Abstract
Purpose
The goal of this study was to determine the effect of combination of intratumoral administration of dendritic cells (DC) and fractionated external beam radiation (EBRT) on tumor-specific immune responses in patients with soft-tissue sarcoma (STS).Methods and material
Seventeen patients with large (>5 cm) high-grade STS were enrolled in the study. They were treated in the neoadjuvant setting with 5,040 cGy of EBRT, split into 28 fractions and delivered 5 days per week, combined with intratumoral injection of 10(7) DCs followed by complete resection. DCs were injected on the second, third, and fourth Friday of the treatment cycle. Clinical evaluation and immunological assessments were performed.Results
The treatment was well tolerated. No patient had tumor-specific immune responses before combined EBRT/DC therapy; 9 patients (52.9%) developed tumor-specific immune responses, which lasted from 11 to 42 weeks. Twelve of 17 patients (70.6%) were progression free after 1 year. Treatment caused a dramatic accumulation of T cells in the tumor. The presence of CD4(+) T cells in the tumor positively correlated with tumor-specific immune responses that developed following combined therapy. Accumulation of myeloid-derived suppressor cells but not regulatory T cells negatively correlated with the development of tumor-specific immune responses. Experiments with (111)In labeled DCs demonstrated that these antigen presenting cells need at least 48 h to start migrating from tumor site.Conclusions
Combination of intratumoral DC administration with EBRT was safe and resulted in induction of antitumor immune responses. This suggests that this therapy is promising and needs further testing in clinical trials design to assess clinical efficacy.Free full text

Combination of External Beam Radiation (EBRT) with Intratumoral Injection of Dendritic Cells as Neo-Adjuvant Treatment of High-Risk Soft Tissue Sarcoma Patients
Abstract
Purpose
The goal of this study was to determine the effect of combination of intratumoral administration of dendritic cells (DC) and fractionated external beam radiation (EBRT) on tumor-specific immune responses in patients with soft tissue sarcoma (STS).
Methods and Material
Seventeen patients with large (>5 cm) high grade STS were enrolled in the study. They were treated in the neoadjuvant setting with 5040 cGy of EBRT, split into 28 fractions and delivered 5 days a week, combined with intratumoral injection of 107 DCs followed by complete resection. DCs were injected on the second, third, and fourth Friday of the treatment cycle. Clinical evaluation and immunological assessments were performed.
Results
The treatment was well tolerated. No patient had tumor-specific immune responses before combined EBRT/DC therapy; nine patients (52.9%) developed tumor-specific immune responses, which lasted from 11 to 42 weeks. Twelve of 17 patients (70.6%) were progression free after one year. Treatment caused a dramatic accumulation of T cells in the tumor. The presence of CD4+ T cells in the tumor positively correlated with tumor-specific immune responses that developed following combined therapy. Accumulation of myeloid-derived suppressor cells but not regulatory T cells negatively correlated with the development of tumor-specific immune responses. Experiments with 111In labeled DCs demonstrated that these antigen presenting cells need at least 48 hr to start migrating from tumor site.
Conclusions
Combination of intratumoral DC administration with EBRT was safe and resulted in induction of antitumor immune responses. This suggests that this therapy is promising and need further testing in clinical trials design to assess clinical efficacy.
Introduction
Intratumoral administration of dendritic cells (DC) is one of the promising methods of induction of therapeutic antitumor immune responses. The main advantage of this approach is that a large variety of tumor-associated antigens present in tumors can be utilized. In addition, patients don’t need to be selected or excluded based on HLA type or the expression of specific antigens. However, immunotherapy alone rarely causes curative anti-tumor effects as the manipulation of tumor microenvironment is necessary to potentiate the effect of DC administration1. Ionizing radiation presents one such powerful intervention. Radiation can not only kill tumor cells releasing tumor antigens, but can also exert various immunomodulatory effects including induction of the expression of cytokines, chemokines, and release of inflammatory mediators 2–4. It also increases the permeability of the local vasculature that leads to recruitment of circulating leukocytes into surrounding tissues including antigen-presenting cells and effector T cells5–7. Thus, the proinflammatory microenvironment within irradiated tumors could provide DCs with maturation-inducing stimuli critical for eliciting effective antigen presentation.
In a number of pre-clinical studies we and others have demonstrated that local tumor irradiation in combination with intratumoral DC administration but not irradiation alone resulted in potent antitumor immune responses that translated into an antitumor effect 7–12. These preclinical studies provided a compelling rationale for testing this approach in the clinic. In a phase I clinical trial of hepatoma, intratumoral administration of DCs in combination with a single high dose of conformal radiation was found to be safe and in some cases resulted in induction of tumor-specific immune responses 13, 14. However, it remains unclear whether conventionally fractionated EBRT coupled with DC can cause a robust immune response in a substantial proportion of patients, whether this response correlates with clinical outcome, and whether the treatment affects immune suppressive cells present in cancer patients. In this study we tested this approach in patients with soft tissue sarcomas (STS).
Sarcomas are relatively rare neoplasms with approximately 10,000+ new cases occurring in the United States every year. Many studies have shown that the preoperative radiotherapy and surgery is an effective strategy to treat many STS with high risk features 15–18. Unfortunately, conventional therapy for large, high grade tumors is frequently systemically ineffective, which makes this a very deadly problem. Indeed, despite a multidisciplinary, multimodality approach employing radiation therapy and at times systemic chemotherapy, approximately 50% of patients with large, high grade STS will go on to develop distant metastasis19. Novel approaches to deal with the systemic potential of this disease are needed.
Materials and Methods
Patient selection and treatment
Seventeen patients with histologically confirmed large high-grade STS of the extremity/trunk/chest wall were enrolled to the study (Table 1). These patients had clinical stage T2N0M0 with a significant (>50%) risk of progressing to distant metastases. All patients provided written informed consent to an Institutional Review Board of University of South Florida approved protocol. Treatment schema is shown in Fig. S1. Patients were treated with external beam radiation (EBRT) combined with experimental intratumoral injection of dendritic cells (DC). Patients received 5040 cGy EBRT in 28 equal fractions. Radiation was delivered 5 days a week (Monday-Friday). DCs (107 cells) were injected intatumorally three times on the second, third, and fourth Friday during the course of radiation. One additional DC injection was given several days prior to surgery to assess DC migration (see below). Tumors were surgically resected 3–6 weeks after the completion of EBRT. Peripheral blood from 15 healthy volunteers (10 females and 5 males age 32–48) was used to establish control values in the analysis of cell phenotype.
Table 1
Patients characteristics and clinical effect of the treatment
| Patient | Age | Sex | Location | Diagnosis | Grade | Max Tumor size prior to therapy on MRI (cm) | Max Tumor size after therapy on MRI (cm) | Tumor size after therapy on pathology (cm) | % Tumor necrosis prior to therapy on MRI | % Tumor necrosis after therapy on MRI | Tumor necrosis/fibrosis/hyalinization (%) PATH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | L pelvis | Undifferentiated pleomorphic sarcoma | HG | 10.4 | 12 | 11 | 10 | 10 | 80 |
| 2 | 81 | M | R flank | Spindle cell sarcoma | HG | 17 | 20 | 19 | 50 | 70 | 70 |
| 3 | 73 | F | L spine | Sarcoma with myxoid and chondroid features | HG | 7.6 | 9.1 | 10 | 50 | 90 | 53 |
| 4 | 54 | M | R shoulder | Monophasic synovial sarcoma | HG | 5 | 4 | 5.3 | 5 | 5 | 10 |
| 5 | 46 | F | R thigh | Spindle/pleomorpic cell sarcoma | HG | 10.7 | 10.1 | 6.5 | 10 | 10 | 70 |
| 6 | 58 | M | L thigh | Undifferentiated pleomorphic sarcoma | HG | 9.7 | 11.5 | 11 | 30 | 80 | 90 |
| 7 | 25 | M | L thigh | Monophasic synovial sarcoma | HG | 8 | 8.4 | 10 | 0 | 40 | 30 |
| 8 | 79 | M | L arm | Spindle/pleomorpic cell sarcoma | HG | 7 | 6.6 | 11 | 10 | 10 | 25 |
| 19 | 53 | M | R groin/thigh | Sclerosing epthelioid fibrosarcoma | HG | 12.6 | 12.2 | 14 | 5 | 5 | 20 |
| 10 | 50 | M | R paraspine | Undifferentiated pleomorphic sarcoma | HG | 15.1 | 15.1 | 15 | 10 | 80 | 70 |
| 11 | 70 | M | L thigh | Undifferentiated pleomorphic sarcoma | HG | 12.2 | 13.7 | 18 | 20 | 50 | 70 |
| 12 | 73 | M | Back | Sclerosing epthelioid fibrosarcoma | HG | 6 | 9.7 | 12 | 0 | 0 | 50 |
| 13 | 82 | M | R arm | Myxoid spindle cell sarcoma | IG | 5.9 | 4.8 | 5 | 0 | 90 | 95 |
| 14 | 60 | M | R leg | Undifferentiated pleomorphic sarcoma | HG | 14 | 7.7 | 7.5 | 5 | 10 | 40 |
| 15 | 69 | F | L knee | Spindle/pleomorpic cell sarcoma | HG | 5.8 | 7.3 | 9 | 40 | 60 | 98 |
| 16 | 34 | M | L knee | Malignant peripheral nerve sheath tumor | HG | 6.2 | 13.0 | 12 | 50 | 50 | 70 |
| 17 | 41 | M | Groin | Myxoid/round cell liposarcoma | IG | 12.1 | 8.8 | 13 | 0 | 10 | 80 |
| Mean | 9.72 | 10.24 | 11.14 | 17.35 | 39.41 | 60.06 | |||||
| Standard Deviation | 3.94 | 4.16 | 4.08 | 18.40 | 33.18 | 28.07 | |||||
HG – high grade; IG – intermediate grade
Preparation and administration of DCs
DCs were prepared using culture of mononuclear cells with GM-CSF and IL-4 as described previously 20. DC phenotype was defined as lineage (CD3, CD14, CD19, CD20, CD56) negative, HLA-DR positive cells. DCs (107 cells) were injected in a total volume of 1 ml.
Evaluation of DC migration
DCs were labeled with 111In and 5×106 cells were injected intratumorally prior surgery. Patients were split into three groups. One group has received injection of labeled DCs 24 hr before surgery, the second group 48 hr, and the third group 72 hr before surgery. DC labeling was performed at AnazaoHealth Corporation (Tampa, FL). The patients were imaged using a standard gamma camera (Siemens e-cam) at 20 minutes post injection and immediately prior to surgery. The camera has a 20% window at the 171 kev Indium energy peak and a 20% window at the 245 kev Indium energy peak. An intraoperative gamma probe (US Surgical Navigator) was used during surgical dissection for localization purposes. Multiple measurements were performed for each patient and the results were averaged. The ratios of tumor to background, and lymph nodes to background were compared. The ratio of counts of cases performed at different times was compared following correction for decay.
Evaluation of immune responses
Peripheral blood mononuclear cells (MNC) were collected from patients at different time points during the treatment (Fig. S1) and kept frozen at −180°C. All samples from one patient were analyzed simultaneously to reduce inter-experimental variability. Tumor cells were harvested by core biopsies of patients’ tumors prior the treatment. Tumor cell lysates (TCL) were prepared as described 21 by repeated snap freeze-thawing cycles and stored in liquid nitrogen. Lysates from patients MNC were used as controls. T-cell responses to TCL were assessed using IFN-γ ELISPOT22, 23 and proliferation assays.
To evaluate T cell response to survivin, DCs were generated from patients MNC using GM-CSF and IL-4 and infected with adenovirus-survivin (Ad-surv) as described previously to serve as stimulator cells 22. As a control we used an adenoviral construct containing empty vector (Ad-c). An individual patient was considered a responder to TCL or survivin if at any time point the response in the IFN-γ ELIPOT assay was higher than 30 spots per 2×105 cells and in the proliferation assay higher than 3000 CPM AND the response in IFN-γ ELISPOT or proliferation assays to TCL or Ad-surv was more than 2 SD higher than the response to the corresponding control lysate or Ad-c at the same time point AND 2 SD higher than the response to the same stimuli before start of the treatment.
Analysis of cell phenotype
Cell phenotype was evaluated by multicolor flow cytometry using a LSR II flow cytometer and indicated monoclonal antibodies obtained from Becton Dickinson. The following combinations of antibodies were used to identify cell populations:
Total population of DCs: Lineage− (Lin) (CD3, 14, 19, 56) HL-DR+
Mature DCs: Lin−HLA-DR+CD86+
Myeloid-derived suppressor cells (MDSC) Lin− HLA-DR− CD33+ and CD11b+ CD14− CD33+
Regulatory T cells (Treg) CD4+CD25+Foxp3+ and CD4+CD27− Foxp3+ cells.
Dead cells were eliminated from the analysis by using DAPI staining and in samples with intracellular staining by using Live/Dead Aqua solution (Invitrogen, Carlsbad, CA).
Histological and immunohistochemical evaluation of tumor tissues
Each tumor case was confirmed by pre-treatment biopsy with histology, immunohistochemistry, cytogenetic or molecular testing. Therapy response assessment included tumor necrosis, fibrosis and hyalinization as well as cytological changes. The viable tumor cells were estimated as 100% minus % of tumor necrosis/fibrosis/hyalinization.
In immunohistochemical studies the following antibodies were used: survivin (Novus Biological, Littleton, CO), CD3 (Ventana), CD4 (Cell Marque, Rocklin, CA), and CD8 (Ventana). The percentage of the immunoreactive cells from the representative tumor section was counted in at least 10 representative areas.
Statistical Analysis
We utilized GraphPad Prizm software for statistical analyses. The Wilcoxon Mann-Whitney test was used to determine a relationship between two groups with continuous variables. Univariate associations between frequency of responders and groups were analyzed by Fisher’s exact test considering small sample sizes. No attempt was made to adjust to multiple tests in this exploratory study. Any results appearing to be statistically significant require further confirmation. Two-sided tests were used for all calculations. The statistical significance for all analyses was determined with p<0.05.
Results
Clinical response to combination of EBRT with intratumoral DC administration
No significant (>= grade 2) toxicity was observed during combination EBRT/DC neo-adjuvant treatment. Post operative wound complications were observed in 5/17 patients (29.41%) defined using NCIC criteria 16. Twelve patients (70.6%) had no evidence of the disease for at least one year after the start of the treatment (time of follow-up). Among those 12 patients, 6 are disease-free for more than 2 years and 4 patients for more than 3 years. There were no local recurrences. Pathological evaluation of specimens collected during the surgery after completion of the EBRT/DC treatment demonstrated tumor necrosis/fibrosis/hyalinization with infiltration of lymphocytes (Fig. S2).
Immune responses in patients treated with EBRT and DC administration
Nine out of seventeen patients (52.9%) demonstrated a positive response to either TCL or survivin on at least one point after the start of treatment. Among nine responders three patients had immune responses detected at only one time point and six patients had more robust response detected at least at two time points (Table 2). Most of the responses to TCL were first detected 4–6 weeks after start of the treatment and became undetectable by weeks 11–16 (6–11 weeks after finish of the therapy). Immune responses to survivin lasted substantially longer (until weeks 22–42). Examples of immune responses are shown in Fig. 1.
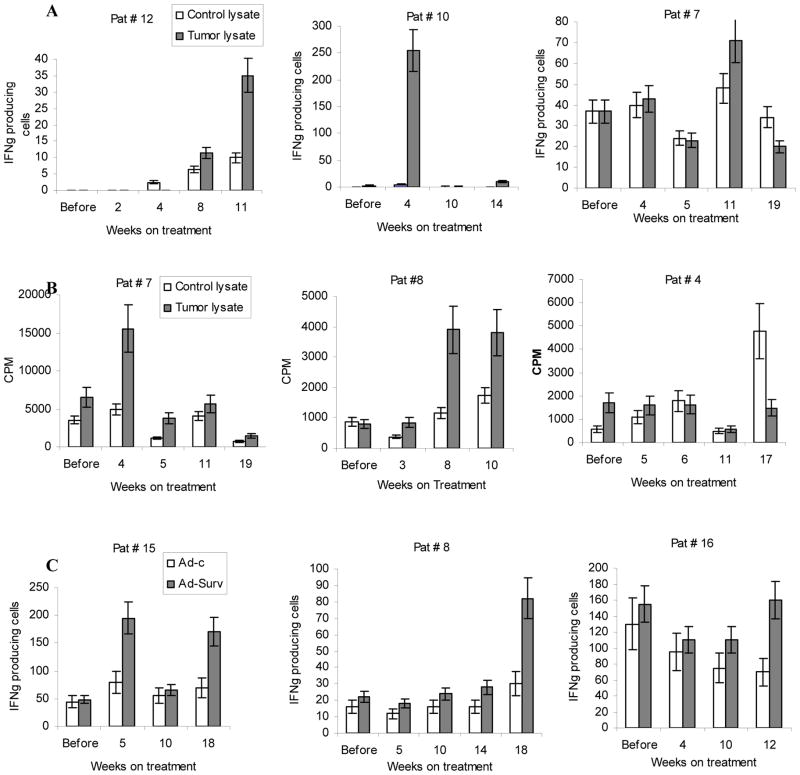
A. IFN-γ producing cells in ELISPOT assay. Each experiment was performed in quadruplicate. Patients 12 and 10 were considered as responders, patient 7 as non-responder. B. T-cell proliferation after stimulation with 100 μg control or tumor cell lysates. Cell proliferation was evaluated by uptake of 3[H]-thymidine. Each experiment was performed in triplicate. Patients 7 and 8 were considered as responders, patient 4 as non-responder. C. MNC from indicated patients were stimulated with DCs infected with control adenovirus (Ad-c) or Ad-surv. The number of IFN-γ producing cells was evaluated in ELISPOT assay. Each experiment was performed in quadruplicate. Patients 15 and 8 were considered as responders, patient 16 as non-responder.
Table 2
Immune responses in patients treated with EBRT and DCs
| Patients | Lysate IFN-γ | Lysate proliferation | Survivin IFN-γ | Overall response |
|---|---|---|---|---|
| 1 | None | None | None | None |
| 2 | None | None | None | None |
| 3 | None | None | None | None |
| 4 | None | None | None | None |
| 5 | None | None | Transient | Transient |
| 6 | None | None | None | None |
| 7 | None | Transient | None | Transient |
| 8 | None | Robust | Transient | Robust |
| 9 | None | Robust | Transient | Robust |
| 10 | Transient | Transient | None | Transient |
| 11 | None | None | Robust | Robust |
| 12 | Transient | Transient | Robust | Robust |
| 13 | None | None | None | None |
| 14 | None | None | None | None |
| 15 | None | None | Robust | Robust |
| 16 | None | None | None | None |
| 17 | Transient | Robust | None | Robust |
None – no immune response was detected at any time point;
Transient immune response – response detected at only one time point;
Robust immune response – response detected at least at two time points;
We asked whether immune responses to survivin correlated with the level of expression of this protein in tumor cells. Patients were split into two groups based on the survivin expression in tumor samples: 6 patients with low level of expression (less than 20% of tumor cells positive) (Fig. 2A right panel) and 10 patients with high level of expression (more than 20%) (Fig. 2A, left panel). None of 6 patients with a low level of survivin expression in tumor cells had detectable responses to survivin after completion of the treatment, whereas 6 out of 10 patients (60%) with high level of expression developed survivin-specific response to the treatment (p=0.026). No correlation between the level of survivin expression and response to TCL was observed (Fig. 2B).
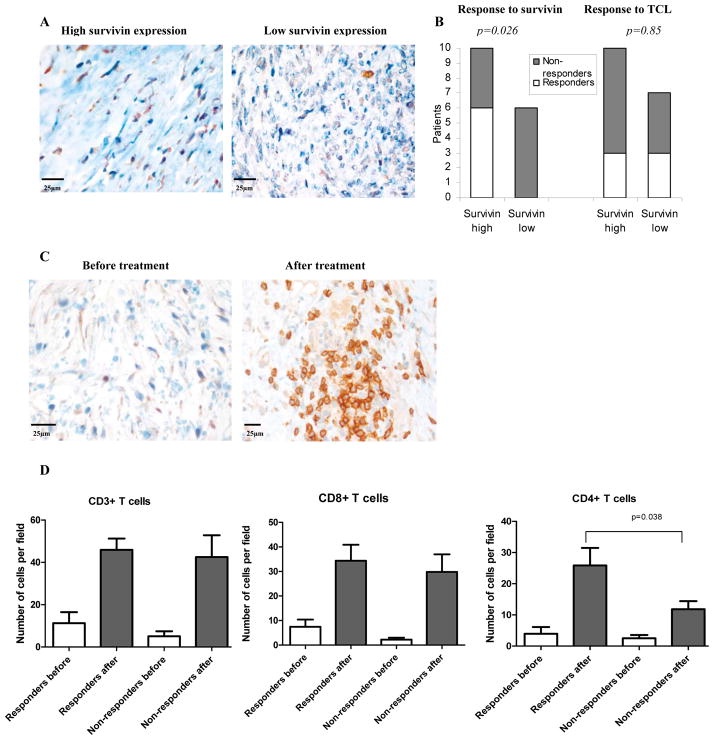
A. Example of staining with anti-survivin antibody of tumor tissues collected prior to start of the treatment. Left panel patient with high expression of surviving (>20%), right panel patient with low expression (<20%). B. Statistical analysis of link between survivin expression in tumors and survivin-specific immune response to the treatment. Patients with less than 20% survivin positive tumor cells were considered “survivin low” and more than 20% as “survivin high”. Non-responders – patients that did not develop immune response to TAA, responders – patients who had detectable response to either TCL or survin. Tumor samples were available from only 16 patients. C. Tumor tissues collected prior to the treatment (left panel) and during the surgery after the treatment (right panel) and stained with anti-CD3 antibody. D. Tumor tissues were collected before start of the treatment and during the surgery (4–5 weeks after the treatment). For the analysis patients were split into three groups: those with robust response to vaccination (n= 5), transient response (n=3) and non-responders (n=5). The number of T-lymphocytes in tumor tissues was evaluated by immunohistochemistry and calculated per high power field (x400) after counting at least 10 randomly selected fields. For each patient group the difference between the number of cells in tumor tissues after the treatment was significantly higher than before (p<0.05). The differences between groups were not statistically significant except as shown in the figure.
Dramatic accumulation of T-lymphocytes in tumor tissues after the treatment was observed (p<0.01) (Fig. 2C, D). Infiltration of CD4+ T cells in tumor tissues from patients who developed an immune response to the treatment was significantly (p=0.026) higher than in patients who did not respond to the treatment (Fig. 2D). No correlation between T cell (including CD4+T cells) infiltration and disease progression was detected.
Correlation of immune responses to the treatment with the levels of immune suppressive myeloid and lymphoid cells
Immune suppressive myeloid cells (MDSC) and lymphocytes (Treg) play a major role in inhibition of immune responses in cancer. We investigated a connection between the levels of MDSC and Tregs and immune responses to TCL and survivin. We used two combinations of markers to identify MDSC: Lin−HLA-DR−CD33+ and CD14−CD11b+CD33+ 24–26. Both of these sets of markers showed similar results. Patients who did not develop immune responses had higher levels of MDSC than healthy volunteers. The differences became significant after start of the treatment. In contrast, the presence of MDSC in patients who developed immune responses remained low. As a result after the treatment the differences in the proportion of MDSC between responders and non-responders were statistically significant (Fig. 3A). The presence of DCs in the patients was not different from control and was similar between responders and non-responders (Fig. 3B). The proportion of Treg in patients was slightly increased. However, this increase was not statistically significant. No differences between responders and non-responders were found (Fig. 3C). Treatment did not significantly affect the absolute number of neutrophils, or lymphocytes. There was only a temporary increase (p=0.03) in the number of monocytes. The differences in the absolute numbers of MDSC, DCs, and Tregs between the groups were similar to those observed in the proportion of those cell populations (Fig. S3).
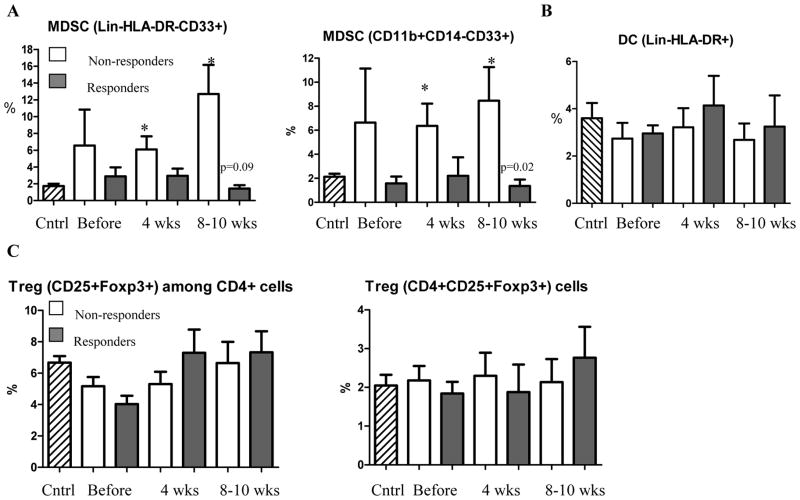
A. Proportion of MDSC in patients. Two antibody cocktails (indicated on the graphs) were used to identify MDSC. Cntrl – values in 12 healthy volunteers. * - statistically significant differences from control. P values for statistically significant differences between the groups are shown on the graph. B. Proportion of DCs in cancer patients. C. Proportion of Treg in patient.
In healthy volunteers the upper 95% confidence interval of the proportion of MDSC was 2.678%. To assess correlation between MDSC level and immune responses all patients were split into two groups based on the values of MDSC in healthy donors: patients who had MDSC proportion within 95% CI of healthy control during or after the treatment and patients who had increased level of MDSC during or after the treatment. Of 7 patients with MDSC proportion within the CI of control group, 6 developed immune response to TCL or survivin. Out of 8 patients with elevated level of MDSC only 1 developed immune response (p=0.01). In two patients analysis could not be performed.
Evaluation of DC migration after intatumoral administration
In order to assess the kinetic of DC migration after intratumoral injection DCs were labeled with 111In that is suitable for analysis of DC migration 27. The maximum duration of observation of DC migratory activity depends on the half-life of the radioisotope (72 hr). To minimize inter-patient variability, radioactivity in the center of the tumor was used as a reference point. As a control in each patient we used the ratio of background radioactivity (distant part of the body) to the center of the tumor. Twenty-four hours after injection of DCs no differences between the level of radioactivity at the edge of the tumor or draining lymph nodes and background was detected, indicating lack of measurable DC migration (Fig. 4A.B). In contrast, 48 hr after DC injection significant radioactivity was detected in both edge of the tumor and lymph nodes (p<0.05). It became significantly higher 72 hr after DC injection indicating migration of DCs from the site of injection (Fig. 4B).
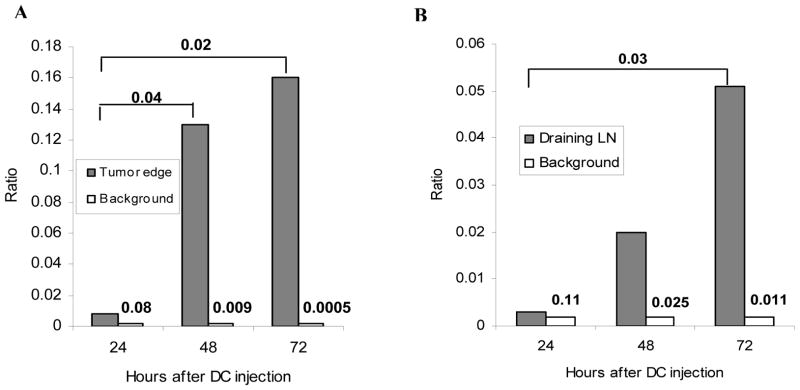
DCs were labeled with 111In and injected intratumorally at different time prior to surgery. 24 hr group included 5 patients, 48 hr group – 4 patients, and 72 hr group – 6 patients. A. Ratio of radioactivity at the edge of the tumor to the center of the tumor. B. Ratio of radioactivity at the draining lymph nodes to the radioactivity at the center of the tumor. Background – ratio of radioactivity at distant site to the radioactivity at the center of the tumor. P values are shown on the graph
Discussion
Herein, we report the clinical and immunologic results of the first phase I/II trial in humans combining localized fractionated EBRT with intratumoral administration of DCs in patients afflicted with high-risk STS. Treatment was well tolerated by all patients. The frequency of wound complications (29.1%) was similar to that reported for EBRT alone 16, 28. The proportion of patients remaining disease free one year after the treatment (70.6%) was similar to that reported for patients with high-grade large STS 16, 29. Thus, preliminary evaluation indicates that addition of DC injection to EBRT did not worsen side effects and clinical outcome of conventionally fractionated pre-operative EBRT followed by surgery.
One important finding of this study is that no patient had significant tumor-specific immune responses before combined EBRT/DC therapy. Only after treatment the development of tumor-specific immune responses was observed in 52.9% of patients. This trial was not designed to test what the individual components of therapy would induce immune responses in vivo in human; however murine data from our and other groups 7–12 suggest that individually either DC or radiation is not sufficient to generate curative tumor-specific immune response or infiltration of tumor site with T-lymphocytes. We believe that these results are promising enough to warrant further trials.
Adequate evaluation of cancer vaccines is impossible without measuring immune responses to tumor-specific antigens. One of the most common approaches is to use whole tumor cell lysates. However, it is more informative to complement this analysis using defined antigens specific for the tumor cells. Survivin can be very effective in this role. Survivin is an antiapoptotic protein30. Overexpression of survivin in tumor cells results in increased expression of survivin epitopes on the tumor cell surface in association with MHC class I molecules, thus representing targets for CTL 22, 31, 32. Survivin is expressed at a high level in many common human cancers but not in normal, terminally differentiated adult tissues 30, 33. In previous studies the expression of survivin has been evaluated in malignant tissue samples from 63 STS patients as well as from a panel of tumor cell lines 34, 35. High survivin levels were detected in tumor samples from more than 75% of patients with stage II and from more than 90% patients with stage III STS 35. In our study 62.5% of patients had high level of survivin expression in tumors. None of the patients with low or undetectable levels of survivin developed immune responses to this protein, whereas 60% of patients with high levels of survivin expression developed specific immune responses. These results are important since overexpression of survivin in cancer patients correlates with more aggressive disease and poor survival 36, 37. Thus, survivin can serve as a good surrogate marker for immunological monitoring of the treatment.
Intratumoral DC administration during the ongoing EBRT treatment raises the concern that DCs subjected to radiation would be functionally impaired. Mouse experiments have shown rapid migration of injected DC’s from the tumor site to regional nodes 7. There are data that radiation doses below 20 Gy do not affect the antigen-presenting function of DCs in vitro38 and that a radiation dose at 7.5 Gy did not affect DC function in vivo 39. The radiation dose in our trial was 1.8 Gy per fraction, which was substantially lower than the amount needed to inactivate DCs. To minimize the possible damage, DCs were injected on Friday mornings after completing the radiation fraction for that day. The next fraction was administered 72 hours later on Monday afternoon. However, it was important to evaluate DC migration in direct experiments. Our data indicate that 24 hr was not enough for significant numbers of DCs to migrate out of tumors. However, after 48 hr more than 10% of DCs were located at the edge of the tumor and 2% in draining lymph nodes. After 72 hr the amount of radioactivity in draining lymph nodes more than doubled indicating that DCs were able to migrate from tumors into lymph nodes. These data were obtained in the absence of radiation. It is possible that migration of DCs from recently irradiated tumors is higher. Our data also suggested that change in fractionation of EBRT may be warranted to provide more time for DC migration out of tumors.
Thus, our data demonstrate that combination of EBRT and intratumoral DC administration could be a promising method of the treatment of patients with high-risk STS and provide rationale for more extensive clinical studies in this and other types of cancer.
Acknowledgments
This work was supported by grant from “Gateway for Cancer Research (to DIG) This manuscript is dedicated in memory of Erin Bryant, our nurse, colleague, and friend
Footnotes
This work was presented at the 51st ASTRO meeting and was awarded clinical winner of the resident clinical/basic science research award.
Conflict of interest notification
No Actual or potential conflicts of interest exist
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ijrobp.2010.12.068
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4241354?pdf=render
Citations & impact
Impact metrics
Article citations
Radiation-induced tumor immune microenvironments and potential targets for combination therapy.
Signal Transduct Target Ther, 8(1):205, 19 May 2023
Cited by: 17 articles | PMID: 37208386 | PMCID: PMC10199044
Review Free full text in Europe PMC
Application of nanotechnology in reversing therapeutic resistance and controlling metastasis of colorectal cancer.
World J Gastroenterol, 29(13):1911-1941, 01 Apr 2023
Cited by: 2 articles | PMID: 37155531 | PMCID: PMC10122790
Review Free full text in Europe PMC
Current research and management of undifferentiated pleomorphic sarcoma/myofibrosarcoma.
Front Genet, 14:1109491, 16 Feb 2023
Cited by: 5 articles | PMID: 36873946 | PMCID: PMC9978151
Review Free full text in Europe PMC
Image-guided intratumoral immunotherapy: Developing a clinically practical technology.
Adv Drug Deliv Rev, 189:114505, 23 Aug 2022
Cited by: 8 articles | PMID: 36007674 | PMCID: PMC10456124
Review Free full text in Europe PMC
Survival comparison between postoperative and preoperative radiotherapy for stage I-III non-inflammatory breast cancer.
Sci Rep, 12(1):14288, 22 Aug 2022
Cited by: 7 articles | PMID: 35995985 | PMCID: PMC9395522
Go to all (75) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Case Report: In Situ Vaccination by Autologous CD16+ Dendritic Cells and Anti-PD-L 1 Antibody Synergized With Radiotherapy To Boost T Cells-Mediated Antitumor Efficacy In A Psoriatic Patient With Cutaneous Squamous Cell Carcinoma.
Front Immunol, 12:752563, 23 Dec 2021
Cited by: 14 articles | PMID: 35003064 | PMCID: PMC8733210
Does an Algorithmic Approach to Using Brachytherapy and External Beam Radiation Result in Good Function, Local Control Rates, and Low Morbidity in Patients With Extremity Soft Tissue Sarcoma?
Clin Orthop Relat Res, 476(3):634-644, 01 Mar 2018
Cited by: 3 articles | PMID: 29443850 | PMCID: PMC6260030
[Management of soft tissue sarcomas in first isolated local recurrence: a retrospective study of 83 cases].
Cancer Radiother, 8(5):279-287, 01 Oct 2004
Cited by: 2 articles | PMID: 15561593
Concurrent ifosfamide-based chemotherapy and irradiation. Analysis of treatment-related toxicity in 43 patients with sarcoma.
Cancer, 92(6):1550-1555, 01 Sep 2001
Cited by: 19 articles | PMID: 11745234
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: K24 CA128953
Grant ID: P30 CA076292





