Abstract
Free full text

DOXORUBICIN-INDUCED CENTRAL NERVOUS SYSTEM TOXICITY AND PROTECTION BY XANTHONE DERIVATIVE OF GARCINIA MANGOSTANA
Abstract
Doxorubicin (Dox) is a potent, broad-spectrum chemotherapeutic drug used around the world. Despite its effectiveness, it has a wide range of toxic side effects, many of which most likely result from its inherent pro-oxidant activity. It has been reported that Dox has toxic effects on normal tissues, including brain tissue. The present study tested the protective effect of a xanthone derivative of Garcinia Mangostana against Dox-induced neuronal toxicity. Xanthone can prevent Dox from causing mononuclear cells to increase the level of tumor necrosis factor-alpha (TNFα). We show that xanthone given to mice before Dox administration suppresses protein carbonyl, nitrotyrosine and 4-hydroxy-2′-nonenal (4HNE)-adducted proteins in brain tissue. The levels of the pro-apoptotic proteins p53 and Bax and the anti-apoptotic protein Bcl-xL were significantly increased in Dox-treated mice compared with the control group. Consistent with the increase of apoptotic markers, the levels of caspase-3 activity and TUNEL-positive cells were also increased in Dox-treated mice. Pretreatment with xanthone suppressed Dox-induced increases in all indicators of injury tested. Together, the results suggest that xanthone prevents Dox-induced central nervous system toxicity, at least in part, by suppression of Dox-mediated increases in circulating TNFα. Thus, xanthone is a good candidate for prevention of systemic effects resulting from reactive oxygen generating anticancer therapeutics.
Doxorubicin (Dox), an antibiotic produced by the fungus Streptomyces peuctius, is a potent anticancer drug commonly used in the treatment of a variety of cancers including breast cancer (Hitchcock-Bryan et al., 1986). However, its clinical effectiveness is limited by its toxic effect on normal tissues (Singal et al., 2000; Oteki et al., 2005) including cumulative, dose-related cardiomyopathy (Singal and Iliskovic, 1998). Recent studies of breast cancer survivors have consistently shown similar changes in their cognitive function following chemotherapy, including memory loss, a tendency for lack of focus, and difficulty in performing simultaneous multiple tasks (Schagen et al., 1999; Brezden et al., 2000). These cognitive problems, collectively called somnolence or cognitive dysfunction, are also reported in cancer patients, especially breast cancer patients, undergoing Dox-based chemotherapy (Schagen et al., 2001; Freeman and Broshek, 2002).
We have previously reported a significant increase in levels of protein oxidation and lipid peroxidation in brains isolated from Dox-treated mice (Joshi et al., 2005). We found that Dox induces circulating tumor necrosis factor-alpha (TNFα) which activates glial cells, leading to increases of TNF in the cortex and hippocampus (the memory function part of the brain). TNF induces mitochondrial dysfunction by its downstream consequences, resulting in further increases in oxidative stress, cytochrome c release, caspase 3 activity, and TUNEL-positive cell death, all of which are suggestive of apoptosis in brain following systemic Dox treatment. The levels of the known pro-apoptotic proteins p53 and Bax and the anti-apoptotic protein Bcl-xL were increased in brain mitochondria following Dox treatment. Blocking circulating TNF with anti-TNF antibody resulted in a significant decrease in TNF alpha levels and mitochondrial dysfunction in brain tissues (Tangpong et al., 2006).
Xanthones, a class of polyphenolic compounds, are biologically active antioxidant phytonutrients that neutralize free radicals and promote healthy cardiovascular, gastrointestinal, respiratory, immune and nervous systems (Jiang et al., 2003; Kondo et al., 2009; Marquez-Valadez et al., 2009; Pedraza-Chaverri et al., 2009). It has been shown that xanthones and xanthone derivatives have antioxidative and cardioprotective effects against isoproterenol-induced myocardial infarction (Devi Sampath and Vijayaraghavan, 2007; Kondo et al., 2009). Alpha-mangostin, an aprenylated xanthone derivative of mangosteen, has been shown to possess diverse pharmacological characteristics, including antioxidant and anti-inflammatory properties (Chen et al., 2008; Pedraza-Chaverri et al., 2008; Kondo et al., 2009). Xanthones have also been found to have the potential to combat Alzheimer’s disease (Urbain et al., 2004).
The present study was undertaken to determine whether chemotherapy-induced neuronal injury can be prevented by xanthone supplementation. The neuroprotective effects of xanthone were investigated in mice by examining the level of TNFα, oxidative damage markers, and apoptotic markers in Dox based chemotherapy.
EXPERIMENTAL PROCEDURES
Animals
Eight-weeks-old male B6C3 mice (25–30 g) were divided into five groups of six mice each and maintained on a 12 h light/dark cycle in a temperature-controlled (23±2 °C) room. The mice had free access to food and water. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Experimental design
Mice were treated with a single dose of 20 mg/kg of Doxorubicin-Adriamycin (Dox) (DOXOrubicin HCl, Bedford Laboratories, Inc., Bedford, OH, USA) via i.p. injection. Xanthone (200 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 2% Gum acacia (Sigma-Aldrich, St. Louis, MO, USA) and administered as i.p. injection 15 min prior to the Dox treatment. Mice were euthanized 72 h after treatment. The experiments were conducted with five independent groups (n=6 per group) as follows:
Group 1: Control (Saline)
Group 2: Dox (20 mg/kg)
Group 3: Vehicle group (2% Gum acacia)
Group 4: Xanthone (200 mg/kg)
Group 5: Xanthone (200 mg/kg) + Dox (20 mg/kg)
Sample collection
Animals were anesthetized using Nembutal\sodium solution (65 mg/kg) (Abbott Laboratories, North Chicago, IL, USA) and blood was obtained via left ventricle puncture. Serum was collected for the macrophage stimulation experiments. Brain tissues were dissected from animals in all treatment groups and placed in ice-cold lysing buffer containing 4 g/ml leupeptin, 4 g/ml pepstatin, 5 g/ml aprotinin, 2 mM ethylenediaminetetraacetic acid (EDTA), 2 mM ethylene glycol-bistetraacetic acid (EGTA), and 10 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES), pH 7.4. The brain tissues were homogenized by 20 passes of a Wheaton tissue homogenizer, and the resulting homogenate was centrifuged at 20,000 g for 10 min and then assayed for protein concentration by the Pierce BCA method (Bradford, 1976).
Macrophage stimulation and cytokine assay
For experiments with macrophages, the mouse macrophage cell line J774A.1 (American Type Culture Collection) was cultured in DMEM supplemented with 10% (vol/vol) FBS, streptomycin (100 µg/ml) and penicillin (100 U/ml). All cultures were incubated at 37 °C in a humidified atmosphere with 5% CO2. The cells were seeded onto a 48-well plate at 5 × 105 cells/well and allowed to grow overnight. Serum collected from the respective groups was inactivated at 56 °C for 30 min and filtered through 0.22 µm membrane and added to the cell cultures. After 24 h, incubation media were collected and TNFα levels were measured with an enzyme-linked immunosorbent assay (mouse TNF-alpha, R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions. The TNFα concentration in the sample was calculated from a recombinant mouse TNFα standard curve. The cells were also processed and measured for protein carbonyls 3-NT (nitrotyrosine) and 4-HNE (hydroxynonenal), as described below.
Measurement of protein carbonyls
Each sample (5 µl) of brain homogenate and macrophage cell lysate was mixed with 12% sodium dodecyl sulfate (SDS; 5 µl) and 10 µl 1:10 diluted 2,4-dinitrophenylhydrazine (DNPH) from 200 mM stock and incubated at room temperature for 20 min, followed by neutralization with 7.5 µl neutralization solution (2 M Tris in 30% glycerol). Protein (250 ng) was loaded into each well on a nitrocellulose membrane using a slot blot apparatus connected to a vacuum source. The membrane was blocked in blocking buffer (3% bovine serum albumin in PBS containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20) for 1 h and incubated with a 1:100 dilution of anti-DNP polyclonal antibody in PBS containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20 for 1 h. After incubation with the primary antibody, the membrane was washed three times in PBS at 5-min intervals then incubated for 1 h with an anti-rabbit IgG alkaline phosphatase secondary antibody diluted in PBS in a 1:8,000 ratio. The membrane was washed three times in PBS for 5 min and developed in substrate solution prepared with Sigma fast tablets (5-bromo-4-chloro-3-indolyl phosphate [BCIP]/nitro blue tetrazolium [NBT] substrate). The membranes were dried, scanned with Adobe Photoshop software, and quantified in Scion Image program (PC version of Macintosh-compatible NIH Image).
Measurement of 4-HNE and 3-nitrotyrosine (3-NT)
Each sample (5 µl) of brain homogenate and macrophage cell lysate was mixed with 12% SDS (5 µl) and 5 µl modified Laemmli buffer containing 0.125 M Tris base, pH 6.8, 4% (v/v) SDS, and 20% (v/v) glycerol, incubated for 20 min at room temperature and loaded (250 ng) into each well on a nitrocellulose membrane in a slot blot apparatus connected to a vacuum source. The membrane was treated as described above except that 1-h primary antibody incubation was replaced with a 90 min incubation with a 1:5,000 dilution of anti-HNE polyclonal antibody in PBS.
Each sample (5 µl) of brain homogenate was also mixed with 12% SDS (5 µl), and modified Laemmli buffer (5 µl) containing 0.125 M Tris base, pH 6.8, 4% (v/v) SDS, and 20% (v/v) glycerol, incubated for 20 min at room temperature and loaded (250 ng) into each well on a nitrocellulose membrane in a slot blot apparatus under vacuum. The membrane was treated as described above except that a 1:2,000 dilution of anti-3-nitrotyrosine polyclonal antibody was used for the primary antibody incubation.
Western blot analysis
Perfused brain homogenates were separated using 12.5% denaturing polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto a nitrocellulose membrane. The membrane was blocked for 1 h at room temperature in blocking solution consisting of 5% nonfat dried milk, 0.5% Tween-20, and Tris-buffered saline (TBST), pH 7.9. After blocking, the membrane was incubated overnight at 4 °C with primary antibodies against TNF (Upstate, Lake Placid, NY, USA), inducible nitric oxide synthase (iNOS), Bax, Bcl-xL (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p53 (Cell signaling, Danvers, MA, USA) and β-actin (Clone AC-74, A5316, Sigma, St. Louis, MO, USA). The membrane was washed twice in TBST and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies in blocking solution. After incubation with secondary antibodies, the membrane was washed twice with TBST and once in TBST without 0.5% Tween-20. Immunoreactivities of the protein bands were detected by enhanced chemiluminescence autoradiography (ECL, Amersham Pharmacia Biotech, Arlington Heights, IL, USA) as described by the manufacturer.
Brain nitric oxide levels
Nitric oxide (NO) production was measured based on the Griess reaction (Fox et al., 1982). A final volume of 200 µl, including 40 µl of sample, 40 µl of nitrate/nitrite assay buffer, and 10 µl each of nitrate reductase cofactor and nitrate reductase enzyme preparation, was incubated covered, at room temperature, for 3 h. After the addition of 50 µl of Griess reagents, the samples were kept at room temperature for 10 min and formation of a deep purple azo compound was detected using a spectrophotometer at 540 nm. All reagents were obtained from Cayman Chemical (Ann Arbor, MI, USA).
Caspase 3 activity assay
Caspase 3 activity assay was performed with the use of a colorimetric substrate in accordance with the protocol supplied by the manufacturer (Sigma, St. Louis, MO, USA). In brief, mice were anesthetized and perfused with 1× PBS to reduce any enzyme activity associated with intravascular blood components. Brain was dissected and homogenized in lysis buffer containing 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4, 5 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 5 mM Dithiothreitol (DTT) plus 1 µg/ml aprotinin and pepstatin. The brain homogenates were incubated on ice for 15 min and centrifuged at 16,000×g for 10 min. The protein concentration was determined by the Bradford method and the caspase 3 activity in the supernatant was measured immediately. 50 µg protein samples in 10 µl were added to 980 µl assay buffer. The reaction was initiated by adding 10 µl of 20 mM of the caspase 3 substrate Ac-DEVD-pNA. The tubes were covered and incubated at 37 °C overnight. Cleavage of the chromophore from the substrate was detected spectrophotometrically at a wave-length of 405 nm.
TUNEL assay
The assay was performed following the manufacturer’s instructions (Promega, Madison, WI, USA). Briefly, the cryosections of brain were fixed with 4% paraformaldehyde, permeabilized with Triton X-100, and incubated with biotinylated nucleotide and recombinant termination deoxynucleotidyltransferase (rTdT) for 1 h at 37 °C. The fragmented DNA labeled at the ends was coated with horseradish peroxidase-labeled streptavidin (streptavidin HRP) and detected as dark brown condensed nuclei, a positive indication of cell death. The sections were counterstained with Methyl-Green by incubating 5 min in Methyl Green staining dye followed by repeated rinsing in distilled water and subsequent quick dehydration using 95% alcohol (10 dips) and two changes of 100% alcohol (10 dips each). The sections were rinsed finally in xylene and mounted with mounting medium. Positive control samples were prepared by incubating sections with DNase I prior to treatment with terminal transferase. Negative controls consisted of specimens in which deoxynucleotidyltransferase were omitted.
Statistical analysis
Statistical analyses were performed using one-way ANOVA followed by Newman–Keuls post-test (GraphPad Prism-4). A P-value of less than 0.05 was considered a significant difference.
RESULTS
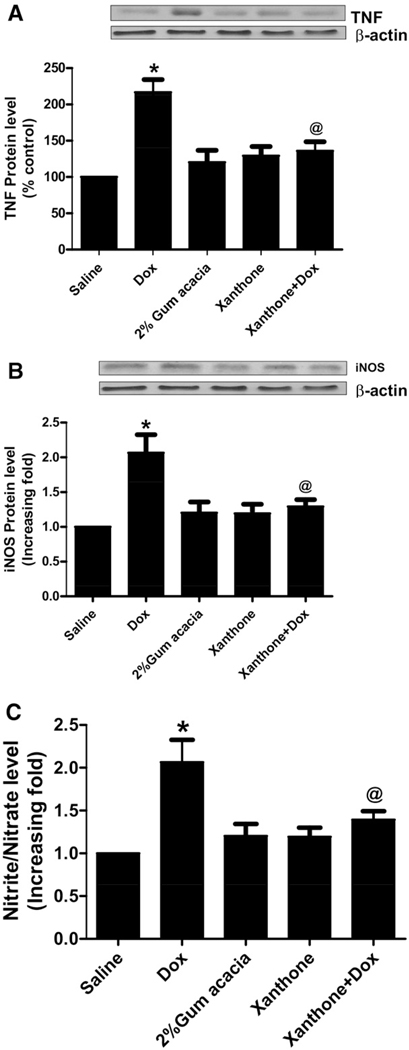
Dox-induced increase in TNF levels, iNOS protein levels and NO production is inhibited by xanthone
We previously have shown that Dox administration in mice increases the levels of the proinflammatory cytokine TNF α (Tangpong et al., 2006). As shown in Fig. 1a, TNF α levels were significantly increased in Dox-treated mice when compared to saline-treated controls. However, xanthone pretreatment for 15 min before Dox administration significantly decreased TNF α level when compared to Dox-only treatment (Fig. 1a, P<0.01). The increase in iNOS protein levels correlated with increased production of NO in brain tissues of Dox-treated mice. Xanthone ameliorated Dox-induced iNOS protein levels and NO production in the group treated with xanthone followed by treatment with Dox when compared to the group treated with Dox alone (Fig. 1b, c, P<0.01).
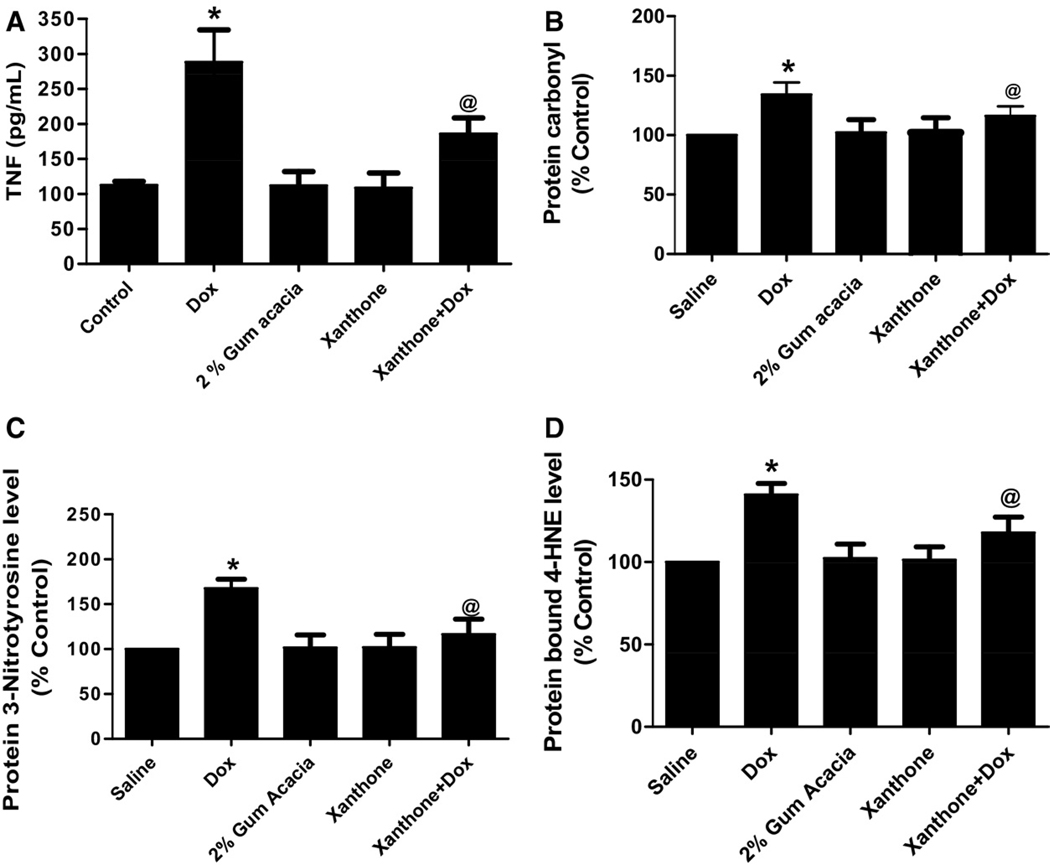
Xanthone reduces Dox-mediated increase in the levels of protein carbonyl, 3-nitrotyrosine and protein bound 4-HNE in brain tissues
Fig. 2 shows the levels of protein carbonyls (markers of protein oxidation), 3-NT (a marker of protein nitration) and protein-bound HNE (a lipid peroxidation product), respectively. In the Dox-treated group, levels of the above markers in brain tissues were significantly elevated as compared with the levels from saline- or xanthone-treated mice (P<0.01), confirming our previous findings (Joshi et al., 2005). Administration of xanthone significantly reduced the levels of protein carbonyls, 3NT, and protein-bound HNE (P<0.01) in brain isolated from mice injected with xanthone prior to the Dox treatment.
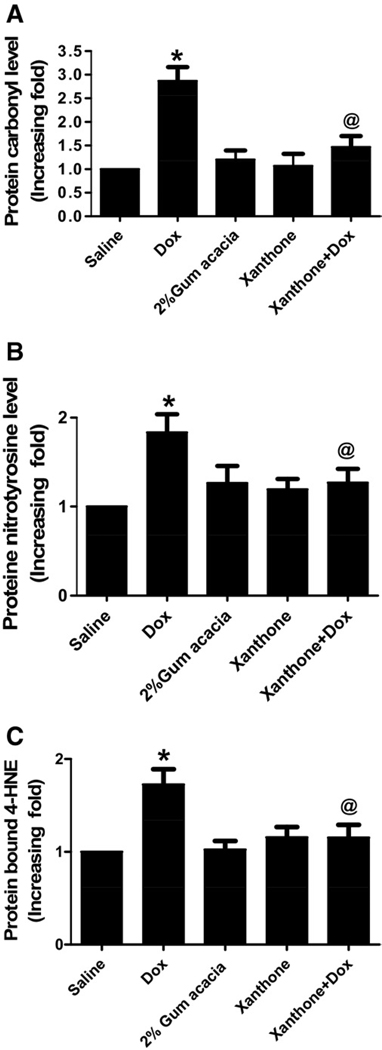
Oxidative stress analysis of brain homogenates showed significant increase in protein carbonyl (a), 3-NT (b) and 4HNE (c) 72 h after a single dose of 20 mg/kg Dox compared with control saline group (* P<0.01) which was significantly decreased by xanthone treatment prior to Dox administration (@ P<0.01). Analysis represents the mean±SEM (n=6) in each group.
Xanthone inhibits DOX-mediated changes in pro- and anti-apoptotic proteins
Increase in oxidative stress leads to increase in the levels of pro-apoptotic protein, p53, Bax and anti-apoptotic protein, Bcl-xL, as detected by Western blot analysis (Fig. 3a–c). The increase of pro- and anti-apoptotic protein were significantly (P<0.01) inhibited by xanthone treatment prior to the Dox administration.
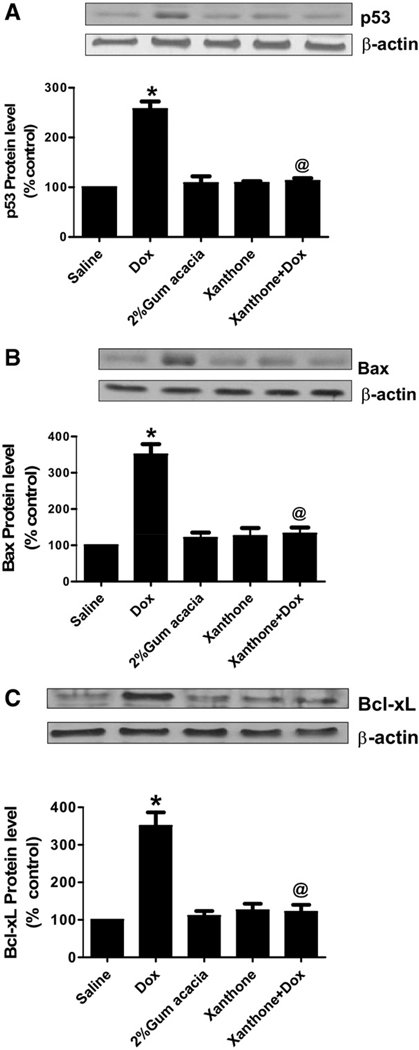
Xanthone inhibits Dox-induced apoptotic proteins. Pro-apoptotic proteins, p53 (a) and Bax (b), and anti-apoptotic protein, Bcl-xL (c), significantly increased in mice 72 h after treatment with a single dose of 20 mg/kg Dox as compared with saline control group (* P<0.01). The levels of p53, Bax and Bcl-xL were inhibited in mice treated with xanthone 15 min prior to Dox administration. Analysis represents the mean±SEM (n=6) in each group.
Xanthone reduces caspase 3 activity and TUNEL-positive apoptotic cells
Apoptotic cell death was represented by an increase in the activity of caspase 3 in Dox-treated mice when compared with the saline-treated mice (P<0.01). The apoptosis in brain was reduced by xanthone administration as revealed by a significant decrease in the elevated activity of caspase 3 following Dox treatment, as shown in Fig. 4a. Dox induces neuronal cell death via an apoptosis-like mechanism in brain tissues. Apoptotic cell death in brain was reduced when xanthone was administered prior to Dox, as revealed by a marked decrease in the elevated number of TUNEL-positive apoptotic cell deaths in comparison to the Dox-treated mice (Fig. 4b, c).
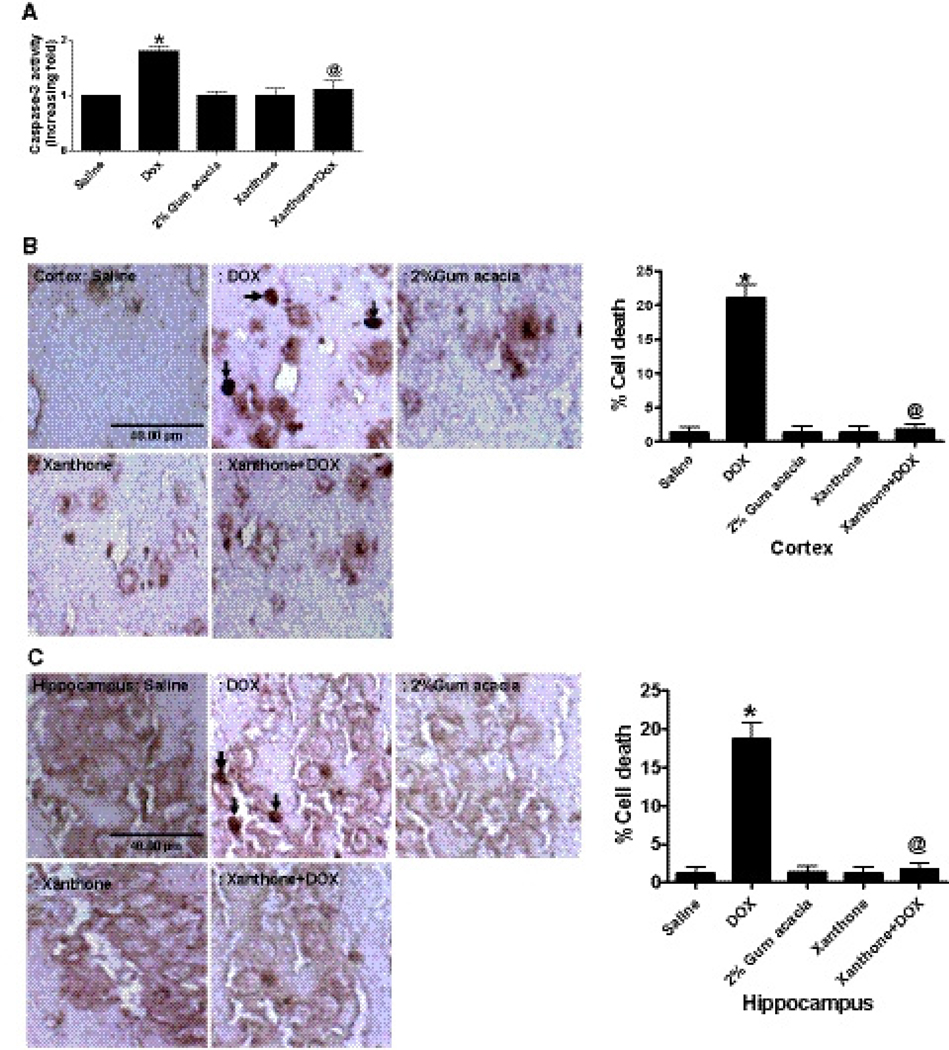
Xanthone inhibits Dox-induced increase in caspase 3 activity (a) and TUNEL-positive apoptotic cell death in cortex (b) and hippocampus (c). Caspase 3 activity was significantly increased in mice 72 h after treatment with a single dose of 20 mg/kg in Dox-treated mice as compared with the untreated mice (* P<0.01). The caspase 3 activity in brain was rescued by xanthone (200 mg/kg) intraperitoneal injection prior to Dox treatment as compared to the mice treated with Dox-only (@ P<0.01). Analysis represents the mean±SEM (n=6) in each group. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Xanthone inhibits Dox-mediated increases in TNFα, protein carbonyl, 3-nitrotyrosine and protein bound 4-HNE levels in macrophages
The mononuclear cell culture treated with serum isolated from Dox-treated mice showed increased levels of TNF, protein carbonyl, 3-NT, and protein bound 4-HNE (Fig. 5a–d [P<0.01]), all of which were significantly inhibited in the mononuclear cell culture treated with the serum isolated from the mice treated with xanthone prior to the Dox administration (P<0.01).
(a) TNF levels in macrophage cells showed a significant increase (* P<0.01) with Dox-treated serum. Cells incubated in serum collected from mice treated with xanthone (@ P<0.01) prior to Dox treatment showed a decrease in TNF production. A similar trend was observed in (b) protein carbonyls, (c) 3-NT and (d) 4-HNE levels. Analysis represents the mean±SEM, n=3 in different experiments.
DISCUSSION
The neurotoxic effects of anticancer drugs that do not cross the blood brain barrier (BBB) have recently received considerable attention. Our group reported the first investigation of potential biochemical pathways for this event, using Dox as a prototype cancer therapeutic (Tangpong et al., 2006). We have also shown that increased protein oxidation and lipid peroxidation in brain isolated from Dox-treated mice could be involved in the symptoms of chemobrain observed in patients treated with Dox (Joshi et al., 2005). Dox does not cross the BBB (Bigotte and Olsson, 1982) but systemic administration of Dox significantly elevates the level of circulating TNF, which does cross the BBB to reside in neurons and increase neuronal-resident TNFα level to cause brain mitochondrial dysfunction (Tangpong et al., 2006, 2007). An increase of oxidative stress in neuronal cells leads to activation of pro-apoptotic protein and migration of p53 to brain mitochondria, which interacts with anti-apoptotic Bcl-xL (Tangpong et al., 2007). The interaction of p53 with anti-apoptotic protein and enhancement of pro-apoptotic protein can cause mitochondrial pore opening and cytochrome c release from mitochondria. Our results coupled with previous reports from other laboratories show that the release of cytochrome c from mitochondria to cytoplasm activates the apoptotic pathway and leads to increased activity of caspase 3 and more TUNEL positive cells (Brookes et al., 2000; Tangpong et al., 2007; Dohare et al., 2008). Our previous studies also demonstrate that the observed increase in 3-NT adducted protein after Dox treatment is mediated, in part, by an increase in NO level. It has been shown that NO-induced apoptosis is related to an increase in the Bax/Bcl-xL ratio, release of cytochrome c and caspase activation (Kolb, 2000). Our current data confirm these previous findings and demonstrate that this pathway may be preventable by systemic administration of xanthone.
The cytotoxic activity of Dox is partly related to its quinone structure. The drug is converted to a semiquinone free radical by NADPH-cytochrome P-450 that is subsequently reoxidized and regenerated by oxygen, leading to the generation of superoxide anions (Goodman and Hochstein, 1977; Olson and Mushlin, 1990; Singal et al., 2000; De Beer et al., 2001). Increased superoxide anions may elevate the level of circulating TNF, which can directly pass through the BBB, as we have demonstrated in both the cerebral cortex and hippocampus, the memory and learning parts of the brain (Tangpong et al., 2007). Dox may exacerbate inflammatory processes, causing excessive release of TNF and resulting in cognitive disruption (Reichenberg et al., 2001). It is also possible that excessive inflammation activates microglia, causing further damage to neurons (Ahles et al., 2002).
The discovery that TNF mediates the toxicity of Dox in the brain suggests that an anti-TNF antibody could be a potential modality to quench oxidative stress in the brain, as recently demonstrated by the preliminary success of our study in a mouse model (Tangpong et al., 2007). Dox induces an imbalance between the generation of reactive oxygen species and reactive nitrogen species, ROS/RNS, and their cellular antioxidant system resulting in oxidative stress, which has been implicated in many neurodegenerative disorders, including Alzheimer’s disease (Butterfield and Lauderback, 2002).
The results of the present study suggest that xanthone, the compound in G. Mangostana, is an antioxidant that acts as a scavenger of free radicals or anti-inflammation agent, which reduces the levels of TNF and prevents increase of iNOS and NO production. Previously, we showed that Dox treatment of mice lacking iNOS does not lead to brain mitochondrial dysfunction (Tangpong et al., 2006). Thus, it is interesting that administration of xanthone prior to Dox treatment significantly prevents an increase in the TNF, iNOS, and NO as compared with mice treated with Dox-only.
The efficacy of xanthone may be related to the anthocyanins found in the proanthocyaniclins in grape seed, a dietary antioxidant supplement that has been shown to enhance the anti-tumor activity of Dox and ameliorate Dox-induced myocardial oxidative stress in tumor-bearing mice (Zhang et al., 2005). The water and 50% ethanol extract of xanthone from G. mangostana exhibit antioxidative and neuroprotective activities in NG-108-15 neuroblastoma cells against H2O2-induced cell damage (Moongkarndi et al., 2004; Chen et al., 2008) and inhibit the lipopolysaccharide-stimulated NO production that inhibits iNOS expression and cytotoxicity in RAW 264.7 cells (Chen et al., 2008). Xanthone also shows a protective effect against lipid peroxidation during isoproterenol-induced myocardial infarction in rats (Devi Sampath and Vijayaraghavan, 2007). These data suggest that xanthone may protect against oxidative stress inducing agents via both direct and indirect action. Our results demonstrate that xanthone suppresses Dox-induced increases in circulating TNFα level and suggest that xanthone can exert an antioxidant effect via reduction of TNFα level. Our finding that serum obtained from animals pretreated with xanthone was inefficient for activating TNFα production by machrophage is consistent with this possibility.
CONCLUSION
In conclusion, our experimental paradigm provides a reproducible model to study the mechanisms of brain dysfunction caused by chemotherapy and to test the potency of possible preventive agents. Our findings suggest that a xanthone derivative isolated from the traditional Thai medicine, magosteen, may be effective for preventing tissue injury resulting from ROS generating chemotherapeutic drugs.
Acknowledgments
This work is supported, in part, by NIH grant CA139843, Walailak University and The Higher Education Parliament, Ministry of Education, Thailand.
Abbreviations
| BBB | blood brain barrier |
| BSA | bovine serum albumin |
| Dox | Doxorubicin |
| HRP | horseradish peroxidase |
| iNOS | inducible nitric oxide synthase |
| NO | nitric oxide |
| PBS | phosphate buffer saline |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| rTdT | recombinant termination deoxynucleotidyltransferase |
| SDS | sodium dodecyl sulfate |
| TBS | Tris-buffered saline |
| TNF | tumor necrosis factor-alpha |
| 3-NT | nitrotyrosine |
| 4HNE | hydroxynonenal |
References
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. [Abstract] [Google Scholar]
- Bigotte L, Olsson Y. Retrograde transport of doxorubicin (adriamycin) in peripheral nerves of mice. Neurosci Lett. 1982;32:217–221. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [Abstract] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–2701. [Abstract] [Google Scholar]
- Brookes PS, Salinas EP, Darley-Usmar K, Eiserich JP, Freeman BA, Darley-Usmar VM, Anderson PG. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J Biol Chem. 2000;275:20474–20479. [Abstract] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. [Abstract] [Google Scholar]
- Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46:688–693. [Abstract] [Google Scholar]
- De Beer EL, Bottone AE, Voest EE. Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: a review. Eur J Pharmacol. 2001;415:1–11. [Abstract] [Google Scholar]
- Devi Sampath P, Vijayaraghavan K. Cardioprotective effect of alpha-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol. 2007;21:336–339. [Abstract] [Google Scholar]
- Dohare P, Garg P, Sharma U, Jagannathan NR, Ray M. Neuroprotective efficacy and therapeutic window of curcuma oil: in rat embolic stroke model. BMC Complement Alternat Med. 2008;8:55. [Europe PMC free article] [Abstract] [Google Scholar]
- Fox JB, Doerr RC, Lakritz L. Interaction between sample preparation techniques and three methods of nitrite determination. J Assoc Off Anal Chem. 1982;65:690–695. [Abstract] [Google Scholar]
- Freeman JR, Broshek DK. Assessing cognitive dysfunction in breast cancer: what are the tools? Clin Breast Cancer 3. 2002 Suppl 3:S91–S99. [Abstract] [Google Scholar]
- Goodman J, Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophys Res Commun. 1977;77:797–803. [Abstract] [Google Scholar]
- Hitchcock-Bryan S, Gelber R, Cassady JR, Sallan SE. The impact of induction anthracycline on long-term failure-free survival in childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1986;14:211–215. [Abstract] [Google Scholar]
- Jiang DJ, Hu GY, Jiang JL, Xiang HL, Deng HW, Li YJ. Relationship between protective effect of xanthone on endothelial cells and endogenous nitric oxide synthase inhibitors. Bioorg Med Chem. 2003;11:5171–5177. [Abstract] [Google Scholar]
- Joshi G, Sultana R, Tangpong J, Cole MP, St. Clair DK, Vore M, Estus S, Butterfield DA. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radic Res. 2005;39:1147–1154. [Abstract] [Google Scholar]
- Kolb JP. Mechanisms involved in the pro- and anti-apoptotic role of NO in human leukemia. Leukemia. 2000;14:1685–1694. [Abstract] [Google Scholar]
- Kondo M, Zhang L, Ji H, Kou Y, Ou B. Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. J Agric Food Chem. 2009;57:8788–8792. [Abstract] [Google Scholar]
- Marquez-Valadez B, Lugo-Huitron R, Valdivia-Cerda V, Miranda-Ramirez LR, Perez-De La Cruz V, Gonzalez-Cuahutencos O, Rivero-Cruz I, Mata R, Santamaria A, Pedraza-Chaverri J. The natural xanthone alpha-mangostin reduces oxidative damage in rat brain tissue. Nutr Neurosci. 2009;12:35–42. [Abstract] [Google Scholar]
- Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 2004;90:161–166. [Abstract] [Google Scholar]
- Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [Abstract] [Google Scholar]
- Oteki T, Nagase S, Yokoyama H, Ohya H, Akatsuka T, Tada M, Ueda A, Hirayama A, Koyama A. Evaluation of adriamycin nephropathy by an in vivo electron paramagnetic resonance. Biochem Biophys Res Commun. 2005;332:326–331. [Abstract] [Google Scholar]
- Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. [Abstract] [Google Scholar]
- Pedraza-Chaverri J, Reyes-Fermin LM, Nolasco-Amaya EG, Orozco-Ibarra M, Medina-Campos ON, Gonzalez-Cuahutencos O, Rivero-Cruz I, Mata R. ROS scavenging capacity and neuroprotective effect of alpha-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp Toxicol Pathol. 2009;61:491–501. [Abstract] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. [Abstract] [Google Scholar]
- Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FS. Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neuro Oncol. 2001;51:159–165. [Abstract] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. [Abstract] [Google Scholar]
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. [Abstract] [Google Scholar]
- Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. [Abstract] [Google Scholar]
- Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St. Clair W, Ratanachaiyavong S, St. Clair DK, Butterfield DA. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100:191–201. [Abstract] [Google Scholar]
- Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St. Clair W, Ratanachaiyavong S, St. Clair DK, Butterfield DA. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23:127–139. [Abstract] [Google Scholar]
- Urbain A, Marston A, Queiroz EF, Ndjoko K, Hostettmann K. Xanthones from Gentiana campestris as new acetylcholinesterase inhibitors. Planta Med. 2004;70:1011–1014. [Abstract] [Google Scholar]
- Zhang XY, Li WG, Wu YJ, Gao MT. Amelioration of doxorubicin-induced myocardial oxidative stress and immunosuppression by grape seed proanthocyanidins in tumour-bearing mice. J Pharm Pharmacol. 2005;57:1043–1052. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.neuroscience.2010.11.007
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3136166?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.neuroscience.2010.11.007
Article citations
Empagliflozin Dampens Doxorubicin-Induced Chemobrain in Rats: The Possible Involvement of Oxidative Stress and PI3K/Akt/mTOR/NF-κB/TNF-α Signaling Pathways.
Mol Neurobiol, 20 Sep 2024
Cited by: 0 articles | PMID: 39302617
Melatonin mitigates doxorubicin induced chemo brain in a rat model in a NRF2/p53-SIRT1 dependent pathway.
Heliyon, 10(19):e38081, 18 Sep 2024
Cited by: 0 articles | PMID: 39386846 | PMCID: PMC11462207
Noni enhances the anticancer activity of cyclophosphamide and suppresses myelotoxicity and hepatotoxicity in tumor-bearing mice.
J Cancer Res Clin Oncol, 150(4):212, 25 Apr 2024
Cited by: 0 articles | PMID: 38662247 | PMCID: PMC11045611
Nicotinic and Muscarinic Acetylcholine Receptor Agonists Counteract Cognitive Impairment in a Rat Model of Doxorubicin-Induced Chemobrain via Attenuation of Multiple Programmed Cell Death Pathways.
Mol Neurobiol, 61(11):8831-8850, 03 Apr 2024
Cited by: 0 articles | PMID: 38568417
Wnt3a/GSK3β/β-catenin Signalling Modulates Doxorubicin-associated Memory Deficits in Breast Cancer.
Mol Neurobiol, 61(8):5441-5458, 10 Jan 2024
Cited by: 0 articles | PMID: 38198045
Go to all (42) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment.
Food Chem Toxicol, 70:151-156, 30 Apr 2014
Cited by: 28 articles | PMID: 24795231
Mangostanaxanthone VII, a new cytotoxic xanthone from Garcinia mangostana.
Z Naturforsch C J Biosci, 73(5-6):185-189, 01 Apr 2018
Cited by: 8 articles | PMID: 29116938
Xanthone-enriched fraction of Garcinia mangostana and α-mangostin improve the spatial learning and memory of chronic cerebral hypoperfusion rats.
J Pharm Pharmacol, 72(11):1629-1644, 02 Aug 2020
Cited by: 6 articles | PMID: 32743849
Xanthones from mangosteen (Garcinia mangostana): multi-targeting pharmacological properties.
J Med Assoc Thai, 97 Suppl 2:S196-201, 01 Feb 2014
Cited by: 23 articles | PMID: 25518194
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: CA139843
Grant ID: R01 CA139843
Grant ID: R01 CA139843-03
National Institutes of Health (1)
Grant ID: CA139843