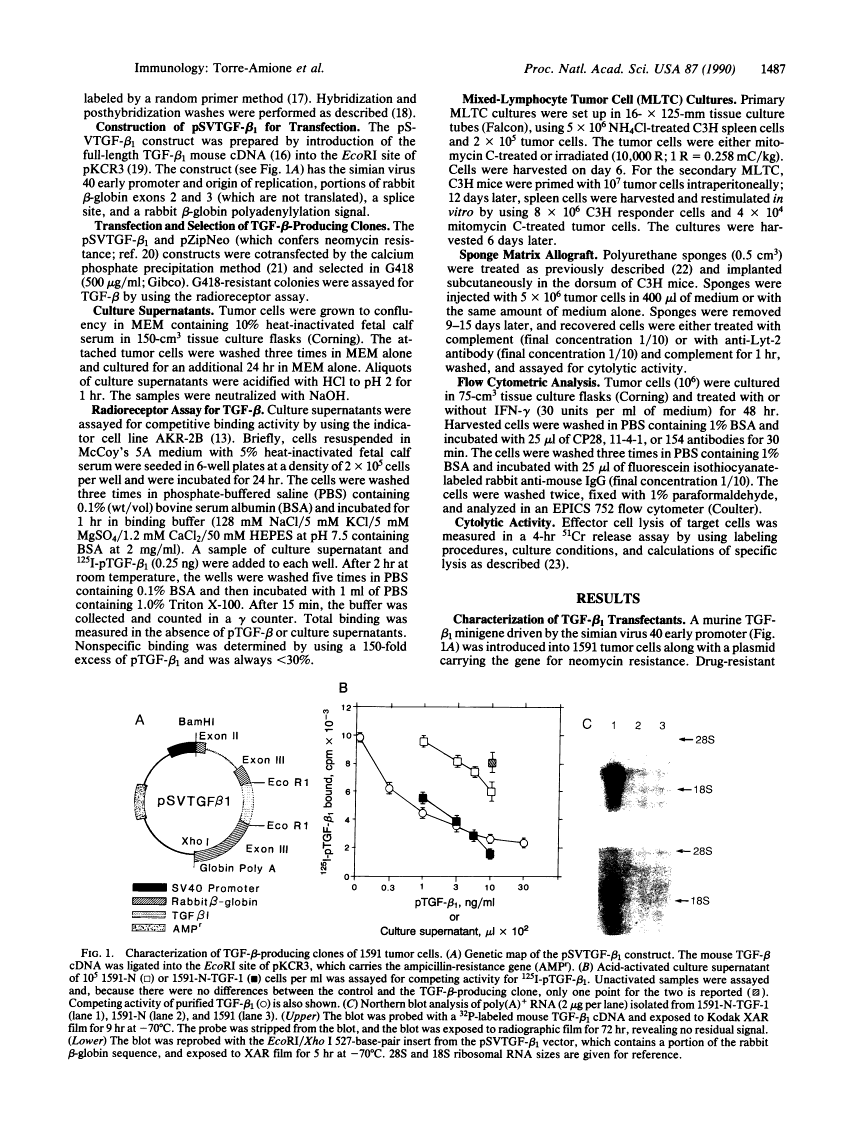
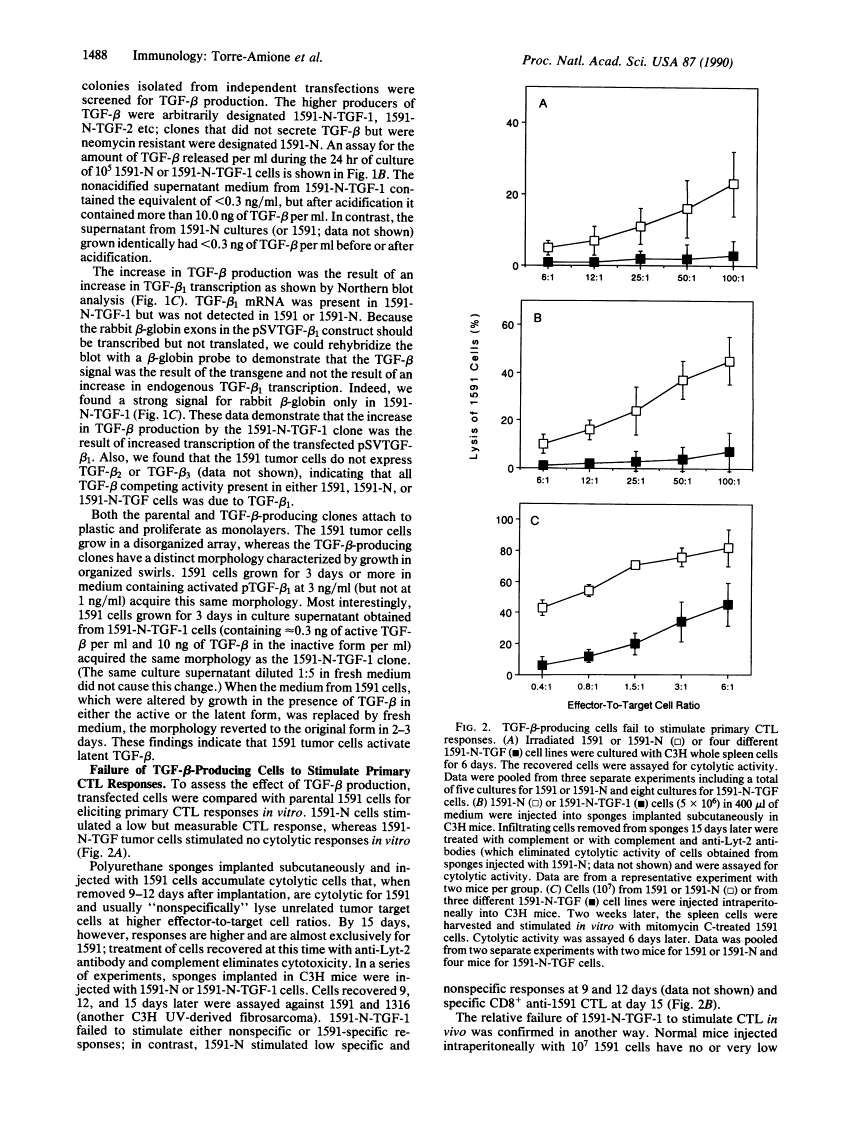
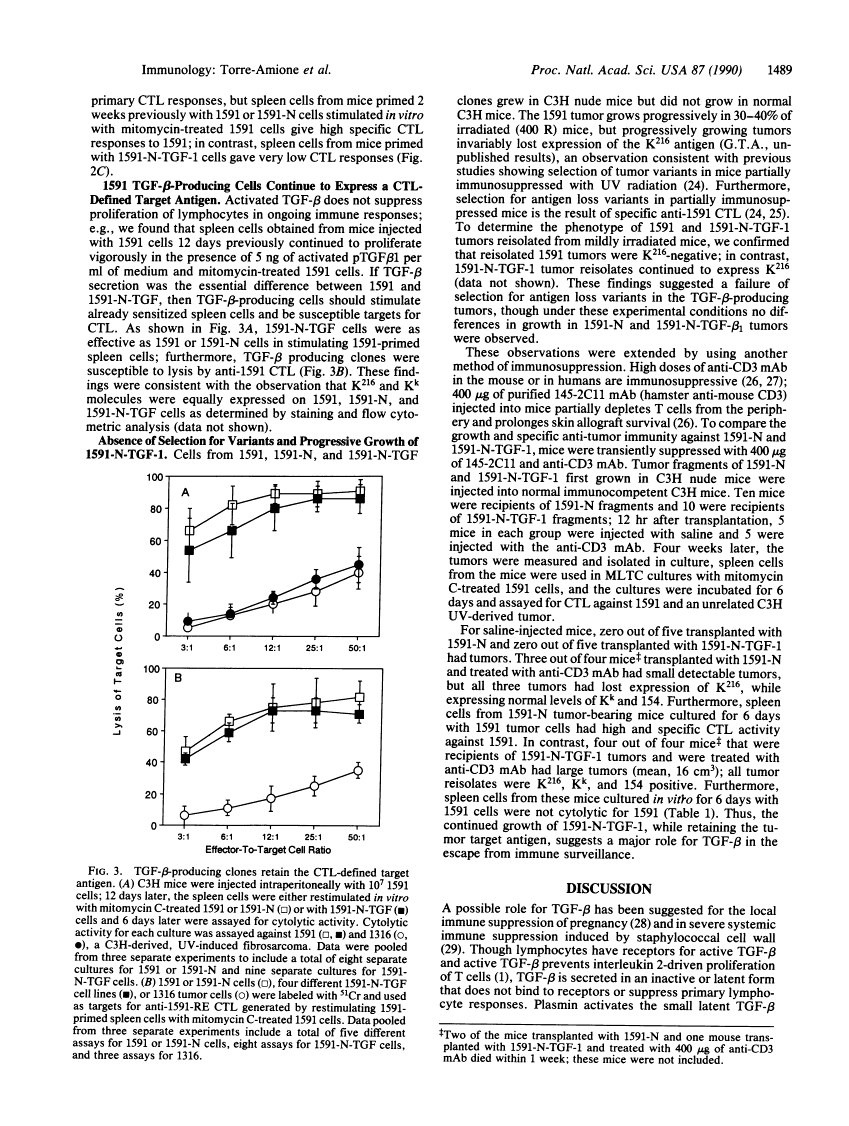
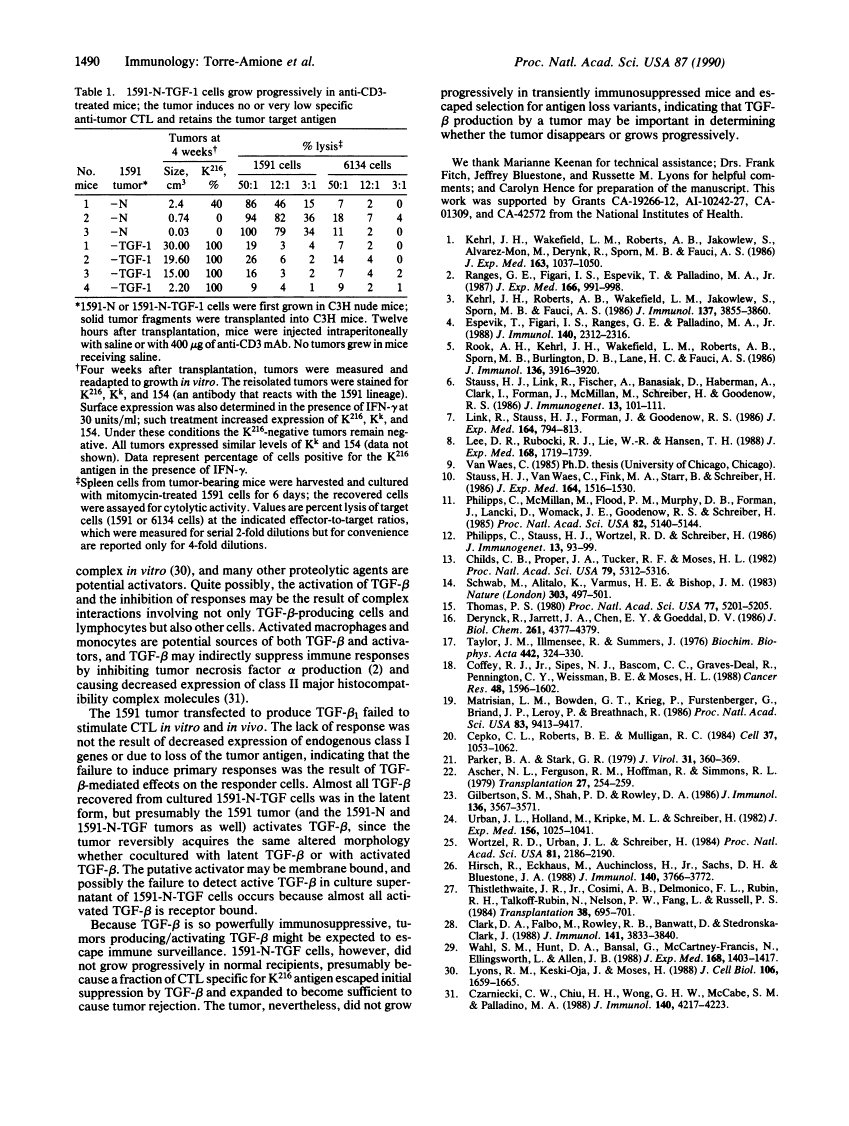
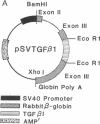
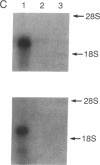
Abstract
Free full text

A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance.
Abstract
A highly immunogenic C3H-derived UV-induced tumor was cotransfected with a murine transforming growth factor type beta 1 (TGF-beta 1) cDNA and a neomycin-resistance gene. Stable clones were isolated and used in vitro and in vivo to determine the effects of endogenously produced TGF-beta on cytolytic T-lymphocyte (CTL) responses. Tumor cells producing TGF-beta, though retaining expression for class I major histocompatibility complex molecules and the tumor-specific antigen, did not stimulate primary CTL responses in vitro and were not effective in vivo for directly stimulating primary CTL or in priming for CTL responses. Furthermore, TGF-beta-producing tumors grew progressively in transiently immunosuppressed mice without losing the tumor antigen; thus, TGF-beta produced by tumors may promote escape from immune surveillance.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. [Europe PMC free article] [Abstract] [Google Scholar]
- Ranges GE, Figari IS, Espevik T, Palladino MA., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. [Europe PMC free article] [Abstract] [Google Scholar]
- Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [Abstract] [Google Scholar]
- Espevik T, Figari IS, Ranges GE, Palladino MA., Jr Transforming growth factor-beta 1 (TGF-beta 1) and recombinant human tumor necrosis factor-alpha reciprocally regulate the generation of lymphokine-activated killer cell activity. Comparison between natural porcine platelet-derived TGF-beta 1 and TGF-beta 2, and recombinant human TGF-beta 1. J Immunol. 1988 Apr 1;140(7):2312–2316. [Abstract] [Google Scholar]
- Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [Abstract] [Google Scholar]
- Stauss HJ, Linsk R, Fischer A, Watts S, Banasiak D, Haberman A, Clark I, Forman J, McMillan M, Schreiber H, et al. Isolation of the MHC genes encoding the tumour-specific class I antigens expressed on a murine fibrosarcoma. J Immunogenet. 1986 Apr-Jun;13(2-3):101–111. [Abstract] [Google Scholar]
- Linsk R, Vogel J, Stauss H, Forman J, Goodenow RS. Structure and function of three novel MHC class I antigens derived from a C3H ultraviolet-induced fibrosarcoma. J Exp Med. 1986 Sep 1;164(3):794–813. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee DR, Rubocki RJ, Lie WR, Hansen TH. The murine MHC class I genes, H-2Dq and H-2Lq, are strikingly homologous to each other, H-2Ld, and two genes reported to encode tumor-specific antigens. J Exp Med. 1988 Nov 1;168(5):1719–1739. [Europe PMC free article] [Abstract] [Google Scholar]
- Stauss HJ, Van Waes C, Fink MA, Starr B, Schreiber H. Identification of a unique tumor antigen as rejection antigen by molecular cloning and gene transfer. J Exp Med. 1986 Nov 1;164(5):1516–1530. [Europe PMC free article] [Abstract] [Google Scholar]
- Philipps C, McMillan M, Flood PM, Murphy DB, Forman J, Lancki D, Womack JE, Goodenow RS, Schreiber H. Identification of a unique tumor-specific antigen as a novel class I major histocompatibility molecule. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5140–5144. [Europe PMC free article] [Abstract] [Google Scholar]
- Philipps C, Stauss HJ, Wortzel RD, Schreiber H. A novel MHC class I molecule as a tumour-specific antigen. Correlation between the antibody-defined and the CTL-defined target structure. J Immunogenet. 1986 Apr-Jun;13(2-3):93–99. [Abstract] [Google Scholar]
- Childs CB, Proper JA, Tucker RF, Moses HL. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5312–5316. [Europe PMC free article] [Abstract] [Google Scholar]
- Schwab M, Alitalo K, Varmus HE, Bishop JM, George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. [Abstract] [Google Scholar]
- Thomas PS. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. [Europe PMC free article] [Abstract] [Google Scholar]
- Derynck R, Jarrett JA, Chen EY, Goeddel DV. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [Abstract] [Google Scholar]
- Taylor JM, Illmensee R, Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. [Abstract] [Google Scholar]
- Coffey RJ, Jr, Sipes NJ, Bascom CC, Graves-Deal R, Pennington CY, Weissman BE, Moses HL. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988 Mar 15;48(6):1596–1602. [Abstract] [Google Scholar]
- Matrisian LM, Bowden GT, Krieg P, Fürstenberger G, Briand JP, Leroy P, Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. [Europe PMC free article] [Abstract] [Google Scholar]
- Cepko CL, Roberts BE, Mulligan RC. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. [Abstract] [Google Scholar]
- Parker BA, Stark GR. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. [Europe PMC free article] [Abstract] [Google Scholar]
- Ascher NL, Ferguson RM, Hoffman R, Simmons RL. Partial characterization of cytotoxic cells infiltrating sponge matrix allografts. Transplantation. 1979 Apr;27(4):254–259. [Abstract] [Google Scholar]
- Gilbertson SM, Shah PD, Rowley DA. NK cells suppress the generation of Lyt-2+ cytolytic T cells by suppressing or eliminating dendritic cells. J Immunol. 1986 May 15;136(10):3567–3571. [Abstract] [Google Scholar]
- Urban JL, Holland JM, Kripke ML, Schreiber H. Immunoselection of tumor cell variants by mice suppressed with ultraviolet radiation. J Exp Med. 1982 Oct 1;156(4):1025–1041. [Europe PMC free article] [Abstract] [Google Scholar]
- Wortzel RD, Urban JL, Schreiber H. Malignant growth in the normal host after variant selection in vitro with cytolytic T-cell lines. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2186–2190. [Europe PMC free article] [Abstract] [Google Scholar]
- Hirsch R, Eckhaus M, Auchincloss H, Jr, Sachs DH, Bluestone JA. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988 Jun 1;140(11):3766–3772. [Abstract] [Google Scholar]
- Thistlethwaite JR, Jr, Cosimi AB, Delmonico FL, Rubin RH, Talkoff-Rubin N, Nelson PW, Fang L, Russell PS. Evolving use of OKT3 monoclonal antibody for treatment of renal allograft rejection. Transplantation. 1984 Dec;38(6):695–701. [Abstract] [Google Scholar]
- Clark DA, Falbo M, Rowley RB, Banwatt D, Stedronska-Clark J. Active suppression of host-vs graft reaction in pregnant mice. IX. Soluble suppressor activity obtained from allopregnant mouse decidua that blocks the cytolytic effector response to IL-2 is related to transforming growth factor-beta. J Immunol. 1988 Dec 1;141(11):3833–3840. [Abstract] [Google Scholar]
- Wahl SM, Hunt DA, Bansal G, McCartney-Francis N, Ellingsworth L, Allen JB. Bacterial cell wall-induced immunosuppression. Role of transforming growth factor beta. J Exp Med. 1988 Oct 1;168(4):1403–1417. [Europe PMC free article] [Abstract] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. [Europe PMC free article] [Abstract] [Google Scholar]
- Czarniecki CW, Chiu HH, Wong GH, McCabe SM, Palladino MA. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988 Jun 15;140(12):4217–4223. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.4.1486
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/87/4/1486.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.87.4.1486
Article citations
TGF-β signaling in health, disease, and therapeutics.
Signal Transduct Target Ther, 9(1):61, 22 Mar 2024
Cited by: 36 articles | PMID: 38514615 | PMCID: PMC10958066
Review Free full text in Europe PMC
Brain type of creatine kinase induces doxorubicin resistance via TGF-β signaling in MDA-MB-231 breast cancer cells.
Anim Cells Syst (Seoul), 26(5):203-213, 31 Aug 2022
Cited by: 2 articles | PMID: 36275445 | PMCID: PMC9586670
Advances in Immunotherapy and the TGF-β Resistance Pathway in Metastatic Bladder Cancer.
Cancers (Basel), 13(22):5724, 16 Nov 2021
Cited by: 12 articles | PMID: 34830879 | PMCID: PMC8616345
Review Free full text in Europe PMC
Application of specialized pro-resolving mediators in periodontitis and peri-implantitis: a review.
Eur J Oral Sci, 129(1):e12759, 09 Feb 2021
Cited by: 10 articles | PMID: 33565133 | PMCID: PMC7986752
Review Free full text in Europe PMC
Transforming growth factor β-induced epithelial-to-mesenchymal signature predicts metastasis-free survival in non-small cell lung cancer.
Oncotarget, 10(8):810-824, 25 Jan 2019
Cited by: 18 articles | PMID: 30783512 | PMCID: PMC6368226
Go to all (216) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Immunodominance deters the response to other tumor antigens thereby favoring escape: prevention by vaccination with tumor variants selected with cloned cytolytic T cells in vitro.
Tissue Antigens, 47(5):399-407, 01 May 1996
Cited by: 14 articles | PMID: 8795140
Immune response to progressor variants derived from transfection of an ultraviolet radiation-induced C3H mouse regressor tumor cell line with activated Harvey-ras oncogene.
Cancer Res, 50(11):3159-3166, 01 Jun 1990
Cited by: 3 articles | PMID: 2185882
Inhibition of tumor-specific cytotoxic T-lymphocyte responses by transforming growth factor beta 1.
Cancer Res, 52(6):1386-1392, 01 Mar 1992
Cited by: 80 articles | PMID: 1531782
MHC class I restricted T cells and immune surveillance against transplanted ultraviolet light-induced tumors.
Semin Cancer Biol, 2(5):321-328, 01 Oct 1991
Cited by: 7 articles | PMID: 1837738
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: CA-19266-12
Grant ID: CA-01309
NIAID NIH HHS (1)
Grant ID: AI-10242-27