Abstract
Free full text

Long-term Outcomes in Children with Pulmonary Arterial Hypertension Treated with Bosentan in Real World Clinical Settings
Abstract
Treatment algorithms in pediatric PAH are derived from clinical trials in adult populations, and from clinical practice, but experience in children is limited. In this retrospective cohort study, we analyzed outcomes in a previously identified cohort of 86 consecutive children with pulmonary arterial hypertension (PAH) treated with bosentan as part of their treatment regimen. All children with idiopathic PAH (IPAH) or heritable PAH, and PAH associated with congenital heart disease (PAH-CHD) or connective tissue disease (PAH-CTD) who started bosentan treatment between May 2001 and April 2003 in two tertiary pediatric referral centers were followed with data collection ending August 2006. 86 children (37 males; 49 females) aged 11±5 years with IPAH/HPAH (n=36), PAH-CHD (n=48) or PAH-CTD (n=2) received bosentan as monotherapy (n=42) or as add-on to pre-existing continuous intravenous epoprostenol or subcutaneous treprostinil (n=44). Median observation period was 39 months (range 2–60). 34 patients (40%) received at least one additional PAH-specific therapy during follow-up. At end of data collection, 25 patients (29%) remained on bosentan, 43 (50%) had stopped bosentan, 11 (13%) had died while on bosentan, and 7 were lost to follow-up. At 4 years, the Kaplan-Meier estimate of disease progression in patients while on bosentan was 54% (7 patients at risk) with a survival estimate of 82% (16 patients at risk). Risk factors significantly associated with survival were WHO FC and indexed PVR. In conclusion, outcome in children with PAH managed with current treatment regimens, appears favorable. However, despite current therapy options, disease progression remains a concern.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by remodeling of the pulmonary vasculature resulting in right ventricular failure and death, if untreated. In children, PAH is most often idiopathic (IPAH), heritable (HPAH) or associated with congenital heart disease (PAH-CHD) (1,2). Bosentan is an orally active dual endothelin receptor antagonist that delays time to clinical worsening, and improves exercise capacity and hemodynamics in adult PAH patients (3,4,5,6). In children, the effectiveness and safety of bosentan is supported by prospective and retrospective studies (7,8). However, long-term outcome data remains limited. In this retrospective cohort study, we describe long-term disease progression and survival in a previously identified cohort of 86 consecutive PAH children in whom treatment with bosentan was initiated as part of their treatment regimens (7) between May 2001 and April 2003 until end of data collection, August 2006. These data provide real-world experience with such treatment regimens in the management of children with PAH.
Methods
This is a retrospective cohort study in 86 consecutive pediatric patients with PAH (≤18 years of age at bosentan initiation) who started bosentan between May 2001 and April 2003 with or without pre-existing continuous parenteral intravenous epoprostenol or subcutaneous treprostinil therapy at two tertiary referral centers in the USA (Columbia University Medical Center, New York, New York and The Children’s Hospital, Denver, Colorado). Follow-up data were collected retrospectively from medical charts; end of data collection was August 31, 2006. The patient characteristics and the treatment algorithms followed in both centers have been described previously (7). PAH was confirmed by right heart catheterization (9). This study was approved by the respective Institutional Review Boards.
Bosentan treatment was initiated as monotherapy or as add-on to pre-existing therapy with intravenous epoprostenol or subcutaneous treprostinil. The maintenance dose of bosentan as well as the dose of pre-existing medications (intravenous epoprostenol, subcutaneous treprostinil), and additional PAH-specific therapies initiated during the observation period were adjusted according to the treating physicians’ clinical judgment. Reasons for addition of PAH-specific therapies were: ‘further improvement desired’, ‘deterioration’, or ‘other’. Cardiopulmonary hemodynamic parameters (cardiac index, mean pulmonary arterial pressure, pulmonary vascular resistance index, and mean right atrial pressure) were obtained by cardiac catheterization (children were anesthetized) within the 3 month-period preceding bosentan initiation. WHO functional class (WHO FC) and 6-minute walk distance [(6MWD), if age appropriate] were assessed prior to starting bosentan treatment and during follow-up. Addition of PAH-specific therapy was recorded. The composite endpoint 'disease progression' was pre-defined as the first occurrence of one or more of the following events: death, atrial septostomy, lung transplantation, deterioration in WHO FC compared to baseline, decrease of at least 15% in 6MWD compared to baseline, discontinuation of bosentan due to PAH deterioration according to the treating physician’s clinical judgment and/or addition of PAH-specific therapy due to PAH deterioration after the initiation of bosentan. Survival status and treatment were assessed for all patients at end of data collection, regardless of whether bosentan was continued or discontinued. Peripheral edema, systemic hypotension and increases in hepatic transaminase levels as well as events leading to discontinuation were collected as safety experience. Due to the small number of patients with PAH associated with connective tissue disease (n=2), only demographics are presented for this subgroup. Due to the retrospective nature of the data collection, statistical analysis was descriptive and exploratory. Person-time incidence rates were calculated by dividing the number of events by the cumulative treatment exposure. P-values are not reported because there was no predefined study hypothesis (10), reflecting STROBE guidance (11). Proportions were reported with 95% 2-sided confidence limits (CL) based on the binomial exact distribution. Patients who died were assigned WHO FC IV. Time to addition of PAH-specific therapy for any reason, time to ‘disease progression’, and time to death were summarized using Kaplan-Meier estimates and 95% CL (patients lost to follow-up were censored). Univariate Cox regression analyses for survival were performed (12) and risk factors significant at the 0.10 level were noted. However, the multivariate Cox proportional-hazards regression analysis was based on a backward selection process with a selection level of 0.0157 (13). Pre-specified risk factors at bosentan initiation were gender, age at PAH diagnosis, time from PAH diagnosis to initiation of bosentan, etiology (e.g. IPAH/HPAH vs. PAH other), WHO FC (i.e. WHO FC I/II vs III/IV), and monotherapy at bosentan initiation (i.e. bosentan without background prostanoid therapy). Hemodynamic variables (CI, PAPm, PVRi, and RAPm) were also included in an additional Cox regression analysis despite a high proportion of missing data. Each continuous risk factor was transformed into a binary variable using its mean as a cut-off.
Results
In this cohort of 86 children with PAH, the median observation period, defined as time between initiation of bosentan and date of last clinical information capture before end of data collection was 39 months (mean ± SD: 35 ± 15, range 2 to 60 months). Demographic characteristics at bosentan initiation have been presented previously (7) (Table 1). Patients ranged in age from 9 months to 18 years at the start of bosentan therapy. More patients with PAH-CHD were female and started bosentan as monotherapy. Congenital heart defects were fully repaired in 19 (40%) patients (including 4 patients on pre-existing prostanoid therapy), or partially repaired or unrepaired for 29 (60%) patients (including 14 patients on pre-existing prostanoid therapy). A higher percentage of patients on pre-existing prostanoid therapy had partially repaired or unrepaired congenital heart defects compared with repaired defects at bosentan initiation than patients not on prostanoid therapy (78% vs 50%). Hemodynamic parameters were also consistent with more severe disease in the subgroup with pre-existing prostaniod therapy at bosentan initiation (Table 1). Congenital heart defects included (repaired / partially repaired and unrepaired): atrial septal defects (1/10), ventricular septal defects (5/9), patent ductus arteriosus (2/5), atrioventricular septal defects (3/0), transposition of the great arteries (3/1), tetralogy of Fallot (3/1), “absent” isolated pulmonary artery (1/1), single ventricle (0/1), total anomalous pulmonary venous return (1/0), and interrupted aortic arch with VSD (0/1). Nineteen patients had Eisenmenger syndrome, defined as unrestrictive and unrepaired CHD with resting systemic arterial oxygen saturation in room air <90%. The median 6MWD was 456 meters (mean ± SD: 473 ± 105, range 201–719) in 53 patients who were old enough to perform the 6MWD.
Table 1
Demographic, hemodynamic, and functional characteristics at bosentan initiation
| All patients* (N = 86) | Patients with IPAH/HPAH (N = 36) | Patients with PAH-CHD (N = 48) | |||
|---|---|---|---|---|---|
| Treatment at bosentan initiation | Monotherapy (n = 11) | Plus prostanoid (n = 25) | Monotherapy (n = 30) | Plus prostanoid (n = 18) | |
| Male:Female | 37:49 | 6:5 | 14:11 | 9:21 | 6:12 |
| Age | |||||
| At bosentan initiation | 11 ± 5 [0–18] | 10 ± 6 [1–16] | 11 ± 4 [4–16] | 10 ± 6 [0–18] | 12 ± 4 [6–18] |
| At pulmonary arterial hypertension diagnosis | 5 ± 5 [0–16] | 8 ± 5 [1–15] | 5 ± 5 [0–16] | 3 ± 4 [0–13] | 6 ± 4 [1–15] |
| Weight at bosentan initiation | 34 ± 16 [5–89] | 35 ± 17 [8–62] | 37 ± 17 [14–89] | 30 ± 16 [5–64] | 37 ± 15 [17–57] |
| Hemodynamic parameters** | |||||
| Mean pulmonary artery pressure (mmHg) | 63 ± 20 (n = 67) | 58 ± 18 (n = 10) | 65 ± 26 (n = 17) | 60 ± 16 (n = 26) | 75 ± 20 (n = 12) |
| Mean pulmonary capillary wedge pressure (mmHg) | 9 ± 3 (n = 67) | 9 ± 2 (n = 10) | 8 ± 3 (n = 17) | 9 ± 4 (n = 26) | 9 ± 3 (n = 12) |
| Mean right atrial pressure (mmHg) | 7 ± 4 (n = 67) | 5 ± 2 (n = 10) | 7 ± 4 (n = 17) | 7 ± 4 (n = 26) | 9 ± 3 (n = 12) |
| Cardiac index (L/min/m2) | 3.6 ± 1.4 (n = 62) | 3.7 ± 1.3 (n = 10) | 3.8 ± 1.2 (n = 17) | 3.3 ± 1.3 (n = 23) | 4.4 ± 2.0 (n = 10) |
| Pulmonary vascular resistance index (U.m2) | 20 ± 14 (n = 66) | 16 ± 13 (n = 10) | 20 ± 17 (n = 17) | 22 ± 14 (n = 25) | 21 ± 12 (n = 12) |
| WHO functional class: I: II: III: IV | 6: 36: 33: 6 | 0: 4: 6: 1 | 3: 8: 10: 2 | 2: 16: 9: 2 | 1: 8: 7: 0 |
Values are number (percentage) of patients or mean ± standard deviation [range].
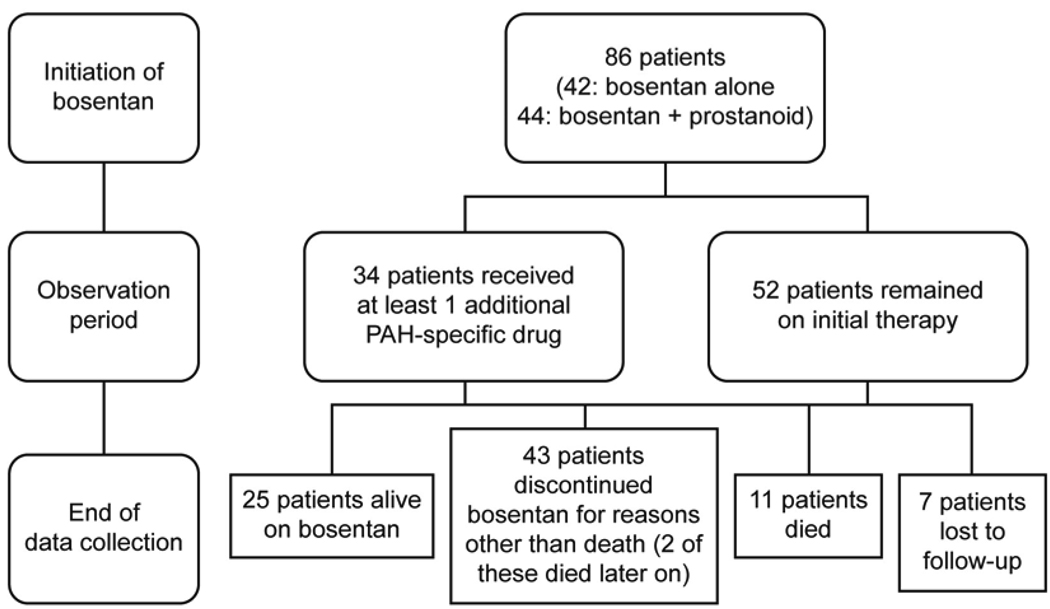
The median bosentan exposure time up to end of data collection was 24 months (mean ± SD: 29 ± 17, range 2 to 63). The treatment patterns of the patients throughout the observation period are outlined in Figure 1. Thirty-four of the 86 patients (40%) had at least one additional PAH-specific therapy added during the follow-up period: PDE-5 inhibitors were added in 30 (88%) of these 34 patients and prostanoids were added in 15 (44%) patients. The most frequent reason for adding PDE-5 inhibitors was ‘further improvement desired’ (n=24); for adding prostanoids it was ‘deterioration’ (n=11). The Kaplan-Meier estimates of time to addition of PAH-specific therapy at 1, 2, 3 and 4 years are shown in Table 2. Overall, the dose of epoprostenol for the 36 children treated with pre-existing epoprostenol at bosentan initiation decreased from 73 ± 42 ng/kg/min (mean ± SD) to 46 ± 45 ng/kg/min at end of data collection; the dose for the 8 patients treated with subcutaneous treprostinil at bosentan initiation decreased from 59 ± 26 ng/kg/min (mean ± SD) to 50 ± 47 ng/kg/ min.
Table 2
Kaplan-Meier estimates
| Year | Time to addition of PAH-specific therapy | Time to ‘disease progression’ |
|---|---|---|
| 1 | 8% (x = 64) | 21% (x = 60) |
| 95% CL [2, 13] | 95% CL [12, 29] | |
| 2 | 36% (x = 26) | 47% (x = 32) |
| 95% CL [24, 47] | 95% CL [35, 58] | |
| 3 | 62% (x = 10) | 56% (x = 20) |
| 95% CL [47, 78] | 95% CL [44, 68] | |
| 4 | 70% (x = 4) | 67% (x = 6) |
| 95% CL [54, 86] | 95% CL [54, 80] |
x: Patients remaining at risk of experiencing the event.
Seventy-eight of the 86 patients who had WHO FC data at bosentan initiation and at least one assessment during the observation period were evaluated for change in WHO FC. Median exposure to bosentan in these patients was 24 months (mean ± SD: 29 ± 17, range 2 to 63). The percentages of patients with improved and worsened WHO FC at the last assessment are displayed in Figure 2. At this time point, 24 patients (31%) had improved (95% CL: 21%, 42%), and 21 patients (27%) had worsened (95% CL: 18%, 38%). WHO FC improved in 44% of IPAH/HPAH patients and in 20% of PAH-CHD patients. At end of data collection, 25 of the 86 patients (29%) remained alive on bosentan (Figure 1 and Table 3). All 25 patients received at least one additional specific [prostanoid (n=18) or PDE-5 inhibitor (n=18)] or nonspecific [warfarin (n=18), oxygen (n=14), diuretics (n=12), calcium channel blocker (n=5), dipyridamole (n=3), L-arginine (n=2)] PAH treatment or underwent a palliative atrial septostomy (n=1). Forty-three of the 86 patients (50%) discontinued bosentan before end of data collection due to: lack of adequate improvement based on parents’/patient’s or physician’s perception (n=19), PAH deterioration (n=9), increases in liver enzymes (n=5), other adverse events (n=5), other reasons (n=4), or transplantation (n=1). Two of the 43 patients who discontinued bosentan before end of data collection died 11 and 34 months after bosentan discontinuation. Twenty-nine (67%) of the 43 patients who discontinued bosentan before end of data collection were treated with up to 3 subsequent or concomitant PAH-specific treatments including prostanoids (n=19), PDE-5 inhibitors (n=17), and endothelin receptor antagonists (n=12, including one patient in whom bosentan was reintroduced). Of the 86 patients, eleven (13%) died while on bosentan. Out of these, 5 patients were on bosentan monotherapy, and 6 patients were on combination therapy: 4 received prostanoids, 1 received sildenafil, and 1 received both a prostanoid and sildenafil in combination with bosentan. Seven of the 86 patients were lost to follow-up.
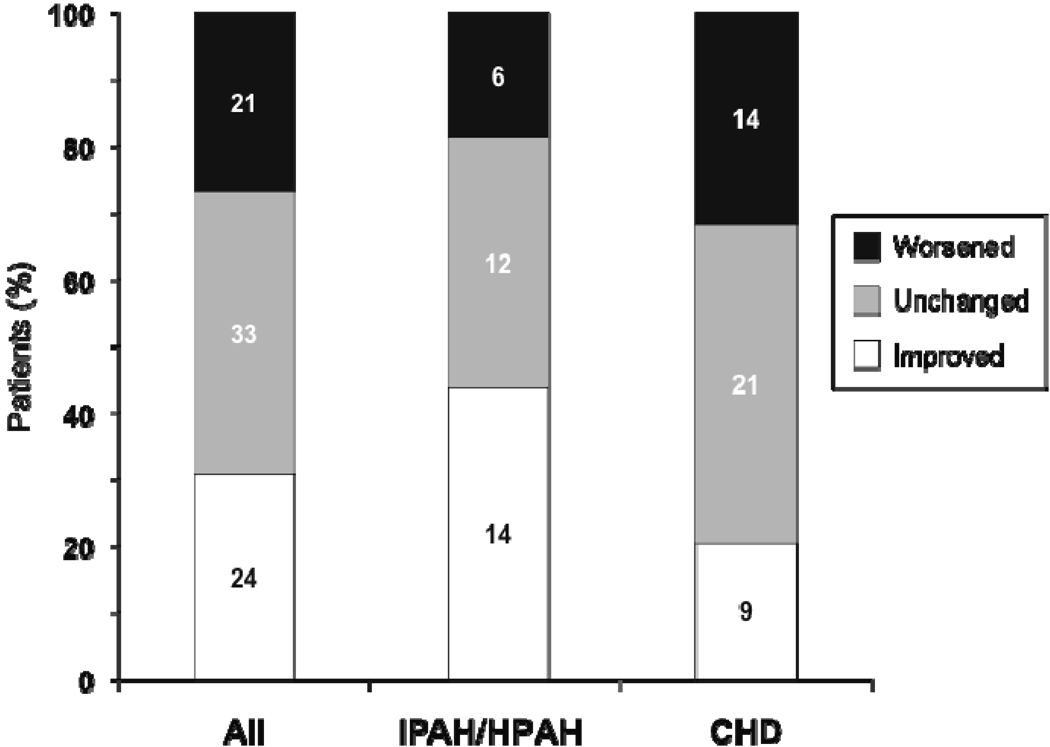
WHO functional class at last assessment for all patients (All), patients with IPAH/HPAH, and patients with PAH-CHD (CHD). Numbers on graph are numbers of patients in each subgroup. The all-patient group (n = 78) includes 2 patients with PAH associated with connective tissue disease. Patients who died were assigned WHO functional class IV.
Table 3
Clinical status at end of data collection
| All patients* (n = 86) | Patients with IPAH/HPAH (n = 36) | Patients with PAH-CHD (n = 48) | |||
|---|---|---|---|---|---|
| Treatment at bosentan initiation | Monotherapy (n = 11) | Plus prostanoid (n = 25) | Monotherapy (n = 30) | Plus prostanoid (n = 18) | |
| Continued bosentan | 25 (29%) | 5 (45%) | 7 (28%) | 6 (20%) | 5 (28%) |
| Discontinued | 43 (50%) | 3 (27%) | 15 (60%) | 17 (57%) | 8 (44%) |
| Increase in liver enzymes | 5 (6%) | 0 (0%) | 2 (8%) | 2 (7%) | 1 (6%) |
| Other adverse events | 5 (6%) | 0 (0%) | 2 (8%) | 3 (10%) | 0 (0%) |
| Transplantation | 1 (1%) | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) |
| Lack of adequate effect | 19 (22%) | 1 (9%) | 6 (24%) | 6 (20%) | 6 (33%) |
| PAH worsening** | 9 (10%) | 2 (18%) | 3 (12%) | 3 (10%) | 1 (6%) |
| Other reasons | 4 (5%) | 0 (0%) | 1 (4%) | 3 (10%) | 0 (0%) |
| Death while on bosentan# | 11 (13%) | 1 (9%) | 3 (12%) | 4 (13%) | 3 (17%) |
| Loss of follow-up | 7 (8%) | 2 (18%) | 0 (0%) | 3 (10%) | 2 (11%) |
Kaplan-Meier estimates of the composite endpoint ‘disease progression’ at 1, 2, 3 and 4 years are shown in Table 2. Kaplan-Meier estimates of survival at 1, 2, 3 and 4 years for patients while on bosentan therapy with or without other PAH-specific therapies were 98% (95% CL: 94, 100; patients remaining at risk: x=69), 88% (95% CL: 79, 96; x=42), 82% (95% CL: 72, 93; x=27) and 82% (95% CL: 72, 93; x=16), respectively. Survival estimates were comparable when considering overall survival (including survival data from patients after discontinuation of bosentan): 98% (95% CL: 94, 100; x=80), 90% (95% CL: 84, 97; x=70), 87% (95% CL: 80, 95; x=67) and 86% (95% CL: 79, 94; x=46), respectively. No differences were observed between the subgroups IPAH/HPAH and PAH-CHD (Figure 3).

Kaplan-Meier estimates of survival for the two etiology subgroups. Survival estimates at 1, 2, 3, 4 years for IPAH/HPAH patients were 100%, 88%, 88%, and 85%, and for PAH-CHD patients 96%, 91%, 87%, and 87%, respectively.
Of the 86 patients, 13 patients died. The univariate Cox regression analysis to predict survival was performed in the patients with information for the single risk factor considered (n between 62 and 67 for hemodynamic variables; n = 81 for WHO FC; n = 86 for the other risk factors). In the univariate analyses, risk factors significantly associated with survival were WHO FC, as reflected by a HR of 5.4 (95% CL: 1.2, 24.6; n=81 with 12 events), and PVRi greater than the mean baseline PVRi of 20 Wood units * m2, as reflected by a HR of 5.4 (95% CL: 1.1, 26.8; n=66). Six (25%) of 24 patients with a PVRi greater than 20 Wood units * m2 died, whereas 2 (4.8%) of the 42 patients with a PVRi equal to or less than 20 Wood units * m2 died. Kaplan-Meier estimates of survival for the two PVRi subgroups are provided in Figure 4. In the 62 patients for whom baseline CI was available, the mean baseline value of 3.7 L/min/m2 appeared to be associated with death since none out of the 31 patients with values higher than the mean died while 7 out of the 31 patients with values lower or equal to the mean died (p=0.0107 for Fisher's exact test). This association cannot be further explored using the Cox proportional-hazards regression analysis since the death risk in patients with CI above the mean is not known. In the multivariate analysis, no baseline risk factor was retained. In the subgroup of PAH-CHD patients, patients with a repaired defect were at higher risk vs unrepaired patients, as reflected by a HR of 0.2 (95% CL: 0.04, 1.12; n=48 with 7 events).

Kaplan-Meier estimates of survival for the two PVRi subgroups (relating to the mean baseline PVRi of 20 Wood units * m2). Survival estimates at 1, 2, 3, 4 years for patients with a PVRi greater than 20 Wood units * m2 were 96%, 83%, 78%, and 74%, and for patients with a PVRi lower than or equal to 20 Wood units * m2 100%, 94%, 94%, and 94%, respectively.
During the study, 8 patients (9%, person-time incidence rate: 4%) experienced worsening peripheral edema and 4 patients (5%, person-time incidence rate: 2%) had systemic hypotension. Increases in liver transaminases (above 3 times the upper limit of normal) were reported for 6 patients (7%, person-time incidence rate: 3%): 3 patients had an elevation 3–5 times the upper limit of normal and 3 patients had an elevation 5–8 times the upper limit of normal. These elevations in liver transaminases were asymptomatic but resulted in bosentan discontinuation in 4 patients. One additional patient discontinued due to an elevation in liver transaminases of 2–3 times the upper limit of normal. In six of the seven patients with elevated liver transaminases, values returned to normal. Thirteen patients died during the study (11 while on bosentan, 2 after discontinuation of bosentan); 6 IPAH/HPAH patients [right heart failure (n=1), sudden death (n=1), worsening pulmonary hypertension (n=1), hemoptysis (n=1), pulmonary hemorrhage (n=1), or thromboembolism (n=1)]; 5 repaired CHD patients [right heart failure or sudden death (n=4) or postoperative multi-organ failure (n=1)]; and 2 unrepaired or partially repaired CHD patients died from sepsis (one Eisenmenger syndrome).
Discussion
In this retrospective cohort study, we extended the observation period by 3 years in a previously identified cohort of 86 consecutive pediatric PAH patients treated with bosentan monotherapy or as add-on to pre-existing parenteral intravenous epoprostenol or subcutaneous treprostinil therapy (7). At the time these patients had bosentan initiated, bosentan was the only approved oral PAH therapy.
Historically, the prognosis for children with PAH appeared even worse than that for adults; the National Institutes of Health registry reported a median survival of 10 months for children with IPAH/HPAH compared with a median survival of 2.8 years for the entire cohort of IPAH/HPAH patients (data were collected 1981–1988) (14). Intravenous epoprostenol (approved in 1995) was available prior to bosentan (approved in 2001). In a previously published cohort of 77 pediatric IPAH/HPAH patients (diagnosed between 1982 and 1995 with follow-up through 2002) (15), 59 patients were considered appropriate to receive long-term intravenous epoprostenol therapy, however, 28 of the patients were unable to receive intravenous epoprostenol due to either unavailability of intravenous epoprostenol for long-term therapy (prior to FDA approval in 1995; n=21) or parental refusal (n=7). The 1-, 2- and 3-year survival estimates in the 31 epoprostenol-treated IPAH/HPAH patients were 100%, 94%, and 94%, respectively, compared to 50%, 43%, and 38%, respectively, in the 28 patients for whom intravenous epoprostenol was considered to be clinically indicated but was not initiated. Recently, a retrospective study of 216 children with IPAH/HPAH or PAH associated with other conditions (APAH), treated with PAH-disease specific therapies between April 2001 and March 2006, reported 1, 3 and 5 year survival rates of 86%, 80% and 72% for IPAH/HPAH, and 92%, 84% and 57%, respectively, for APAH. The authors concluded that the availability of the newer PAH-specific treatments had improved survival outcomes in children as they had in adults (16).
The children in our retrospective cohort study, who initiated bosentan with or without pre-existing prostanoids, had similar survival estimates at 1, 2, 3 and 4 years, i.e., 98%, 90%, 87% and 86%, respectively, with no differences noted between the IPAH/HPAH cohort and the overall PAH-CHD (repaired and unrepaired defects). Age at diagnosis has previously been reported to be a survival parameter in children with IPAH/HPAH (15). For our current study, the univariate Cox hazard regression analysis suggests that patients in WHO FC III/IV at bosentan initiation have increased mortality compared with patients in WHO FC I/II at bosentan initiation, and patients with a PVRi higher than 20 Wood units * m2 at bosentan initiation have increased mortality compared with patients with a PVRi lower than 20 Wood units times m2 at bosentan initiation. A normal or high CI at bosentan initiation also seems to be predictive of long-term survival. In this current study, where half of the children were WHO FC I or II at bosentan initiation, WHO FC improved in 31% of patients and remained unchanged in 42% of patients. A previous review also suggests that WHO FC for most children treated with bosentan either improves or remains unchanged (8).
The children enrolled in this current study were treated according to investigators’ judgment, current treatment recommendations and drug availability (1). Patients received additional PAH-specific treatments as new therapies continued to become available during the observation period of this study. Combination treatment has become more frequent in clinical practice (17,18–22) and is often considered an attractive therapeutic option to address the postulated multiple pathobiologic mechanisms involved in PAH (23). In the current study, the addition of PAH-specific therapies for the reason of ‘further improvement desired’ may have contributed to the observed improvement in clinical status in many of the patients as well as the overall favorable survival. The mean dose of parenteral prostanoids for the 44 children on pre-existing prostanoids at the time of bosentan initiation decreased following bosentan initiation, whereas dose escalation is not infrequent with prostanoid monotherapy, especially during the first one to two years of initiating continuous parenteral prostanoid therapy (24). Furthermore, although not analyzed in this study, the potential for less associated side effects related to a decrease in prostanoid dose may improve overall quality of life, however, the goal of combination therapy in PAH should be to address inadequate disease improvements, and not to minimize side effects.
The retrospective design of this study permits descriptive analyses of long-term outcomes in a rare pediatric disease. Patients were included based on the initiation of bosentan during a restricted time period in each of the two tertiary referral centers. Thus, these study results should not be generalized to all pediatric PAH patients. Furthermore, the population described here might be affected by a survival bias due to a notable time between diagnosis and bosentan initiation. For patients who discontinued bosentan before the end of data collection, only treatment and survival status were collected. Despite the challenges associated with longitudinal follow-up in rare pediatric diseases, this study demonstrates the potential utility of long-term data acquisition with current disease management strategies in pediatric PAH.
In this real-world clinical setting, pediatric PAH patients managed with current treatment regimens, including bosentan, show favorable survival. However, even with current therapy options, disease progression remains a concern. In order to evaluate the impact of early therapeutic interventions and early combination therapies on disease progression and survival, further investigation is warranted in the pediatric PAH population. Recognizing the challenges inherent in pediatric PAH clinical trial research, data from open label uncontrolled observational studies could support the development of data driven treatment guidelines.
Acknowledgments
The authors thank Sylvie Ertel for medical writing assistance funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Grant support: This research was supported by grant number M01 RR00069, General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health, and by Actelion Pharmaceuticals Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Ivy has served on advisory boards for Actelion, Gilead, Pfizer, and United Therapeutics, and receives grant support from Gilead. Dr. Berman Rosenzweig receives grant support from Actelion, Eli Lilly, Pfizer, Gilead and United Therapeutics and is a consultant for Actelion and United Therapeutics. Mr. Lemarié is a consultant for Actelion. Mrs. Brand and Dr. Rosenberg are employees of Actelion. Dr. Barst is a consultant for Actelion, Eli Lilly, Gilead, GSK, Novartis, and Pfizer.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.amjcard.2010.06.064
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2966874?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.amjcard.2010.06.064
Article citations
Embracing the challenges of neonatal and paediatric pulmonary hypertension.
Eur Respir J, 64(4):2401345, 31 Oct 2024
Cited by: 2 articles | PMID: 39209483 | PMCID: PMC11525338
Review Free full text in Europe PMC
Actigraphy methodology in the Kids Mod PAH trial: Physical activity as a functional endpoint in pediatric clinical trials.
Pulm Circ, 14(1):e12339, 01 Jan 2024
Cited by: 2 articles | PMID: 38464344 | PMCID: PMC10923039
Long-term outcome of children with newly diagnosed pulmonary arterial hypertension: results from the global TOPP registry.
Eur Heart J Qual Care Clin Outcomes, 10(1):66-76, 01 Jan 2024
Cited by: 5 articles | PMID: 36972621 | PMCID: PMC10785586
Monitoring of Hemodynamics With Right Heart Catheterization in Children With Pulmonary Arterial Hypertension.
J Am Heart Assoc, 12(7):e029085, 28 Mar 2023
Cited by: 3 articles | PMID: 36974756 | PMCID: PMC10122902
Pharmacotherapy for Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia: Past, Present, and Future.
Pharmaceuticals (Basel), 16(4):503, 28 Mar 2023
Cited by: 6 articles | PMID: 37111262 | PMCID: PMC10141152
Review Free full text in Europe PMC
Go to all (72) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of long-term bosentan in children with pulmonary arterial hypertension.
J Am Coll Cardiol, 46(4):697-704, 01 Aug 2005
Cited by: 126 articles | PMID: 16098438
Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open-label extensions.
Ann Rheum Dis, 65(10):1336-1340, 22 Jun 2006
Cited by: 126 articles | PMID: 16793845 | PMCID: PMC1798307
Long-term efficacy of bosentan in treatment of pulmonary arterial hypertension in children.
Eur Respir J, 38(1):70-77, 22 Dec 2010
Cited by: 44 articles | PMID: 21177841
Endothelin receptor antagonist therapy in congenital heart disease with shunt-associated pulmonary arterial hypertension: a qualitative systematic review.
Can J Cardiol, 25(3):e63-8, 01 Mar 2009
Cited by: 5 articles | PMID: 19279988 | PMCID: PMC2691700
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: M01 RR00069
Grant ID: M01 RR000069
NHLBI NIH HHS (1)
Grant ID: P50 HL084923