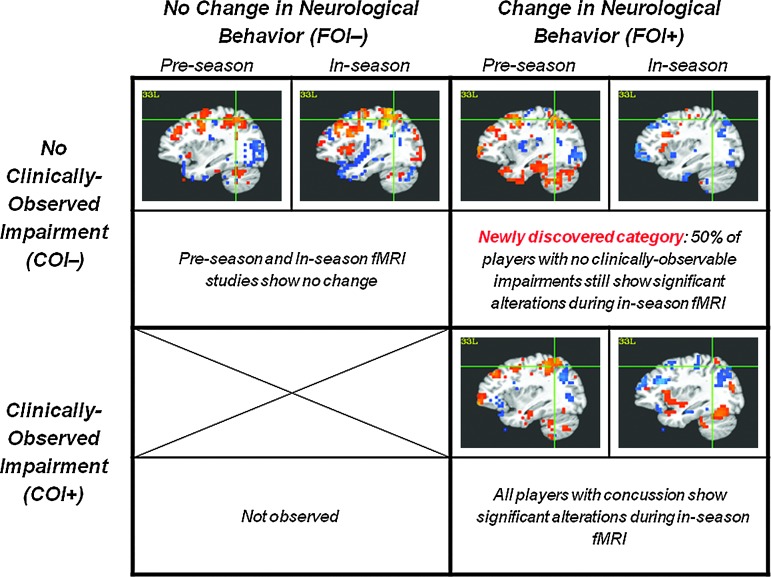
Summary of observed player categories, with representative functional magnetic resonance imaging (fMRI) observations. Categories are based on both clinical observation by the team physician of impairment associated with concussion (clinically-observed impairment; COI+ or COI−), and the presence or absence of significant neurocognitive impairment via ImPACT (functionally-observed impairment; FOI+ or FOI−). fMRI activations are depicted for all players using a sagittal slice through the left inferior parietal lobule (L IPL), to illustrate the presence of many changes, relative to pre-season assessment, for FOI+ players. Details of the depicted fMRI activations can be seen in Figures 2 and and3.3. (Bottom right) As expected, all (3/3) players who were diagnosed by the team physician as having experienced a concussion (COI+) were also found to exhibit significantly reduced ImPACT scores (FOI+), and are categorized as COI+/FOI+ (see also Fig. 2). (Top left) Half (4/8) of players brought in for assessment ostensibly for control purposes (i.e., presenting with no clinically-observable impairments, COI−) were found to be neurocognitively consistent with pre-season assessment (FOI−), and are categorized as COI−/FOI−. (Top right) The other half (4/8) of the intended control group, studied in the absence of diagnosed concussion (COI−), were found to exhibit significantly impaired ImPACT performance (FOI+), and are categorized as COI−/FOI+ (see also Fig. 3). This group represents a newly-observed category of possible neurological injury. (Bottom left) No players who were diagnosed with a concussion (COI+) were found to exhibit ImPACT scores consistent with pre-season assessments (FOI−). Color image is available at www.liebertpub.com/neu
Statistical analysis
Three categories of data were evaluated in this work: neurocognitive scores (ImPACT), collision events (HIT System), and neurophysiological signal changes (fMRI). In addition, the statistical significance of the observed frequencies of player categories was evaluated.
The consequences of the multiple environments in which ImPACT testing was performed were modeled via regression to most accurately identify abnormal re-test performance; the documented range of reliable test/re-test performance is based on a single site.33 Verbal and Visual Memory Composite scores from re-tests for which ImPACT did not indicate performance outside the reliability range were regressed on a population basis to compute the effect of site, permitting prediction of re-test performance at either site. Population variances were computed for ImPACT scores based on the pre-season tests conducted at the high school; 99% confidence intervals were generated around each player's re-test scores, on a site-specific basis, using the pre-season test variances scaled to account for observation of a higher mean for MRI Facility re-tests. Verbal and Visual Memory Composite re-test scores outside of these intervals were deemed to be “abnormal” (see Table 1). Note that this approach is conservative, being biased toward non-detection of abnormal re-test performance.
Collision events recorded by the HIT System for each player were analyzed using a one-way analysis of variance (ANOVA) to identify differences between categories of players (see above). Observed differences were assessed for significance using a Bonferroni-corrected one-tailed t-test, with the alternative hypothesis being that the COI−/FOI+ group exhibited the highest number of events under given location and magnitude constraints.
The fMRI data were analyzed using AFNI.32 Pre-processing included slice timing correction, motion correction, normalization to Talairach space, and 8-mm gaussian smoothing for inter-subject comparison. Individual runs (no more than one per subject) were discarded if extensive mid-sagittal ventricular activity was observed, suggestive of stress-induced, stimulus-correlated changes in physiological behavior (e.g., cardiac rate and respiratory cycle), likely arising from participants being uncomfortable in the MRI environment. Final analysis for each subject was effected on concatenated data, using a general linear model approach with Gamma Variate hemodynamic response function (without derivatives). The contrast of interest is a comparison between 2-back and 1-back working memory tasks, with statistically significant activation identified using a threshold of p<0.05, corrected for false-discovery rate (FDR). Changes in fMRI activation were assessed using the 116 anatomically-defined regions of interest (ROIs) from MarsBaR.34 For each player category (see below), a given ROI was said to exhibit significantly altered neurophysiological activity if the mean t-statistic fell outside the 99.9% confidence interval derived for that ROI using the pre-season data (23 players) for both (a) the group fixed-effects mean, and (b) a majority of players within the group.
Cross-modality analyses were performed to assess whether subsequently observed changes in fMRI assessment of physiology were correlated with head collision events. To evaluate possible short-term neurophysiological effects of head-collision events, alterations of hemodynamic response signal amplitudes observed during in-season fMRI relative to that observed within the same subject in the pre-season assessment (i.e., %SignalChangeIn-Season – %SignalChangePre-Season), was compared to the number of head-collision events measured by the HIT System in the week prior to the in-season assessment. This assessment was performed both on an anatomical ROI basis (i.e., for all 116 anatomical ROIs), and on a more global basis, for an aggregated ROI encompassing nearly the entirety of the frontal lobe, excluding only the precentral gyrus (i.e., the motor cortex, which is expected to be equally active in all tasks).
To document that the designated player categories were statistically meaningful, the predictive power of player category with respect to fMRI activation was evaluated using a one-tailed version of Fisher's exact test.35 Fisher's exact test is a more conservative version of the chi-square test that is appropriate for smaller study populations. A 3×2 implementation of the test was used, in which the player categories are considered the treatments, and the observation is either a decrease or a non-decrease in the in-season frontal lobe hemodynamic response signal amplitude, relative to that obtained from the pre-season assessment, evaluated on a per-subject basis. The alternative was that the COI−/FOI+ player category was significantly associated with an increased probability of observation of decreases in the aggregate frontal lobe response amplitude. The null hypothesis is that observation of a decrease in aggregate frontal lobe response amplitude is not associated with the COI−/FOI+ categorization. Note that categorization was made from ImPACT scores without knowledge of the aggregate frontal lobe signal change.
Results
Four of the 21 full-season participants were diagnosed with a concussion (i.e., were COI+) as a consequence of activities related to a practice or a game. Three of these players participated in an in-season assessment within 72 h of the diagnosis. One player (#100) was obligated to cease participating in football due to persistent symptoms following the injury. A second player (#118) was injured near the end of the season and was not cleared to play prior to the last game. A third player (#103) missed one game and returned to play the following week. As expected, all three of these COI+ players examined within 72
h of the diagnosis. One player (#100) was obligated to cease participating in football due to persistent symptoms following the injury. A second player (#118) was injured near the end of the season and was not cleared to play prior to the last game. A third player (#103) missed one game and returned to play the following week. As expected, all three of these COI+ players examined within 72 h of diagnosis of concussion were found to exhibit significantly lower neurocognitive performance on one or both of the Verbal and Visual Memory Composite scores on ImPACT. Based on joint observation of impairment by the team physician and the athlete's neurological assessment scores, these players are categorized as COI+/FOI+. fMRI data for these players revealed alterations in the pattern and amplitude of signal differences observed when contrasting the 2-back and 1-back memory tasks, particularly in the posterior middle and superior temporal gyri, regions associated with accessing linguistic representations of external stimuli (Fig. 2).
h of diagnosis of concussion were found to exhibit significantly lower neurocognitive performance on one or both of the Verbal and Visual Memory Composite scores on ImPACT. Based on joint observation of impairment by the team physician and the athlete's neurological assessment scores, these players are categorized as COI+/FOI+. fMRI data for these players revealed alterations in the pattern and amplitude of signal differences observed when contrasting the 2-back and 1-back memory tasks, particularly in the posterior middle and superior temporal gyri, regions associated with accessing linguistic representations of external stimuli (Fig. 2).
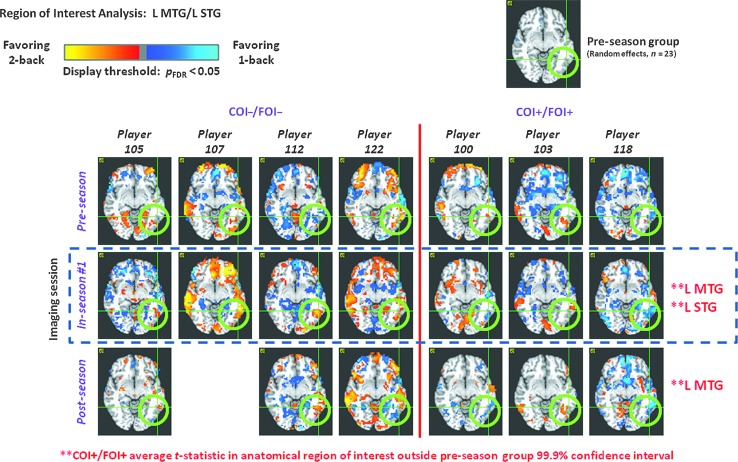
Significant alterations in functional magnetic resonance imaging (fMRI) activation during in-season assessment were found in the left middle and superior temporal gyri (L MTG and L STG; green circles) under a MarsBaR-based (Brett et al. 2002) region-of-interest (ROI) analysis for COI+/FOI+ players, but not for COI−/FOI− players. For the COI+/FOI+ group, in-season assessments took place within 72 h of diagnosis of concussion by the team physician. fMRI activations are depicted for a contrast between 2-back and 1-back working memory tasks, with observation of greater activation for the 2-back (1-back) task indicated using the red-yellow (blue-cyan) color scale, thresholded at a statistical significance level of p
h of diagnosis of concussion by the team physician. fMRI activations are depicted for a contrast between 2-back and 1-back working memory tasks, with observation of greater activation for the 2-back (1-back) task indicated using the red-yellow (blue-cyan) color scale, thresholded at a statistical significance level of p <
< 0.05, corrected for false-discovery rate. MarsBaR ROIs were said to exhibit significant alteration if the mean t-statistic fell outside the ROI-specific 99.9% confidence interval (derived from the pre-season data for 23 players), for both the group fixed-effects mean, and in a majority of the players within the group. For the COI+/FOI+group, the L MTG and L STG were two of five ROIs that exhibited such significant alterations. Other anatomical ROIs were left middle occipital gyrus, left cerebellum 10, and right cerebellum 3. The L MTG persisted in exhibiting significant deviation in the post-season data. For the COI−/FOI− group, only the right cerebellum 3 anatomical ROI was found to exhibit significant deviation during in-season assessment. It is important to note that all players performed the working-memory task at a consistent near-ceiling level over all sessions (COI, clinically-observed impairment; FOI, functionally-observed impairment). Color image is available at www.liebertpub.com/neu
0.05, corrected for false-discovery rate. MarsBaR ROIs were said to exhibit significant alteration if the mean t-statistic fell outside the ROI-specific 99.9% confidence interval (derived from the pre-season data for 23 players), for both the group fixed-effects mean, and in a majority of the players within the group. For the COI+/FOI+group, the L MTG and L STG were two of five ROIs that exhibited such significant alterations. Other anatomical ROIs were left middle occipital gyrus, left cerebellum 10, and right cerebellum 3. The L MTG persisted in exhibiting significant deviation in the post-season data. For the COI−/FOI− group, only the right cerebellum 3 anatomical ROI was found to exhibit significant deviation during in-season assessment. It is important to note that all players performed the working-memory task at a consistent near-ceiling level over all sessions (COI, clinically-observed impairment; FOI, functionally-observed impairment). Color image is available at www.liebertpub.com/neu
Four (#105, #107, #112, and #122) of the eight players invited to undergo in-season assessment in the absence of a clinical diagnosis of concussion (i.e., designated as COI−) exhibited no statistically significant deviations in ImPACT (Table 1). These players were categorized as COI−/FOI−. The in-season fMRI data for this group remained consistent with the pre-season evaluation in 115 of the 116 regions of interest, both on a within-player basis, and relative to the group random effects analysis (Figs. 2 and and3).3). The only exception was in right cerebellum 3, which exhibited decreased activation in three players. Three of the four COI−/FOI− players completed participation in a post-season assessment, at which time ImPACT scores and task performance were again found to be within test/re-test limits.
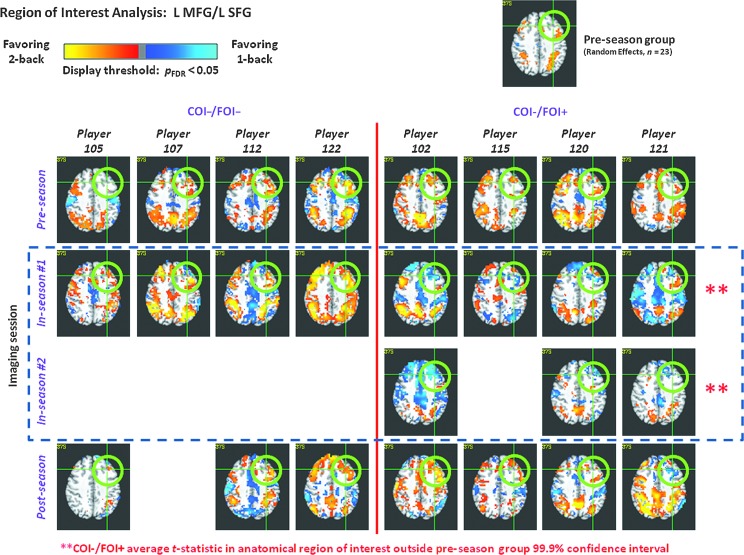
Significant alterations in functional magnetic resonance imaging (fMRI) activation during in-season assessment were found in left middle and superior frontal gyri (L MFG and L SFG; green circles) under a MarsBaR-based (Brett et al., 2002) region-of-interest (ROI) analysis for COI−/FOI+ players, but not for COI−/FOI− players. These ROIs represent much of dorsolateral prefrontal cortex (DLPFC). Coupled with observations of significantly reduced ImPACT scores, the COI−/FOI+ group represents a newly observed category of neurological injury, as these players are functionally impaired, yet do not exhibit symptoms associated with a clinical diagnosis of concussion. See Figure 2 for information regarding the depicted fMRI activations. For the COI−/FOI+ group, the L MFG and L SFG were two of eight ROIs that exhibited significant alterations in multiple in-season assessments. Other anatomical ROIs were right middle and superior frontal gyrus (R MFG and R SFG), both the right and left superior parietal lobules (R SPL and L SPL), the right pars triangularis, and the right cerebellum crus 1. Three of these ROIs (L MFG, L SFG, and R SFG) exhibited deviant activation in all in-season assessments for all COI−/FOI+ subjects. For the COI−/FOI− group, only the right cerebellum 3 anatomical ROI was found to exhibit significant deviation during in-season assessment. It is important to note that all players performed the working-memory task at a consistent near-ceiling level over all sessions (COI, clinically-observed impairment; FOI, functionally-observed impairment). Color image is available at www.liebertpub.com/neu
Unexpectedly, four (#102, #115, #120, and #121) of the eight COI− players evaluated during the season, while exhibiting no symptoms that would prompt evaluation for concussion by the team healthcare personnel, were found to exhibit statistically significant reductions in ImPACT scores (Verbal and/or Visual Memory Composite scores; Table 1). On this basis, these players are categorized as COI−/FOI+. This finding was augmented by observation (Fig. 3), in all such individuals at all in-season assessments (7 total, across 4 players), of significantly decreased fMRI activation levels in the dorsolateral prefrontal cortex (DLPFC; middle and superior frontal gyri) and cerebellum, regions of the brain strongly associated with working memory.36,37 In particular, when the 2-back and 1-back working memory conditions were contrasted, activation in the DLPFC changed from favoring (i.e., being greater for) the 2-back condition, to favoring the 1-back condition (Fig. 2). Note that the DLPFC has previously been documented to favor the 2-back condition in healthy controls,32 and was also found to favor the 2-back condition in our participants who did not exhibit deviant ImPACT performance (i.e., COI−/FOI−; Figs. 2 and and3).3). When compared with those players clinically diagnosed as having been concussed (i.e., COI+/FOI+), the COI−/FOI+ players were found to be at least as impaired (as demonstrated by both ImPACT and fMRI measures) as the known-concussed group.
The observed player categories were found to be statistically meaningful, with the null hypothesis rejected at the p<0.04 level (Fisher's exact test). Therefore, the COI−/FOI+ category is a justifiable segmentation of the subjects with respect to fMRI signal change.
Evaluation of HIT System data indicated that the COI−/FOI+ group was different from the other two groups with regard to the total number and distribution of collision events. The 21 players participating in our study throughout the season experienced 15,264 collision events (i.e., a motion/action during which at least one accelerometer registered a magnitude in excess of 14.4g),26 across 48 practices and games (varsity and junior varsity, including pre-game warm-up sessions), an average of 15.5 collision events per player per organized activity. Among players who started for either the varsity or junior varsity teams, per-player collision event totals ranged from a high of 1855 (player #121, COI−/FOI+, 38.6 events per session) down to a low of 226 (player #107, COI−/FOI−, 4.7 events per session). The total number of collision events experienced by the COI−/FOI+ group was significantly greater than that of any other group. This difference becomes even more pronounced when the number of collision events is examined on the basis of both region and magnitude (Fig. 4). Specifically, the COI−/FOI+ group exhibited more high magnitude (>80g) collision events directed to the top front of the helmet immediately above the DLPFC, in which functional changes were observed (Fig. 3).
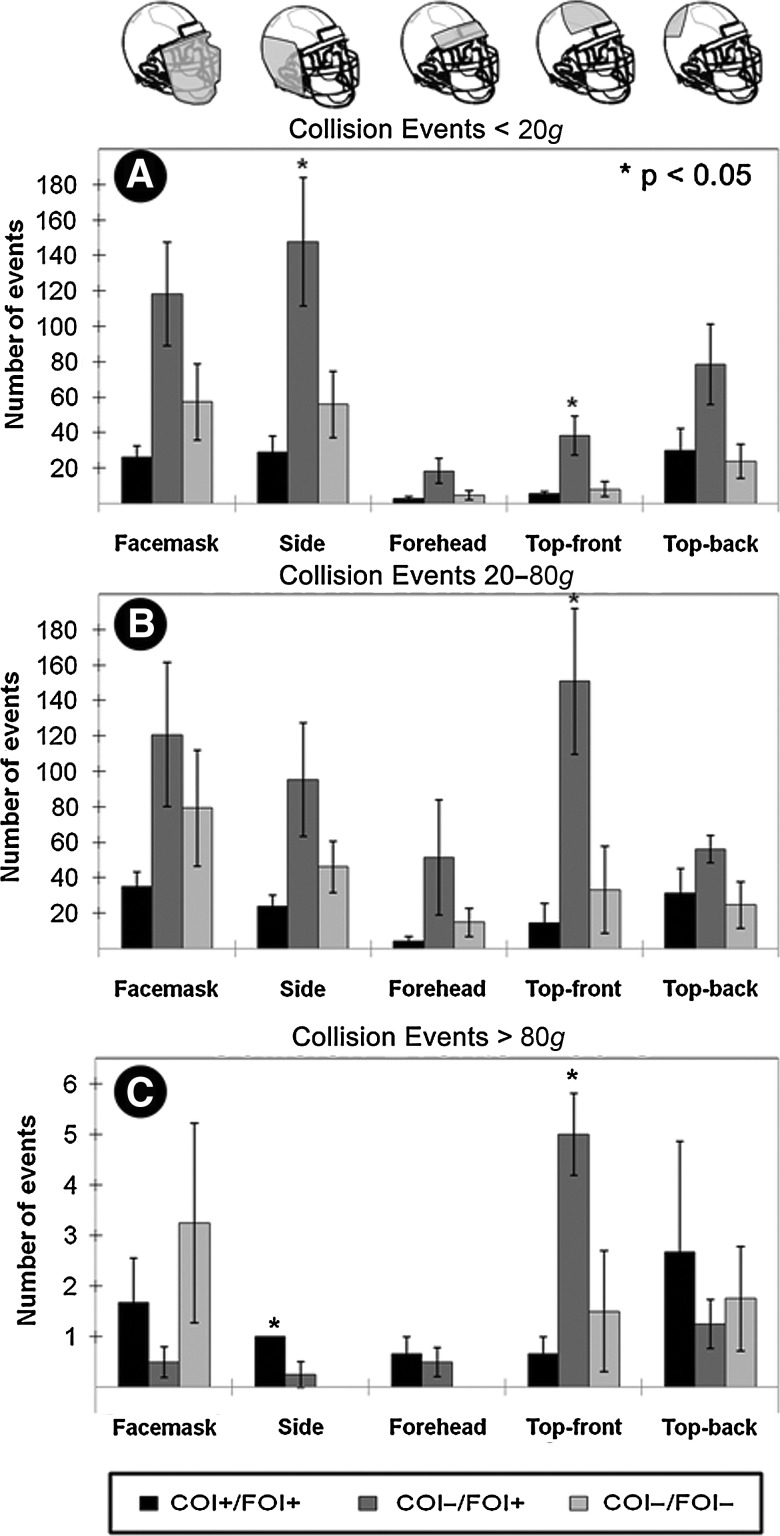
While peak linear acceleration was not predictive for neurological trauma, the pattern of average collision events per individual was significantly related to the concussion group as identified using neurocognitive testing (ImPACT). Each participant wore a Riddell® Revolution™ helmet (Riddell, Elyria, OH) outfitted with a sensor array (HIT System) to record head accelerations in practices and games once the season started (Crisco et al., 2004). Overall the COI−/FOI+ group experienced the greatest number of collision events to each region of the helmet. The COI−/FOI− group accumulated more collisions per player in each location than did the COI+/FOI+ group, suggesting that the latter group's injuries were not the result of the number of blows, but likely were due to a single or a small number of particularly deleterious collisions. (A) For collision events less than 20g, the COI−/FOI+ group experienced significantly more collision events to the side and the top-front of the helmet (p <
< 0.05, group-wise one-way ANOVA and Bonferroni-corrected one-tailed t-test). (B) Between 20 and 80g, the COI−/FOI+ group experienced a greater number of collision events to each region of the helmet, but the only location where it reached significance was the top-front. (C) Above 80g, the COI+/FOI+ group experienced significantly more blows to the side of the head, while the COI−/FOI+ group continued to experience a statistically greater number of blows to the top-front of the helmet (COI, clinically-observed impairment; FOI, functionally-observed impairment; ANOVA, analysis of variance).
0.05, group-wise one-way ANOVA and Bonferroni-corrected one-tailed t-test). (B) Between 20 and 80g, the COI−/FOI+ group experienced a greater number of collision events to each region of the helmet, but the only location where it reached significance was the top-front. (C) Above 80g, the COI+/FOI+ group experienced significantly more blows to the side of the head, while the COI−/FOI+ group continued to experience a statistically greater number of blows to the top-front of the helmet (COI, clinically-observed impairment; FOI, functionally-observed impairment; ANOVA, analysis of variance).
Further evaluation of the head-collision events experienced by the 11 players assessed during the season revealed that the number of events experienced in the week immediately preceding an in-season assessment (n=14) was significantly correlated with changes in fMRI activation for the 2-back versus 1-back contrast of interest. At the level of anatomical ROIs (Table 2), statistically significant (p<0.05; |r|≥0.53) correlations were observed for collision events with the deviation of the 2-back versus 1-back signal change from that observed for the individual in the pre-season assessment. For all of the ROIs listed in Table 2, fMRI signal changes became less biased in the favor of the 2-back task (i.e., lesser activation for the 2-back task, relative to that evidenced for the 1-back task), as the number of head-collision events increased. While the most consistent changes in activation were located in the DLPFC, the majority of anatomical structures associated with this region (e.g., L MFG, L SFG, and R SFG) are not indicated in Table 2. However, comparison of collision events to calculated changes in hemodynamic response signal amplitude for the aggregated frontal lobe ROI yields a trend that is well described by a linear regression model (R2=0.46; Fig. 5).
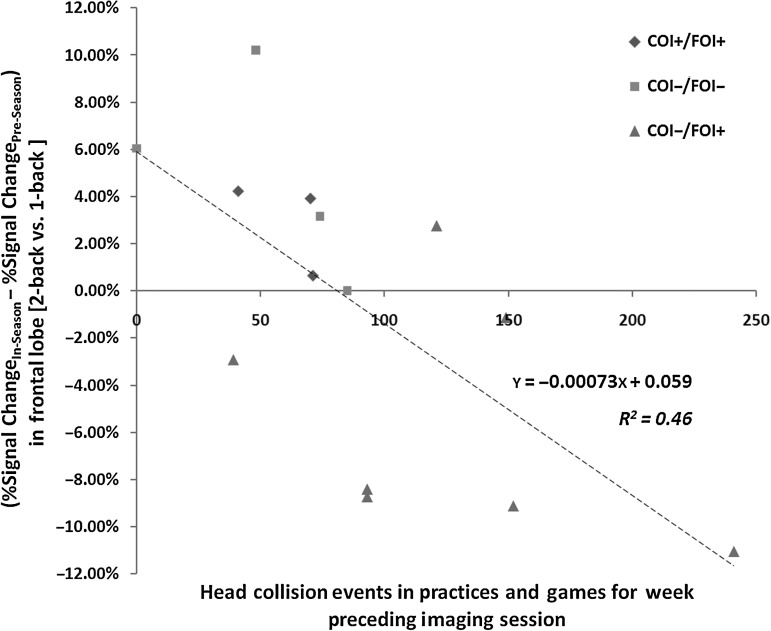
The number of head collision events exceeding 14.4g experienced by a player was found to be inversely correlated with the fMRI contrast (2-back versus 1-back) observed during in-season assessments. All frontal regions of interest in the MarsBaR (Brett et al., 2002) were aggregated to obtain a frontal lobe region of interest (ROI), from which an average percent signal change was computed for this contrast in each of the 14 completed in-season fMRI assessments, and the corresponding pre-season assessment for the same subject. The difference between these assessments is plotted against the total number of HIT-System-reported collision events. Different symbols have been used to identify the three groups (COI−/FOI−, COI−/FOI+, and COI+/FOI+) of players, illustrating the greater number of collision events for the COI−/FOI+ group (see Fig. 4). The regression line across the 14 comparisons achieves R2 =
= 0.46, indicating that the recent accrual of head collision events is meaningfully related to the degree of change in neurophysiological response (COI, clinically-observed impairment; FOI, functionally-observed impairment; fMRI, functional magnetic resonance imaging).
0.46, indicating that the recent accrual of head collision events is meaningfully related to the degree of change in neurophysiological response (COI, clinically-observed impairment; FOI, functionally-observed impairment; fMRI, functional magnetic resonance imaging).
Table 2.
Anatomical Regions of Interest (ROIs) from MarsBaRa Exhibiting Statistically Significant* Correlations Between the Total Number of Head Collision Eventsb Experienced by a Player in the Week Prior to In-Season Assessment and the fMRI Percent Signal Change Observed During That Assessment for the Two-Back Versus One-Back Contrast
| Anatomical region of interest (MarsBaR ROI #) | Correlation between fMRI contrast and collision events |
|---|---|
| Frontal medial orbital L (41) | −0.70** |
| Frontal medial orbital R (42) | −0.72** |
| Frontal middle R (46) | −0.55 |
| Frontal superior orbital L (50) | −0.71** |
| Fusiform R (54) | −0.56 |
| Hippocampus L (57) | −0.60 |
| Hippocampus R (58) | −0.68** |
| Parahippocampal R (76) | −0.58 |
| Rectus L (89) | −0.59 |
| Rectus R (90) | −0.62 |
| Temporal superior pole L (103) | −0.59 |
| Temporal superior pole R (104) | −0.53 |
 <
< 0.05; n
0.05; n =
= 14.
14. <
< 0.01.
0.01.fMRI, functional magnetic resonance imaging.
Discussion
The goal of this study was to evaluate neurocognitive and neurophysiological deficits in high school football players as a function of head-collision events, using pre-season baselines to quantify observed deficits. Athletes with collision event distributions similar to those diagnosed with concussion were intended as controls—as opposed to non-athletes—representing the first time that such a control population has been examined. Unexpectedly, half of these controls demonstrated both neurocognitive and neurophysiological deficits, prompting the designation of a new group without observable signs of concussion who nevertheless exhibited cognitive impairments (COI−/FOI+ players; Fig. 1).
Athletes are a particularly high-risk population for TBI, especially amateur hockey and football players.37,39 Of the two, football is the more commonly played sport, with approximately 1.1 million high school participants in the United States during 2008–2009 (http://www.nfhs.org). Each year, between 43,000 and 67,000 of these players are diagnosed with concussions.3,4 Unfortunately, many young athletes do not appreciate the seriousness of concussion and fail to self-report symptoms—sometimes intentionally, as they seek to remain on the field—likely doubling the number of actual concussions.5 Those with undiagnosed impairments who are not removed from play are of critical concern, because they continue to experience repeated head-collision events.
In addition to players that do not report their symptoms, the results presented here indicate that additional athletes—those that would be considered COI−/FOI+—may be accruing damage that does not immediately result in symptoms that are typically observed by a clinician. The analysis performed using Fisher's exact test demonstrates that the COI−/FOI+ category is statistically distinct from the a priori expected COI+/FOI+ and COI−/FOI− categories. This analysis examined the probability of decreased aggregate frontal lobe activation, which is a region associated with working memory.36,37 The frontal lobe was a structure of interest both because working memory scores from ImPACT were used to make the original categorizations, and because frontal lobe damage has received considerable attention in the literature.12,25,40 It is therefore notable that significant changes in signal amplitude in working memory structures were statistically more frequent in COI−/FOI+ subjects. This correspondence is consistent with the hypothesis of the existence of the COI−/FOI+ category.
While our data do not provide statistical confidence that 50% of all COI− subjects will exhibit functional impairment, as this proportion has inherent scatter that must be measured through several replicates across different player populations, we nevertheless observed that 4 of the original 23 volunteer subjects, about 17%, were COI−/FOI+, which is still a sufficiently large proportion to raise concern. Further, the use of our method for identifying COI−/FOI+ players based on decreases in ImPACT scores, indicates that 12 of the 23 non-concussed contact sport athletes studied by Beckwith et al.41 would be considered COI−/FOI+. This substantiates the proportion we have observed, and indicates that the actual proportion of functional impairment among players who do not exhibit signs of concussion (i.e., COI− players) may indeed be quite high. It should also be noted that there is expected to be considerable variation in neurological response based on player size, speed, skill level, position, and technique, as well as field conditions and level of competition. Consequently, additional studies are needed to elucidate the effects of these parameters and further generalize the results.
It is suspected that the COI−/FOI+ group comprises players who experienced neurological trauma arising from repeated, sub-concussive head-collision events, each of which likely produces sub-clinical stress on neural tissue.42 In this case, the players failed to accrue sufficient short-term damage to integrative neural systems that they exhibited externally observable symptoms. As such, these players continued to participate in practices and games throughout the season, with neurocognitive and neurophysiological impairments persisting over time (Fig. 3), but never exhibiting symptoms that would trigger evaluation by a healthcare professional. These players not only may be representative of the group associated with “unreported” concussions, but are also likely to meet the criterion of McKee and associates,12 whereby having received repetitive, sub-concussive blows to the head, they may have an increased likelihood of long-term neurodegeneration.8,42
Of particular interest, this functionally (but not clinically) impaired group was primarily comprised of linemen, who experience helmet-to-helmet contact on nearly every play from scrimmage, often to the top front of the head. This finding of degraded neurological performance in the absence of classical symptoms of concussion is consistent with prior observations of CTE in the absence of a commensurate history of concussion in two ex-NFL offensive linemen and a defensive back.9,10 Given the dire outcomes observed as a consequence of CTE, and given that such a functional observation suggests that present clinical practice does not succeed in detecting the neurological deficits in these individuals, it is important that we develop a means to detect when such injury occurs, or perhaps more importantly, to predict and prevent injuries of this nature.
Our observation of two groups (COI+/FOI+ and COI−/FOI+) exhibiting neurocognitive and neurophysiological impairment that is distinguished by the presence or absence of externally-observable behavioral symptoms, implies that these groups have experienced injuries that differ by mechanism and associated locations of damage. The COI+/FOI+ group exhibits an onset and extent of behavioral deficits consistent with damage to integrative centers of the brain associated with auditory (especially language) processing, with such damage likely produced in locations unique to each individual by a singular, deleterious collision event. In contrast, the COI−/FOI+ group predominantly exhibits behavioral deficits in working memory (predominantly visual), that likely are produced by repeated sub-clinical trauma to specific locations in the brain, though the nature of the trauma is as yet unclear.
Regardless of the uncertainty surrounding the specific injury incurred in the COI−/FOI+ group, the similarities of fMRI impairment associated with members of this group (Fig. 3), suggests that future work may be able to identify the underlying causes of deficits within this population. It is worth noting that previous studies involving positron emission tomography (PET) have found that the changes in metabolism associated with TBI are spatially diffuse relative to the actual site of mechanically-induced injury,43 and not necessarily localized to regions experiencing transient ischemia.44 Therefore, alterations in fMRI signal changes may not take place at the precise location of mechanically-induced injury, but these alterations would be an expected consequence of the changes in metabolism associated with damage. Thus players experiencing clinically-diagnosable concussions (i.e., COI+/FOI+) due to subject-specific injuries would not be expected to exhibit group-wise consistency in the alteration of fMRI activations, but players experiencing a common injury (i.e., possibly the COI−/FOI+ group) would.
Initial assessment (Fig. 4) of the mechanical insults (as assessed by the HIT System) to the athletes in the two FOI+ groups indicates that they did in fact experience different collision event histories, and supports the above hypotheses regarding the potential for identifying a common underlying injury in the COI−/FOI+ group. Note that these data also support the argument that peak acceleration is not a sufficient measure to predict cognitive deficit.7,8,24,26 Currently, the location of the postulated injuries in the COI−/FOI+ group (working memory networks, including DLPFC), and their apparent focal behavioral effect, make it difficult to identify this group on-site. If an individual has not suffered damage to integration centers associated with language, or to auditory processing pathways, he is unlikely to exhibit the symptoms necessary for identification as being concussed. Further, if working memory deficits are sufficiently small, the individual may not be aware of the additional effort required to complete everyday tasks, perhaps only becoming aware that a deficit is present under the duress of probes such as neurocognitive tests.
The findings of this study suggest that functional MRI can be a valuable tool for detecting neurophysiological deficits after head injury, as has been previously suggested,15 and as supported by the PET literature related to the study of changes in metabolism seen following TBI.43–45 To better evaluate the structure-function relationships that cause neurological damage, it will be essential to expand the range of neurological testing done within the MRI, and to add structural assessments such as diffusion tensor imaging,46 and susceptibility-weighted imaging47 modalities. In addition, better characterization of the mechanical insults, and the complex, heterogeneous effects they have on the brain itself, must be developed.
This study was strengthened by acquisition of baseline data prior to the commencement of athletic activities, greatly increasing the ability to detect changes at both an individual and group level. Despite the small sample size, a precise correlation was found between the deficits observed using an established neurocognitive assessment tool (ImPACT), and the neurophysiological changes observed with fMRI during a verbal working memory task. Consistent with the hypothesis that the different observed cognitive and neurophysiological deficits arise from distinct mechanical insult histories, significant differences were observed between groups of players categorized by changes in ImPACT scores.
Therefore, these data indicate the presence of a previously unknown, but suspected (McKee et al., 2009), group of athletes exhibiting neurocognitive deficits that persist over time, but which does not present with observable symptoms. This group continues to participate in contact sports, and may be at risk of further long-term neurological injury. Consequently, high priority should be given to the development of procedures that lead to the identification of these at-risk individuals.
Acknowledgments
This work was supported by grants from the Indiana State Department of Health Spinal Cord and Brain Injury Research Fund, and General Electric Healthcare. The authors thank Dr. Charles A. Bouman and Mr. Dennis A. Miller of Purdue University, Dr. Randall R. Benson of Wayne State University, and Drs. Michael W. Collins and Mark J. Lovell of the University of Pittsburgh Medical Center, for their feedback and encouragement. The authors additionally thank Dr. Gregory G. Tamer, Jr., for his assistance with data collection.
References
Articles from Journal of Neurotrauma are provided here courtesy of Mary Ann Liebert, Inc.
Full text links
Read article at publisher's site: https://doi.org/10.1089/neu.2010.1512
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3922228?pdf=render
Citations & impact
Impact metrics
Article citations
Potential of Soft-Shelled Rugby Headgear to Lower Regional Brain Strain Metrics During Standard Drop Tests.
Sports Med Open, 10(1):102, 27 Sep 2024
Cited by: 0 articles | PMID: 39333426 | PMCID: PMC11436562
Uncovering the hidden effects of repetitive subconcussive head impact exposure: A mega-analytic approach characterizing seasonal brain microstructural changes in contact and collision sports athletes.
Hum Brain Mapp, 45(12):e26811, 01 Aug 2024
Cited by: 0 articles | PMID: 39185683 | PMCID: PMC11345636
Sub-concussive head impacts from heading footballs do not acutely alter brain excitability as compared to a control group.
PLoS One, 19(8):e0306560, 01 Aug 2024
Cited by: 0 articles | PMID: 39088385 | PMCID: PMC11293750
Cognitive and Salience Network Connectivity Changes following a Single Season of Repetitive Head Impact Exposure in High School Football.
AJNR Am J Neuroradiol, 45(8):1116-1123, 09 Aug 2024
Cited by: 0 articles | PMID: 39054293
Head Kinematics and Injury Analysis in Elite Bobsleigh Athletes Throughout a World Cup Tour.
J Athl Train, 59(6):584-593, 01 Jun 2024
Cited by: 1 article | PMID: 37648215
Go to all (256) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Post-concussion cognitive declines and symptomatology are not related to concussion biomechanics in high school football players.
J Neurotrauma, 28(10):2061-2068, 29 Aug 2011
Cited by: 56 articles | PMID: 21644811 | PMCID: PMC4346373
Association between recurrent concussion and late-life cognitive impairment in retired professional football players.
Neurosurgery, 57(4):719-26; discussion 719-26, 01 Oct 2005
Cited by: 609 articles | PMID: 16239884
Modifiable Risk Factors for Poor Cognitive Function in Former American-Style Football Players: Findings from the Harvard Football Players Health Study.
J Neurotrauma, 38(2):189-195, 04 Aug 2020
Cited by: 16 articles | PMID: 32640866 | PMCID: PMC8182470
Abnormal white matter integrity related to head impact exposure in a season of high school varsity football.
J Neurotrauma, 31(19):1617-1624, 14 Jul 2014
Cited by: 126 articles | PMID: 24786802 | PMCID: PMC4170811
 1,,2
1,,2 




