Abstract
Objectives
The radiobiological modelling of all types of protracted brachytherapy is susceptible to uncertainties in the values of tissue repair parameters. Although this effect has been explored for many aspects of pulsed brachytherapy (PB), it is usually considered within the constraint of a fixed brachytherapy treatment time. Here the impact of repair parameter uncertainty is assessed for PB treatments of variable duration. The potential use of "block-schemes" (blocks of PB pulses separated by night-time gaps) is also investigated.Methods
PB schedule constraints are based on the cervical cancer protocols of the Royal Marsden Hospital (RMH), but the methodology is applicable to any combination of starting schedule and treatment constraint. Calculations are performed using the biologically effective dose (BED) as a tissue-specific comparison metric. The ratio of normal tissue BED to tumour BED is considered for PB regimens with varying total pulse numbers and/or "block-schemes".Results
For matched brachytherapy duration, PB has a good "window of opportunity" relative to the existing RMH continuous low dose rate (CLDR) practice for all modelled repair half-times. The most clear-cut route to radiobiological optimisation of PB is via modest temporal extension of the PB regimen relative to the CLDR reference. This option may be practicable for those centres with scope to extend their relatively short CLDR treatment durations.Conclusion
Although daytime-only "block-scheme" PB for cervical cancer has not yet been employed clinically, the possibilities appear to be theoretically promising, providing the overall (external beam plus brachytherapy) treatment duration is not extended relative to current practice, such that additional tumour repopulation becomes a concern.Free full text

Pulsed brachytherapy: a modelled consideration of repair parameter uncertainties and their influence on treatment duration extension and daytime-only “block-schemes”
Abstract
Objectives
The radiobiological modelling of all types of protracted brachytherapy is susceptible to uncertainties in the values of tissue repair parameters. Although this effect has been explored for many aspects of pulsed brachytherapy (PB), it is usually considered within the constraint of a fixed brachytherapy treatment time. Here the impact of repair parameter uncertainty is assessed for PB treatments of variable duration. The potential use of “block-schemes” (blocks of PB pulses separated by night-time gaps) is also investigated.
Methods
PB schedule constraints are based on the cervical cancer protocols of the Royal Marsden Hospital (RMH), but the methodology is applicable to any combination of starting schedule and treatment constraint. Calculations are performed using the biologically effective dose (BED) as a tissue-specific comparison metric. The ratio of normal tissue BED to tumour BED is considered for PB regimens with varying total pulse numbers and/or “block-schemes”.
Results
For matched brachytherapy duration, PB has a good “window of opportunity” relative to the existing RMH continuous low dose rate (CLDR) practice for all modelled repair half-times. The most clear-cut route to radiobiological optimisation of PB is via modest temporal extension of the PB regimen relative to the CLDR reference. This option may be practicable for those centres with scope to extend their relatively short CLDR treatment durations.
Conclusion
Although daytime-only “block-scheme” PB for cervical cancer has not yet been employed clinically, the possibilities appear to be theoretically promising, providing the overall (external beam plus brachytherapy) treatment duration is not extended relative to current practice, such that additional tumour repopulation becomes a concern.
There have been a number of earlier theoretical analyses of pulsed brachytherapy (PB). At the start of the 1990s, various groups [1,2] predicted that the radiobiological effects of PB should be similar to those of CLDR, provided that the hourly dose, total dose and treatment duration remained unchanged and pulses were small (0.5–3 Gy) and frequent (hourly).
In subsequent work [3,4] it was suggested that, as radiobiological data for repair kinetics pointed to longer repair half-times for healthy tissue (1.5–4 h), PB schedules with longer interpulse intervals (of up to 3–4 h) should produce results that are radiobiologically similar, or even superior, to CLDR.
Visser et al [4] concluded that attempts to reduce the overall PB treatment time while maintaining equivalent tumour effect were very likely to result in increased biologically effective dose (BED) values for normal tissue. This is because normal tissue generally possesses a lower α/β ratio (has greater sparing due to fractionation) than tumour tissue, such that temporal compounding of sublethal radiation damage is likely to lead to a relative increase in normal tissue damage over tumour damage. Conversely, Visser et al [4] noted that the choice of a somewhat longer overall treatment time for the PB schedule could lead to reduced effects in normal tissues in comparison with the reference CLDR schedule.
In 1997, Fowler and Van Limbergen [5] explored the possibility that, because of high instantaneous dose rates, PB may exert an increased radiobiological effect on tissues with short half-times of repair (half-times of a few minutes) relative to CLDR.
It is clear that, at present, theoretical regimen optimisation for PB is limited by the uncertainties associated with repair times, particularly those of the healthy tissues. On the basis that few human values have been estimated from brachytherapy clinical data sets, (The Groupe Européen de Curiethérapie and the European Society for Therapeutic Radiology and Oncology) Brachytherapy Committee have GEC-ESTRO the suggested that: “as there are no better estimates at the moment, a value of T1/2 of 1.5 h is recommended for all tissues involved” [6].
At present T1/2 values remain contentious, especially as more than one repair component may be operative in some normal tissues. With specific relation to cervical carcinoma, Fowler [7], based on the clinical data reported by Newman [8], estimated that T1/2 for normal structures (the bladder and the rectum) most likely lies between 1.5 h and 2.5 h, assuming an α/β ratio of 2–4 Gy. Fowler said “[for healthy tissue a T1/2 of ] 1.5 h is a value that has been assumed for many years, with no precise justification except its failure to contradict clinical information. It does not seem to have been too misleading. However if T1/2 should be 2 or 2.5 h instead of 1.5 h it could make a significant difference when equal-effect calculations were made”. Analysis of morbidity for patients treated with the continuous hyperfractionated accelerated radiotherapy (CHART) regimen estimated long half-times of repair (2.5–4.9 h) for certain normal cell types (subcutaneous fibrosis and skin telangiectasia). In 2001, Orton [9] published an analysis that used these data to explain the potential superiority of fractionated high dose rate (FHDR) over CLDR in cervical carcinoma.
Based on their analysis of the Manchester trials in which different doses of 137Cs were compared with those used in earlier 226Ra brachytherapy implants, Roberts et al [10] found that a value of α/β=3 Gy implies a repair half-time of around 0.5 h. Pos et al [11] also suggested a short repair half-time of <1 h for late-responding normal bladder tissue, based on analysis of both clinical and mouse data. In 2006 Guerrero and Li [12] made a further attempt to resolve the inconsistency existing in the repair half-time for the bladder and the rectum. By reconciling clinical data from cervical brachytherapy for different dose rates, they estimated that the most likely value of the repair half-time for bladder and rectum was short, 0.2–0.4 h, assuming α/β=2–4 Gy. Thus, their analysis did not support the long repair half-times reported previously for the bladder, rectum and other normal structures.
The lack of consensus on this issue has an important implication for centres considering implementing PB: to replace an existing treatment, the alternative PB schedule must appear radiobiologically sound, irrespective of the parameter uncertainties. A number of studies have investigated the impact of parameter selection on the modelled therapeutic potential of PB relative to CLDR. Pop et al [13] highlighted the fact that extrapolations based on longer half-times of repair in CLDR schedules could lead to the calculation of dangerously high PB doses. Based on further analysis, Sminia et al [14] concluded that advantages in normal tissue sparing could be achieved if pulse frequencies were tailored to the repair kinetics of the normal tissue exposed. In this work the effect of repair parameter uncertainty is investigated with particular regard to the possibility of radiobiological optimisation via temporal extension of PB treatments. In each case, the therapeutic potential of PB relative to CLDR is considered for a broad range of T1/2 values (from 5 min to 6 h for both normal and cancerous tissue types).
Because of staffing/safety concerns, there are often temptations to avoid night-time therapy where possible. Protocols for daytime-only PB were first proposed by Brenner et al [15] in 1997. PB is currently being implemented on a daytime-only basis for the treatment of certain tumours (e.g. breast [16] and recurrent oesophageal cancer [17]), but not for cervical cancer. The possible use of daytime-only block-schemes for cervical cancer is investigated here. It is stressed that the term “block-scheme” is used throughout this paper to refer to blocks of pulsed brachytherapy separated by overnight gaps: the “block-overnight gap-block…” pattern is the important issue, rather than the fractionation pattern within the PB blocks. The latter is discussed in references 1–5.
Methods and materials
Calculations were performed assuming the PB BED equations derived by Dale et al [18] that have been used extensively by earlier investigators of PB scheduling:
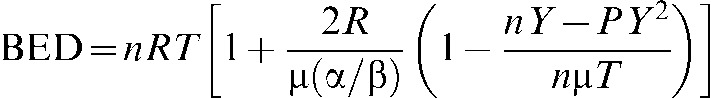
where n is the number of pulses; R is the dose rate within each pulse; T is the pulse duration; and α/β is specific to the tissue under consideration;
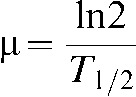
T1/2 is the half-time of repair of sublethal damage,
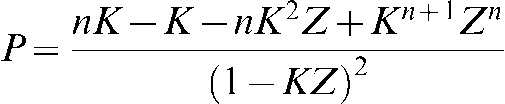
Z=e–μT, Y=1 – Z, K=e–μX and X is the radiation-free interval between pulses.
To establish a clinically feasible dose rate, the Royal Marsden Hospital (RMH) planning system was used to calculate the duration of a 1 Gy treatment pulse, delivered using a 2Ci 192Ir source and a standard treatment plan. This duration was then used to calculate the average dose rate within the pulse at the prescription point, which was found to be 10.27 Gy h−1. 10 Gy h−1 was thus adopted as the standard dose rate for this analysis.
In designing a PB regimen it is prudent to begin by limiting the prescribed dose according to a normal tissue 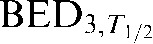 (subscripts are used throughout this work to represent the modelled α/β ratio and T1/2 value respectively (e.g. BEDα/β,T1/2)) constraint, drawn from previous CLDR practice. Since T1/2 values cannot yet be ascertained on a patient-by-patient basis, the dose prescription for all patients must be performed assuming a constant T1/2. In this case a normal tissue T1/2 of 1.5 h was assumed, in-line with the GEC-ESTRO recommendations [6].
(subscripts are used throughout this work to represent the modelled α/β ratio and T1/2 value respectively (e.g. BEDα/β,T1/2)) constraint, drawn from previous CLDR practice. Since T1/2 values cannot yet be ascertained on a patient-by-patient basis, the dose prescription for all patients must be performed assuming a constant T1/2. In this case a normal tissue T1/2 of 1.5 h was assumed, in-line with the GEC-ESTRO recommendations [6].
Using the CLDR BED equation (here Equation (4)) from Reference 19, BED calculations were performed for the RMH CLDR treatment schedule (Table 1), to establish the BED3,1.5 constraint of 64.2 Gy3,1.5. Equation (4) is preferred for this purpose since the duration of the reference CLDR schedule in this case is 15 h, a time that is not quite long enough to justify use of the simplified version of Equation (4), in which the inner-bracketed term is omitted.
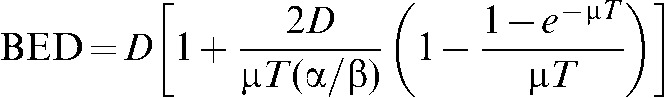
Table 1

Subscripts are used to indicate the α/β ratio used (3 Gy for normal tissue, 10 Gy for tumour tissue) followed by the half-time of repair (here taken to be 1.5 h for both normal tissue and tumour).
where D is the total dose, T is the total treatment time and μ is given by Equation (2).
For pulse numbers (n) in the range 10–72, the pulse dose required to meet the CLDR BED3,1.5 constraint of 64.2 Gy3,1.5 is then calculated and is plotted as a function of pulse number in Figure 1. As a standard protocol, the Figure 1 pulse doses, rather than randomly selected doses, are used as the physical dose input data for the model. For a range of different T1/2 values,  /
/ ratios are calculated for both the PB schedule under consideration and the RMH CLDR reference, such that the window of opportunity for successful use of the PB alternative can be identified.
ratios are calculated for both the PB schedule under consideration and the RMH CLDR reference, such that the window of opportunity for successful use of the PB alternative can be identified.
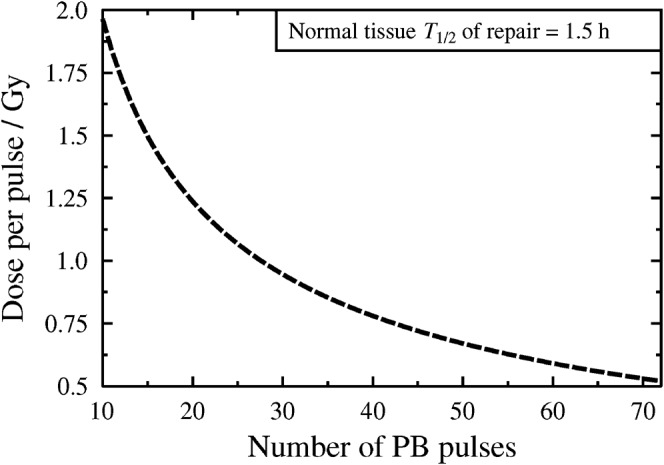
Plot to show the physical pulse doses prescribed under the CLDR BED3,1.5 constraint of 64.2 Gy3,1.5 for pulse numbers in the range 10–72. BED, biologically effective dose; PB, pulsed brachytherapy.
As already noted by Visser et al [4], the variable with the largest influence on the BED ratio is the overall duration of the PB. In this work the effect of PB duration extension is explored for various half-times of repair for both the normal tissue and the tumour. The interval between pulse starts is always maintained at 1 h (in line with other theoretical analyses [1,2], standard clinical practices and the GEC-ESTRO recommendations [6]). If accurate and precise T1/2 data were to be established in the future, then the interpulse interval could be utilised as an additional variable for radiobiological optimisation (it has already been shown that longer interpulse intervals are likely to benefit normal tissues with longer repair half-times [3,4,14]).
Block-scheme modelling
For modelling purposes it was initially assumed that a sufficient gap is left between fractions to allow complete sublethal damage repair for the full range of T1/2s. This allows BEDs to be added linearly for separate fractions. In reality this condition would require interfraction intervals of greater than 24 h for the longest assumed normal tissue T1/2 (6 h). In clinical contexts, however, it is likely that shorter interfraction intervals (of 15, 12 or even 6 h) would be preferable, such that night-time treatments could be avoided at little penalty in terms of overall treatment time extension. Thus, additional modelling was employed to consider the uncertainty in dose prescription that would accompany such a transition. It is clear that for scenarios with shorter interfraction intervals, the correct pulse dose prescription must lie between the two extremes: (a) X pulses, delivered hourly in a single fraction, and (b) X pulses delivered over two fractions, separated by an interfraction interval sufficient to allow complete sublethal damage repair. Thus, if the difference between pulse dose prescriptions derived from considerations (a) and (b) is smaller than the required precision of the pulse dose prescription for clinical delivery, then the conclusion is that block intervals smaller than the theoretically ideal value may be considered. This means that night-time gaps, of, for example, 6, 12 or 15 h duration may be implemented. If the difference is larger than the stipulated clinical precision then this approach should be used with caution since, in such cases, the required pulse dose is not uniquely identified by the method described.
Results
Figure 2 shows that if the GEC-ESTRO T1/2 values (1.5 h for both the normal tissue and the tumour [6]) are accepted, then the most clear-cut route to radiobiological improvement (reduction of the BED ratio plotted on the y-axis) is via temporal extension of the PB regimen. For PB regimen durations of at least 20 h, the resulting treatment is therapeutically superior to the existing CLDR case.
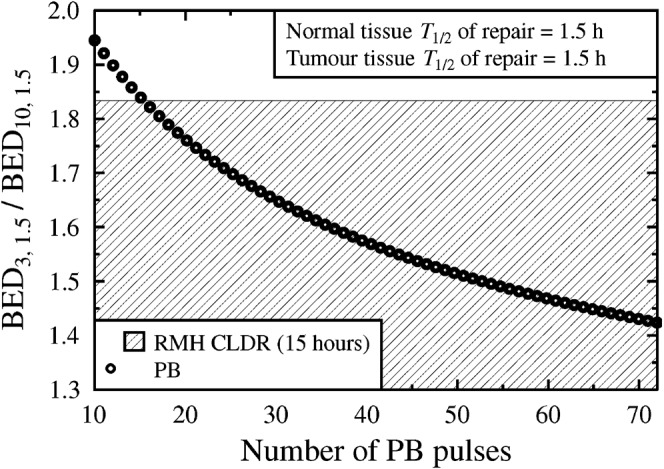
Variation of the biologically effective dose (BED) ratio with increasing pulse number (n=10:72). T1/2 values of 1.5 h are assumed for both normal tissue and tumour. Pulsed brachytherapy (PB) data points within the shaded region represent modelled improvement over the current CLDR practice for the same assumed T1/2 values.
Figures 3 and and44 show modelled BED ratios resulting from the same physical dose regimens as in Figure 2, but considering different values for the T1/2 parameters. (In all cases the selected T1/2 parameters are used to first calculate the BED ratio for the CLDR case and are then applied to the PB alternatives using the pulse sizes derived from Figure 1.)
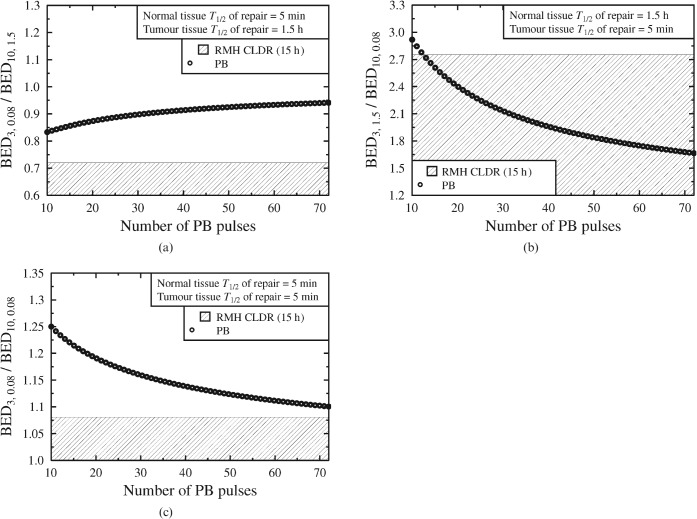
(a–c) Considering the possible effects of shorter T1/2 values, for both the normal tissue and the tumour. Schedules within the shaded region represent an improvement on the Royal Marsden Hospital (RMH) 15 h CLDR practice. BED, biologically effective dose; PB, pulsed brachytherapy.
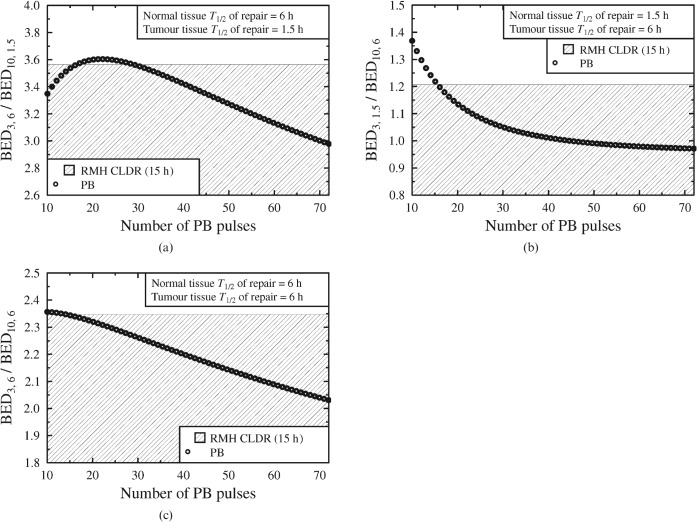
(a–c) Considering the possible effects of longer T1/2 values, for both the normal tissue and the tumour. Pulsed brachytherapy (PB) data points within the shaded regions represent an improvement on the Royal Marsden Hospital (RMH) 15 h CLDR practice. BED, biologically effective dose.
Figure 3a–c highlights the dependence of optimal brachytherapy schedule design on T1/2 values. In Figure 3a, when the normal tissue repair of half-time is very short relative to that of the tumour (5 min against 1.5 h), short treatment durations give the lowest BED ratio. However, if the normal tissue repairs with a half-time equal to or greater than the half-time of the tumour (e.g. Figure 3b,c), extended schedules will remain the most appropriate.
Figure 4 shows that if the T1/2 value is long (6 h) for the tumour, the normal tissue or both, then the model again predicts a trend of radiobiological improvement (over the CLDR reference) as soon as the PB treatment duration is extended beyond that of the CLDR reference.
Implications of block-scheme design
As discussed in the methods section, block-scheme modelling which assumes complete repair between fractions may be performed via linear addition of BED values for the separate fractions. However, in reality fractionation schemes which seek to reduce night-time staffing concerns may involve gaps as short as 6 h, i.e. gaps of insufficient duration to allow complete sublethal damage repair. The pulse dose difference between a regimen with a complete repair interfraction interval and a single-fraction regimen (as described in the methods and materials section) is plotted as a function of total pulse number in Figure 5.
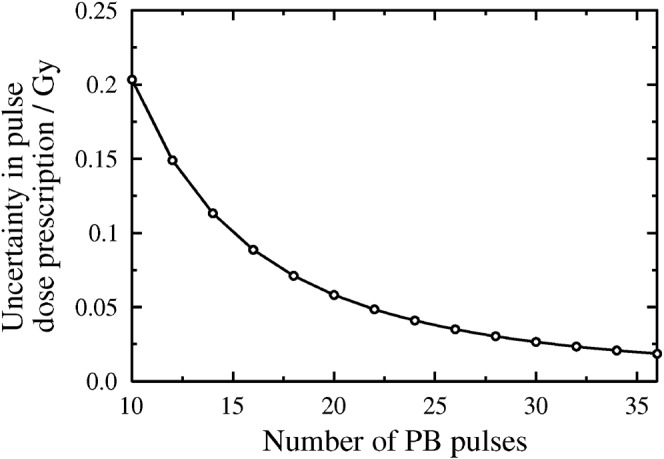
For given pulse numbers, a plot to show the difference between prescribed pulse doses (in Gy) for (a) single fraction delivery and (b) two fraction delivery assuming complete repair between the fractions (plotted as “Uncertainty in pulse dose prescription” on the y-axis). In each case the maximum dose per pulse has been calculated under the Royal Marsden Hospital BED3 constraint of 64.2 Gy3,1.5. PB, pulsed brachytherapy.
If the example of a total pulse number of 22 is considered, for single-block delivery the RMH dose-pulse prescription would be 1.15 Gy, whereas for two-block delivery (with each block containing 11 pulses and assuming complete repair between blocks) the RMH pulse-dose prescription would be 1.20 Gy. As shown in Figure 5 (the 22 pulse data point), the difference between these two prescriptions is 0.05 Gy or 4.3%: the uncertainty in pulse dose prescription can be reduced to ≤0.05 Gy (i.e. ≤5%) per pulse for total pulse numbers of 22 or more. That is, in the RMH case, for the higher total pulse numbers it should be possible to introduce night-time gaps of any duration without introducing an error of greater than 5% in the pulse dose precision.
For simplicity, all further mathematical modelling assumes complete repair between fractions, but the reader is reminded that shorter block intervals should not prove seriously problematic in terms of deriving a dose prescription provided that the total pulse number is kept high (from here on only total pulse numbers of 24+ are considered). Thus, in Figure 6 the block-scheme modelling results can be extrapolated to practical cases, e.g. an interval of around 12 h (as might occur in a “daytime-only” schedule) between 2 fractions of 12 hourly treatment pulses. Such a scheme would theoretically be better than a scheme with a single fraction of 24 hourly treatment pulses, but not as good as 36 (hourly) pulses with no gap (Figure 6). This finding was observed for all except the cases involving the longest tumour T1/2 investigated, and the shortest two healthy tissue T values (Figure 7).
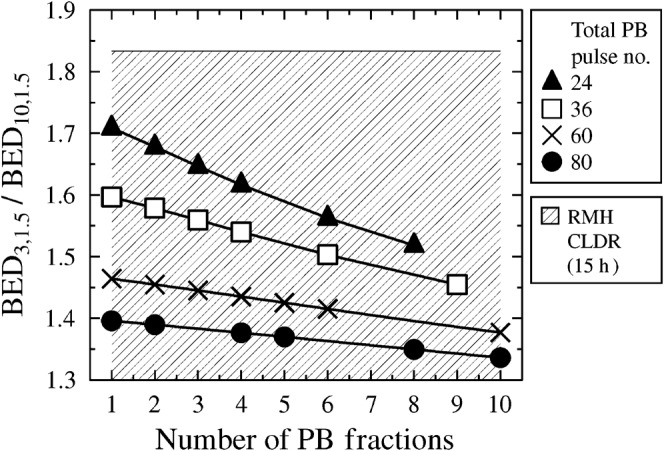
Exploring the relationship between the biologically effective dose (BED) ratio and the number of blocks over which the total pulse number is spread. For example, the points on the curve marked by black triangles represent 1 block of 24 pulses, 2 blocks of 12 pulses, 3 blocks of 8 pulses, 4 blocks of 6 pulses, 6 blocks of 4 pulses and 8 blocks of 3 pulses. PB, pulsed brachytherapy.
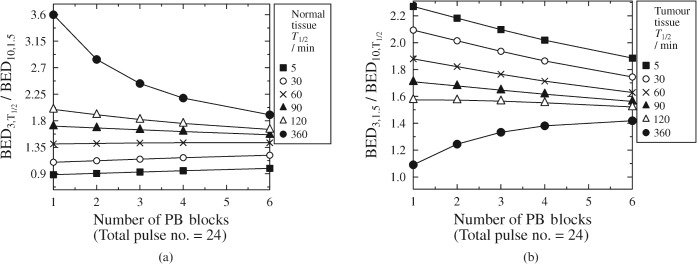
The effect of fractionation on the biologically effective dose (BED) ratio for a 24 hourly pulse schedule. Various T1/2 values are considered. (a) Varying the normal tissue T1/2 for a constant tumour T1/2 of 1.5 h. (b) Varying the tumour T1/2 for a constant normal tissue T1/2 of 1.5 h. PB, pulsed brachytherapy.
It is important to note that the improvement brought about by excessive increase in total pulse number becomes negligible (Figure 6). For high total pulse numbers it is likely that the BED ratio improvement brought by fractionation would be outweighed by detriment owing to applicator movement and (in extreme cases involving many fractions) the possibility of tumour repopulation.
Discussion
In this paper PB pulse doses were derived to match the normal tissue CLDR BED according to an assumed T1/2 of 1.5 h. BED ratios for both CLDR and PB were then calculated for a range of possible T1/2 values. As neither normal tissue nor tumour T1/2 values are known with certainty, a degree of inherent uncertainty is usually present in radiobiological calculations which purport to intercompare treatments. However, this work shows that, for PB regimens of duration approximately equal to the CLDR reference, it is possible to closely approximate the radiobiological efficacy of the reference CLDR schedule, regardless of the assumed T1/2. This finding is consistent with the work of Brenner and Hall [1] and Fowler and Mount [2].
Considering PB in isolation from repopulation effects, the most clear-cut route to radiobiological improvement is via a modest temporal extension of the treatment duration relative to that of the CLDR reference. Such extension would be beneficial to all cases except those combining very short normal tissue T1/2s (less than 20 min) with much longer tumour T1/2s (around 1.5 h). Extending the treatment duration carries the additional advantage of reducing the effect of repair half-time uncertainty on the modelled BED outcome.
This study also investigated the potential for PB block-schemes, such that night-time treatment could be avoided. Although the modelling assumed complete sublethal damage repair between fractions, it was shown that the compounding effects of incomplete repair were reduced with increasing pulse number. For the selected RMH starting-point, pulse numbers of at least 22 should be sufficient to introduce an overnight gap of any duration into the PB regimen at little cost in terms of pulse dose prescription uncertainty. Block-scheme design was thus found to be positively beneficial for a wide range of repair half-times and without the need to utilise excessively large total pulse numbers.
The amount of tumour repopulation during treatment will be governed by the overall treatment time (duration of the external beam treatment course plus duration of the brachytherapy treatment course) [20]. Thus, if a centre could incorporate PB block-schemes into its treatment schedule at no penalty to the overall therapy duration, additional repopulation effects ought not to occur. However, if a move to PB block-scheme design necessitates extension of the total treatment time relative to CLDR, the additional tumour repopulation could offset the PB advantages.
Numerous retrospective studies have investigated the effect of overall treatment (external beam plus brachytherapy) duration on cervical cancer control. In 1992, Keane et al [21] and Fyles et al [22] proposed a linear relationship: a 0.7% decrease in control per day of treatment prolongation beyond 30 days for Stages I and II, rising to 1.2% per day for Stages III and IV. Lanciano et al [23] demonstrated a highly significant decrease in survival (p=0.0001) and pelvic control (p=0.0001) as the total treatment time was increased from <6 to 10+ weeks for cervical cancer patients. In this case, the majority of the adverse effects from the prolongation of total treatment time were observed in Stage III patients [23]. From analysis of 386 patients with Stage IIB and III cervical carcinoma, Grinksy et al [24] found that loss of local control and overall survival, when treatment exceeded 52 days, was approximately 1% per day in both cases. Similarly, from analysis of 209 patients, Petereit et al [25] found that survival decreased by 0.6% per day and pelvic control decreased by 0.7% per day for each additional day of treatment beyond 55 days for all stages of disease. A 229 patient study by Gasinka et al [26] concluded that “the overall time factor in cervical squamous cell carcinoma treatment is very important. It is essential to initiate RT as soon as possible and complete it as quickly as acute tolerance allows.” Gasinka et al [26] suggest that, for conventional RT coupled with medium dose rate brachytherapy, an overall treatment time of 50–60 days seems reasonable. Their statistical analysis suggested that tumour proliferation was not important for short overall treatment times (<60 days) [26]. However, this result contradicted data from the Tsang et al [27], who found that a lower tumour proliferation rate in the cervix was a prognostic factor for patients treated with overall treatment times ≤70 days (median 45 days). Although many of these studies suggest that carefully designed prospective randomised trials would be necessary to determine whether treatment duration is a truly independent prognostic factor, most recommend that unnecessary treatment extensions should be avoided, particularly in patients with Stage III and IV disease. This recommendation is also reflected elsewhere [28].
Quantitative repopulation modelling is explored by Fowler [29,30], but accurate data regarding tumour proliferation kinetics would be required to apply mathematical models in this case. Thus, for the present modelling purposes, the assumption is made that the entire fractionated treatment is completed within a sufficiently short overall time period that repopulation ought not to be significant (considering the conclusion of Fyles et al [22], a period of 30 days could be considered as a conservative estimate for such an overall treatment time). Many centres currently schedule 25 or more fractions of external beam radiotherapy for cervical cancer, so that they cannot complete the entire treatment (including brachytherapy) within 30 days. In such cases, overall temporal extension of treatments should be avoided, at least until the timescales of cervical carcinoma repopulation are better understood. However, the implementation of extended PB treatments or PB block-schemes may still prove viable and desirable, if the gap between external beam radiotherapy and PB can be reduced.
It should also be noted that, theoretically at least, continuous pulse delivery (i.e. through both the day and night) is preferable to block-scheme delivery, since the total pulse number can be increased to bring added benefit to all cases bar those involving the shortest normal tissue half-times of repair (<20 min) when coupled with relatively long tumour half-times of repair (around 1.5 h). Assessment of the clinical effects of different block fractionation patterns would require careful analysis of data from large-scale PB clinical trials. However, although the relative importance of BED ratio reductions, staffing concerns and patient comfort must be a local issue for individual treatment centres, modelling has a role to play in guiding those discussions.
Pulsed brachytherapy is a treatment modality that is attracting rapidly growing clinical and commercial interest (the availability of CLDR equipment now being very limited). Promising initial clinical results are coupled with good theoretical outcome predictions across a range of possible repair parameters. The results of this study suggest that radiobiological improvement is likely to be achieved via modest temporal extension of PB therapy duration (an increase in the total number of hourly pulses delivered), provided that the overall (external beam plus brachytherapy) treatment time is not extended such that additional tumour repopulation becomes a concern. If the total pulse number is kept high (in the case of the RMH starting point >22), repair parameter uncertainty need not be an obstacle to designing block-scheme daytime-only PB schedules, which are more convenient and at least as good (in terms of therapeutic index) as existing schedules. Furthermore, as more accurate T1/2 data become available, PB will offer increased scope for optimisation, especially when used in conjunction with MRI treatment planning.
References
Articles from The British Journal of Radiology are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1259/bjr/58276427
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3473649?pdf=render
Free after 24 months at bjr.birjournals.org
http://bjr.birjournals.org/cgi/content/full/84/1001/449
Free after 24 months at bjr.birjournals.org
http://bjr.birjournals.org/cgi/reprint/84/1001/449.pdf
Free to read at bjr.birjournals.org
http://bjr.birjournals.org/cgi/content/abstract/84/1001/449
Citations & impact
Impact metrics
Article citations
Radiation repair models for clinical application.
Br J Radiol, 92(1093):20180070, 28 Feb 2018
Cited by: 5 articles | PMID: 29470100 | PMCID: PMC6435088
[Radiobiology in brachytherapy].
Cancer Radiother, 17(2):81-84, 04 Apr 2013
Cited by: 7 articles | PMID: 23562380
Review
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical implications of incomplete repair parameters for rat spinal cord: the feasibility of large doses per fraction in PDR and HDR brachytherapy.
Int J Radiat Oncol Biol Phys, 51(1):215-226, 01 Sep 2001
Cited by: 9 articles | PMID: 11516872
Office hours pulsed brachytherapy boost in breast cancer.
Radiother Oncol, 59(3):273-280, 01 Jun 2001
Cited by: 6 articles | PMID: 11369068
Pulsed-dose-rate brachytherapy: design of convenient (daytime-only) schedules.
Int J Radiat Oncol Biol Phys, 39(4):809-815, 01 Nov 1997
Cited by: 24 articles | PMID: 9369127
Optimizing the time course of brachytherapy and other accelerated radiotherapeutic protocols.
Int J Radiat Oncol Biol Phys, 29(4):893-901, 01 Jul 1994
Cited by: 30 articles | PMID: 8040040
Review



