Abstract
Free full text

celB, a gene coding for a bifunctional cellulase from the extreme thermophile "Caldocellum saccharolyticum".
Abstract
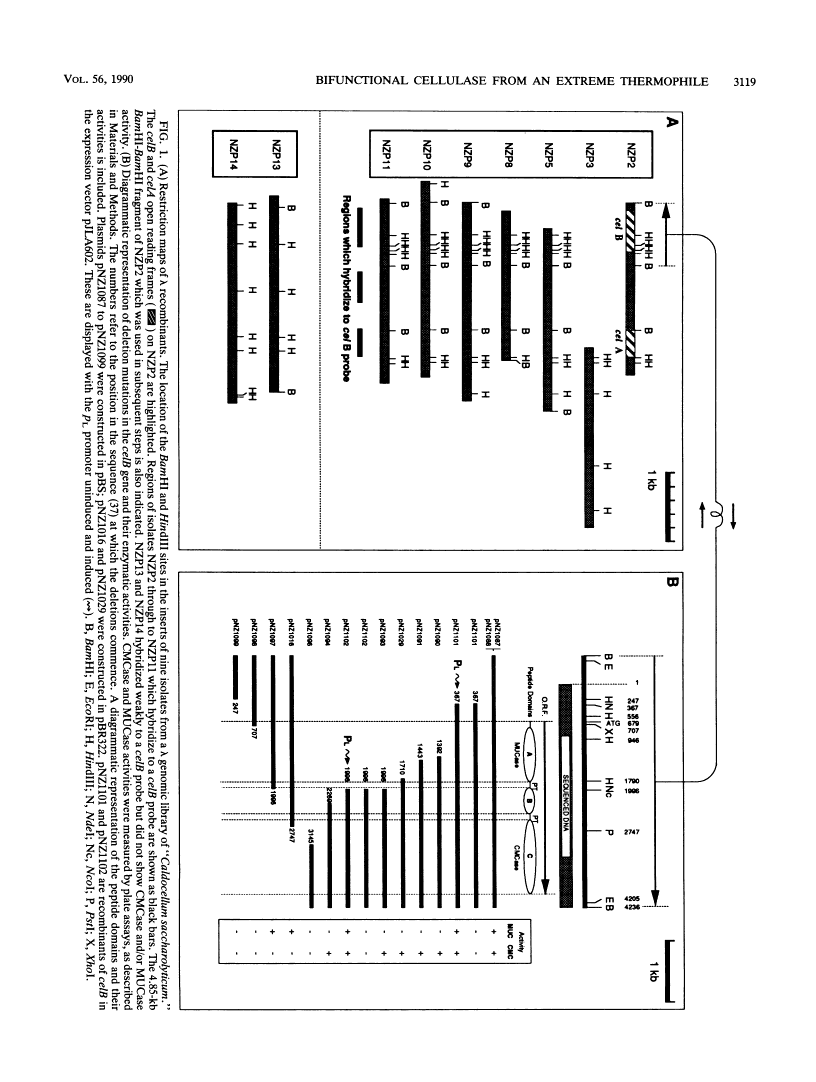
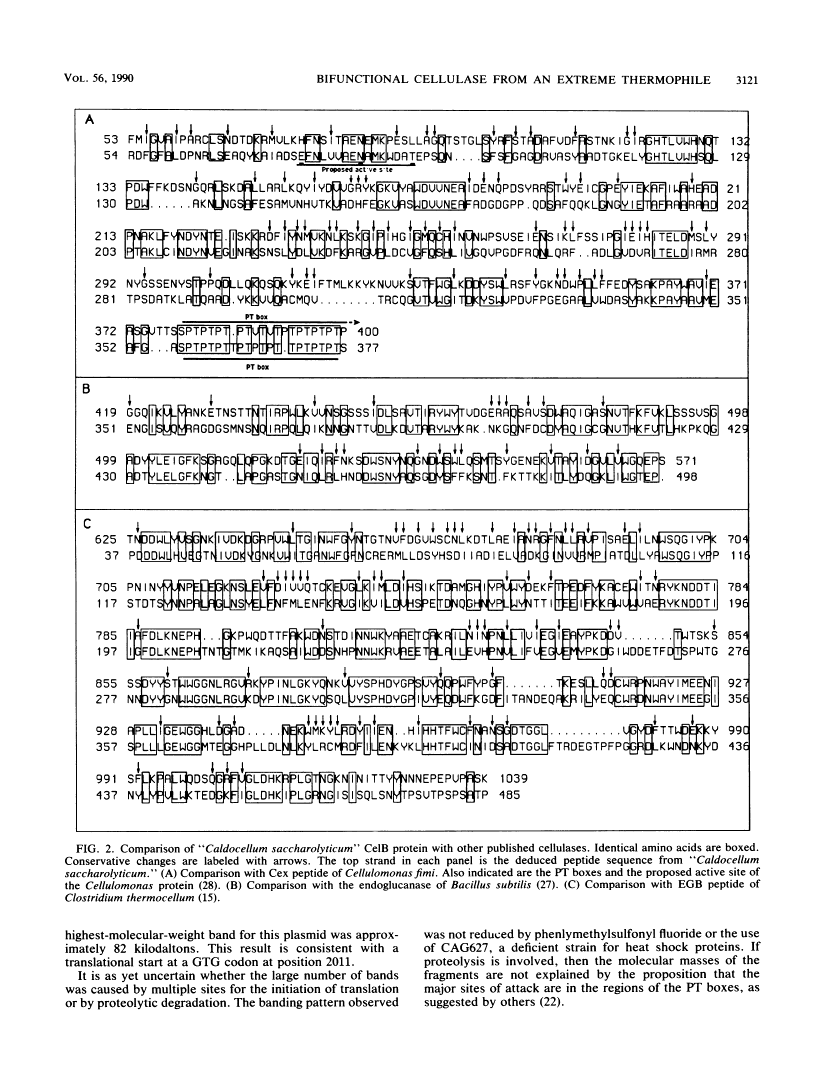
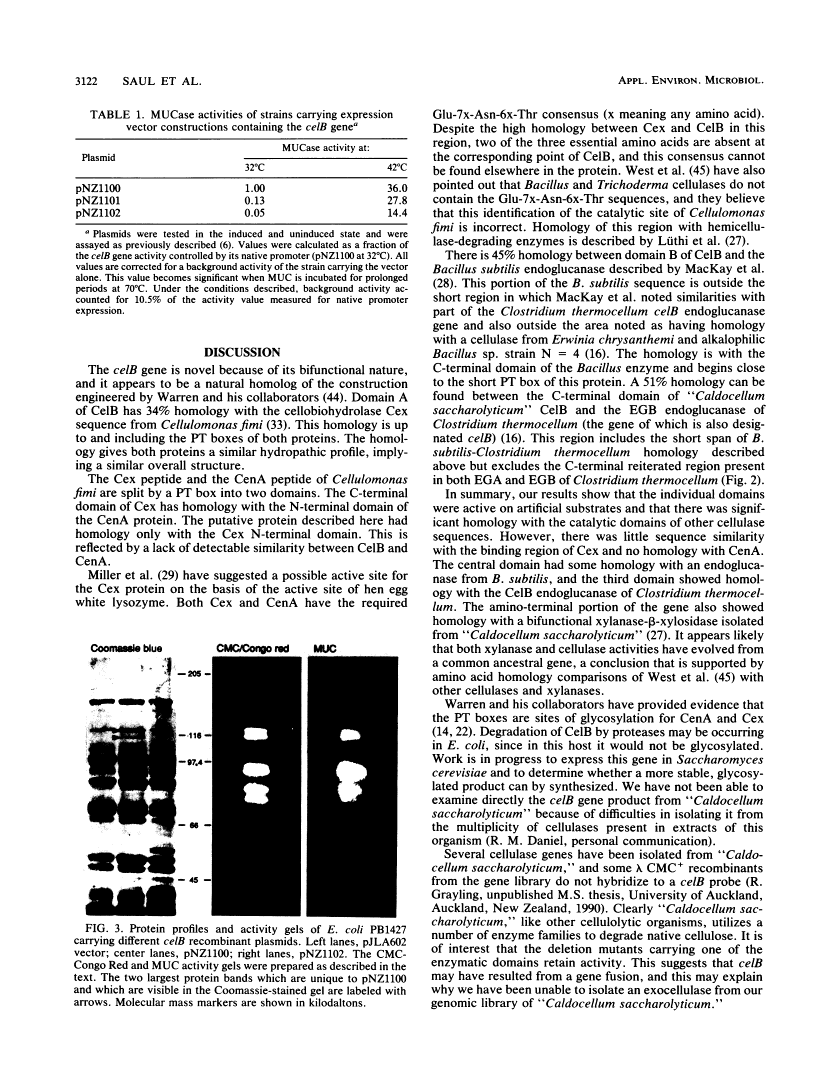
"Caldocellum saccharolyticum" is an obligatory anaerobic thermophilic bacterium. A gene from this organism, designated celB, has been cloned in Escherichia coli as part of a bacteriophage lambda gene library. This gene produces a thermostable cellulase that shows both endoglucanase and exoglucanase activities on test substrates and is able to degrade crystalline cellulose to glucose. The sequence of celB has homology with both exo- and endoglucanases described by others. It appears to have a central domain without enzymatic activity which is joined to the enzymatic domains by runs of amino acids rich in proline and threonine (PT boxes). Deletion analysis shows that the exoglucanase activity is located in the amino-terminal domain of the enzyme and that endoglucanase activity is located in the carboxy-terminal domain. There are internal transcriptional and translational start sites within the gene. The intact gene has been cloned into a temperature-inducible expression vector, pJLA602, and overexpressed in E. coli. Polyacrylamide gel electrophoresis showed that celB produced a protein with a molecular weight of 118,000 to 120,000. A number of smaller proteins with activity against carboxymethyl cellulose and 4-methyl umbelliferyl-beta-D-cellobioside were also produced. These are believed to be the result of alternative translational start sites and/or proteolytic degradation products of the translated gene product.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. [Abstract] [Google Scholar]
- Béguin P, Cornet P, Aubert JP. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. [Europe PMC free article] [Abstract] [Google Scholar]
- Bhikhabhai R, Johansson G, Pettersson G. Isolation of cellulolytic enzymes from Trichoderma reesei QM 9414. J Appl Biochem. 1984 Oct-Dec;6(5-6):336–345. [Abstract] [Google Scholar]
- Chen EY, Seeburg PH. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. [Abstract] [Google Scholar]
- Chernoglazov VM, Jafarova AN, Klyosov AA. Continuous photometric determination of endo-1,4-beta-D-glucanase (cellulase) activity using 4-methylumbelliferyl-beta-D-cellobioside as a substrate. Anal Biochem. 1989 May 15;179(1):186–189. [Abstract] [Google Scholar]
- Chou PY, Fasman GD. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. [Abstract] [Google Scholar]
- Clarke L, Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. [Abstract] [Google Scholar]
- Dierstein R, Wickner W. Requirements for substrate recognition by bacterial leader peptidase. EMBO J. 1986 Feb;5(2):427–431. [Europe PMC free article] [Abstract] [Google Scholar]
- Garnier J, Osguthorpe DJ, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. [Abstract] [Google Scholar]
- Gilkes NR, Warren RA, Miller RC, Jr, Kilburn DG. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988 Jul 25;263(21):10401–10407. [Abstract] [Google Scholar]
- Grépinet O, Béguin P. Sequence of the cellulase gene of Clostridium thermocellum coding for endoglucanase B. Nucleic Acids Res. 1986 Feb 25;14(4):1791–1799. [Europe PMC free article] [Abstract] [Google Scholar]
- Guiseppi A, Cami B, Aymeric JL, Ball G, Creuzet N. Homology between endoglucanase Z of Erwinia chrysanthemi and endoglucanases of Bacillus subtilis and alkalophilic Bacillus. Mol Microbiol. 1988 Jan;2(1):159–164. [Abstract] [Google Scholar]
- Hall J, Hazlewood GP, Barker PJ, Gilbert HJ. Conserved reiterated domains in Clostridium thermocellum endoglucanases are not essential for catalytic activity. Gene. 1988 Sep 15;69(1):29–38. [Abstract] [Google Scholar]
- Joliff G, Béguin P, Aubert JP. Nucleotide sequence of the cellulase gene celD encoding endoglucanase D of Clostridium thermocellum. Nucleic Acids Res. 1986 Nov 11;14(21):8605–8613. [Europe PMC free article] [Abstract] [Google Scholar]
- Karn J, Brenner S, Barnett L, Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Langsford ML, Gilkes NR, Singh B, Moser B, Miller RC, Jr, Warren RA, Kilburn DG. Glycosylation of bacterial cellulases prevents proteolytic cleavage between functional domains. FEBS Lett. 1987 Dec 10;225(1-2):163–167. [Abstract] [Google Scholar]
- Love DR, Fisher R, Bergquist PL. Sequence structure and expression of a cloned beta-glucosidase gene from an extreme thermophile. Mol Gen Genet. 1988 Jul;213(1):84–92. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Lüthi E, Love DR, McAnulty J, Wallace C, Caughey PA, Saul D, Bergquist PL. Cloning, sequence analysis, and expression of genes encoding xylan-degrading enzymes from the thermophile "Caldocellum saccharolyticum". Appl Environ Microbiol. 1990 Apr;56(4):1017–1024. [Europe PMC free article] [Abstract] [Google Scholar]
- MacKay RM, Lo A, Willick G, Zuker M, Baird S, Dove M, Moranelli F, Seligy V. Structure of a Bacillus subtilis endo-beta-1,4-glucanase gene. Nucleic Acids Res. 1986 Nov 25;14(22):9159–9170. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Neill G, Goh SH, Warren RA, Kilburn DG, Miller RC., Jr Structure of the gene encoding the exoglucanase of Cellulomonas fimi. Gene. 1986;44(2-3):325–330. [Abstract] [Google Scholar]
- Rackwitz HR, Zehetner G, Frischauf AM, Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Saul D, Spiers AJ, McAnulty J, Gibbs MG, Bergquist PL, Hill DF. Nucleotide sequence and replication characteristics of RepFIB, a basic replicon of IncF plasmids. J Bacteriol. 1989 May;171(5):2697–2707. [Europe PMC free article] [Abstract] [Google Scholar]
- Saul DJ, Williams LC, Love DR, Chamley LW, Bergquist PL. Nucleotide sequence of a gene from Caldocellum saccharolyticum encoding for exocellulase and endocellulase activity. Nucleic Acids Res. 1989 Jan 11;17(1):439–439. [Europe PMC free article] [Abstract] [Google Scholar]
- Schauder B, Blöcker H, Frank R, McCarthy JE. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52(2-3):279–283. [Abstract] [Google Scholar]
- Schwarz WH, Gräbnitz F, Staudenbauer WL. Properties of a Clostridium thermocellum Endoglucanase Produced in Escherichia coli. Appl Environ Microbiol. 1986 Jun;51(6):1293–1299. [Europe PMC free article] [Abstract] [Google Scholar]
- Short JM, Fernandez JM, Sorge JA, Huse WD. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. [Europe PMC free article] [Abstract] [Google Scholar]
- Warren RA, Gerhard B, Gilkes NR, Owolabi JB, Kilburn DG, Miller RC., Jr A bifunctional exoglucanase-endoglucanase fusion protein. Gene. 1987;61(3):421–427. [Abstract] [Google Scholar]
- West CA, Elzanowski A, Yeh LS, Barker WC. Homologues of catalytic domains of Cellulomonas glucanases found in fungal and Bacillus glycosidases. FEMS Microbiol Lett. 1989 May;50(1-2):167–172. [Abstract] [Google Scholar]
- Wong WK, Gerhard B, Guo ZM, Kilburn DG, Warren AJ, Miller RC., Jr Characterization and structure of an endoglucanase gene cenA of Cellulomonas fimi. Gene. 1986;44(2-3):315–324. [Abstract] [Google Scholar]
Associated Data
Articles from Applied and Environmental Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aem.56.10.3117-3124.1990
Read article for free, from open access legal sources, via Unpaywall:
https://aem.asm.org/content/aem/56/10/3117.full.pdf
Free to read at aem.asm.org
http://aem.asm.org/cgi/content/abstract/56/10/3117
Free after 4 months at aem.asm.org
http://aem.asm.org/cgi/reprint/56/10/3117
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aem.56.10.3117-3124.1990
Article citations
Insights into Thermophilic Plant Biomass Hydrolysis from Caldicellulosiruptor Systems Biology.
Microorganisms, 8(3):E385, 10 Mar 2020
Cited by: 6 articles | PMID: 32164310 | PMCID: PMC7142884
Review Free full text in Europe PMC
Genomic and physiological analyses reveal that extremely thermophilic Caldicellulosiruptor changbaiensis deploys uncommon cellulose attachment mechanisms.
J Ind Microbiol Biotechnol, 46(9-10):1251-1263, 07 Aug 2019
Cited by: 3 articles | PMID: 31392469
Creation of a functional hyperthermostable designer cellulosome.
Biotechnol Biofuels, 12:44, 28 Feb 2019
Cited by: 12 articles | PMID: 30858881 | PMCID: PMC6394049
Expression of Heterologous Cellulases in Thermotoga sp. Strain RQ2.
Biomed Res Int, 2015:304523, 26 Jul 2015
Cited by: 11 articles | PMID: 26273605 | PMCID: PMC4529897
Carbohydrate hydrolysis and transport in the extreme thermoacidophile Sulfolobus solfataricus.
Appl Environ Microbiol, 78(22):7931-7938, 31 Aug 2012
Cited by: 6 articles | PMID: 22941087 | PMCID: PMC3485927
Go to all (52) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
celA, another gene coding for a multidomain cellulase from the extreme thermophile Caldocellum saccharolyticum.
Appl Microbiol Biotechnol, 43(2):291-296, 01 May 1995
Cited by: 33 articles | PMID: 7612247
Properties and gene structure of a bifunctional cellulolytic enzyme (CelA) from the extreme thermophile 'Anaerocellum thermophilum' with separate glycosyl hydrolase family 9 and 48 catalytic domains.
Microbiology (Reading), 144 ( Pt 2):457-465, 01 Feb 1998
Cited by: 75 articles | PMID: 9493383
Characterization of a bifunctional cellulase and its structural gene. The cell gene of Bacillus sp. D04 has exo- and endoglucanase activity.
J Biol Chem, 270(43):26012-26019, 01 Oct 1995
Cited by: 38 articles | PMID: 7592793
The beta-mannanase from "Caldocellum saccharolyticum" is part of a multidomain enzyme.
Appl Environ Microbiol, 58(12):3864-3867, 01 Dec 1992
Cited by: 42 articles | PMID: 1476429 | PMCID: PMC183195