Abstract
Free full text

Targeting IL-12/IL-23 by Employing a p40 Peptide-Based Vaccine Ameliorates TNBS-Induced Acute and Chronic Murine Colitis
Abstract
Interleukin (IL)-12 and IL-23 both share the p40 subunit and are key cytokines in the pathogenesis of Crohn’s disease. Previously, we have developed and identified three mouse p40 peptide-based and virus-like particle vaccines. Here, we evaluated the effects and immune mechanisms of the optimal vaccine in downregulating intestinal inflammation in murine acute and chronic colitis, induced by intrarectal administrations of trinitrobenzene sulfonic acid (TNBS). Mice were injected subcutaneously with vaccine, vaccine carrier or saline three times, and then intrarectally administered TNBS weekly for 2 wks (acute colitis) or 7 wks (chronic colitis). The severity of colitis was evaluated by body weight, histology and collagen and cytokine levels in colon tissue. Th1 and Th17 cells in mesenteric lymph nodes (MLN) were determined. Our results showed the vaccine induced high level and long-lasting specific IgG antibodies to p40, IL-12 and IL-23. After administrations of TNBS, vaccinated mice had significantly less body weight loss and a significant decrease of inflammatory scores, collagen deposition and expression of p40, IL-12, IL-23, IL-17, TNF, iNOS and Bcl-2 in colon tissues, compared with carrier and saline groups. Moreover, vaccinated mice exhibited a trend to lower percentages of Th1 cells in acute colitis and of Th17 cells in chronic colitis in MLN than in controls. In summary, administration of the vaccine induced specific antibodies to IL-12 and IL-23, which was associated with improvement of intestinal inflammation and fibrosis. This suggests that the vaccine may provide a potential approach for the long-term treatment of Crohn’s disease.
INTRODUCTION
Studies have provided evidence that the pathogenesis of inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, is associated with genetic factors, environmental factors, enteric flora and immunological abnormalities (1). It has been accepted widely that Crohn’s disease is caused by an overly aggressive Th1 immune response and, recently discovered, excessive IL-23/Th17 pathway activation to bacterial antigens in genetically predisposed individuals (1–3). These Th1 cytokines, such as IL-12 and the IL-23/ Th17 pathway, could be critical in generating and perpetuating the chronic intestinal inflammation of Crohn’s disease (3,4).
In active Crohn’s disease, the expression of IL-12 receptors on mucosal cells is increased (5). Various immunohistological studies indicate that in situ IL-12 is overproduced by macrophages in Crohn’s disease, and that macrophages isolated from the inflammatory lesions of patients with Crohn’s disease produce increased amounts of IL-12 ex vivo (6). In TNBS-induced colitis mice, administration of an IL-12 antagonist (the IL-12p40-IgG2b fusion protein) (7) or anti-mouse IL-12 antibodies (8) abrogates mucosa inflammation, prevents body weight loss and restores a nearly normal histological appearance of the colon, through a mechanism of induction of Fas-mediated apoptosis of Th1 cells (8). This treatment also reduces TNF levels and increases IL-10 secretion in mice (7).
More recently, studies have highlighted the roles of IL-23/Th17 pathway in the pathogenesis of Crohn’s disease (9–13). Yen and his colleagues have demonstrated that the development of colitis is suppressed by IL-23p19 (p19 is the IL-23 specific subunit) deficiency but not IL-12p35 (p35 is the IL-12 specific subunit) deficiency in IL-10−/−mice, and that administration of IL-23 accelerates the onset of colitis and promotes inflammation through an IL-17-and IL-6-dependent mechanism (9). Anti-IL-23 monoclonal antibody can prevent and reverse active colitis in a T-cell-mediated colitis mouse model, with the downregulation of a broad array of inflammatory cytokines and chemokines in the colon (14). Recently, a genome-wide association study has reported IL-23R as an IBD gene that is highly associated with Crohn’s disease (15). Even so, these findings do not exclude the role of an exaggerated Th1 response in Crohn’s disease, since IL-12/ IFN-γ and IL-23/IL-17 may be parallel pathways involved in inflammatory responses (2).
These observations in mice are supported by successful clinical trials. Monoclonal antibodies (mAb) against those overproduced endogenous Th1 and Th17 cytokines have emerged as a potential treatment in IBD (16–18). Administration of a human mAb against p40, the subunit shared by IL-12 and IL-23 (4), induced significant clinical responses and remissions in patients with active Crohn’s disease (19). Although the therapy with mAb may be useful clinically (3,16), these agents all act as passively administered antagonists with short half-lives. Repeated intravenous (i.v.) or subcutaneous (s.c.) injections with large doses of mAb are required to maintain the effects. Side effects include high rates of acute infusion reactions and the development of antibodies to the infused mAb, which reduces the effectiveness of the treatment and occurs in 61% of the patients receiving infliximab (an mAb to TNF) therapy (20,21) and also in patients receiving the mAb to IL-12/IL-23 p40 treatment (19). Another highly relevant concern is the extremely high cost associated with such passively administered therapeutics.
To overcome these disadvantages, a new active immunization strategy, employing vaccines that target overex-pressed endogenous molecules, is being investigated. This new strategy may offer long-term efficacy with fewer adverse effects (22,23). To break immune tolerance, these vaccines generally are made by inserting heterogeneous T cell epitopes into the target self-cytokine (24), or linking the intact self cytokine to a foreign carrier protein (25).
Previously, we have developed and identified three recombinant mouse IL-12/IL-23 p40 peptide-based virus-like particle vaccines (C, D and F), which induce high levels of specific antibodies against p40, IL-12 and IL-23 (26). The vaccine is constructed by inserting a peptide derived from mouse p40 subunit into a carrier protein, hepatitis B core antigen (HBcAg), using gene engineering methods. In this study, we systemically evaluated the effectiveness of the best vaccine (F) in the attenuation of TNBS-induced both acute and chronic murine colitis. We further explored the possible immune mechanisms of vaccine-mediated improvement, especially Th1 and IL-23/Th17 responses.
MATERIALS AND METHODS
Animals
Female BALB/c mice (7–8 wks old), purchased from Charles River Laboratories (Saint-Constant, Sherbrooke, QC, Canada), were maintained at the Central Animal Care Services, University of Manitoba. All protocols used were approved by the University Animal Ethics Committee.
Protocols of Vaccine Immunization and Induction of Colitis
Vaccine F, which contains a peptide of nine amino acids and a carrier protein HBcAg, was prepared using the methods previously described (26) and used in TNBS-induced colitis, a widely used animal model that exhibits characteristics of Crohn’s disease (27).
The vaccine was evaluated firstly in vivo in a TNBS-induced acute colitis in which mice were injected s.c. 3× at a 2-wk interval with vaccine, vaccine carrier HBcAg or saline (100 μg, 25 μg and 25 μg in 200 μL, respectively) (n = 20). Two weeks later, mice were intrarectally challenged with TNBS (Sigma-Aldrich, Oakville, ON, Canada) twice (1.5 mg and 2.0 mg, respectively) at a 1-wk interval to induce acute colitis (Figure 1A) according to the previous description (27).
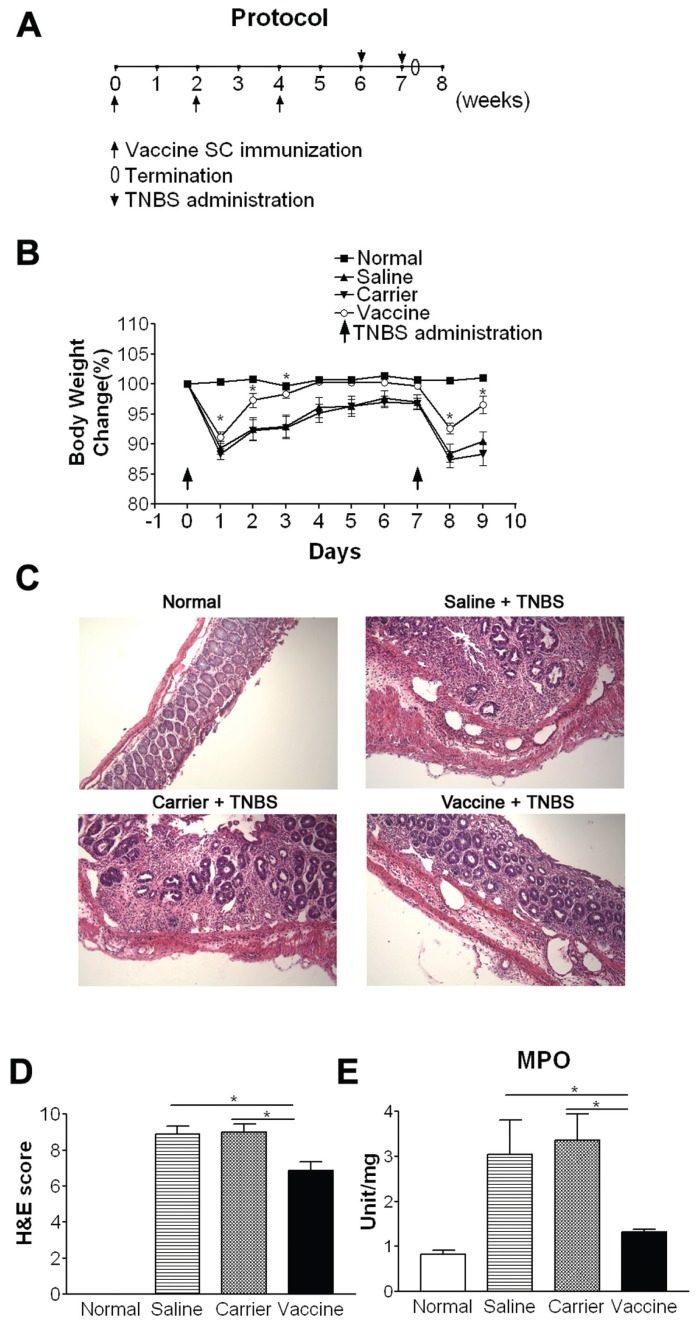
Vaccine ameliorates TNBS-induced acute colitis. (A) Protocol. (B) Body weight. (C) Colon tissue sections (H&E staining). (D) Semiquantitative analysis of histological inflammation. (E) MPO activity. *P < 0.05; **P < 0.01; ***P < 0.001.
The vaccine was further evaluated in TNBS-induced chronic colitis, in which mice were immunized 4× with vaccine or carrier (doses described in the legend of Figure 2A) or saline (n = 12). Two weeks after the third vaccination, chronic colitis was induced by seven weekly administrations of increasing doses of TNBS (1.0–2.5 mg) (Figure 2A).
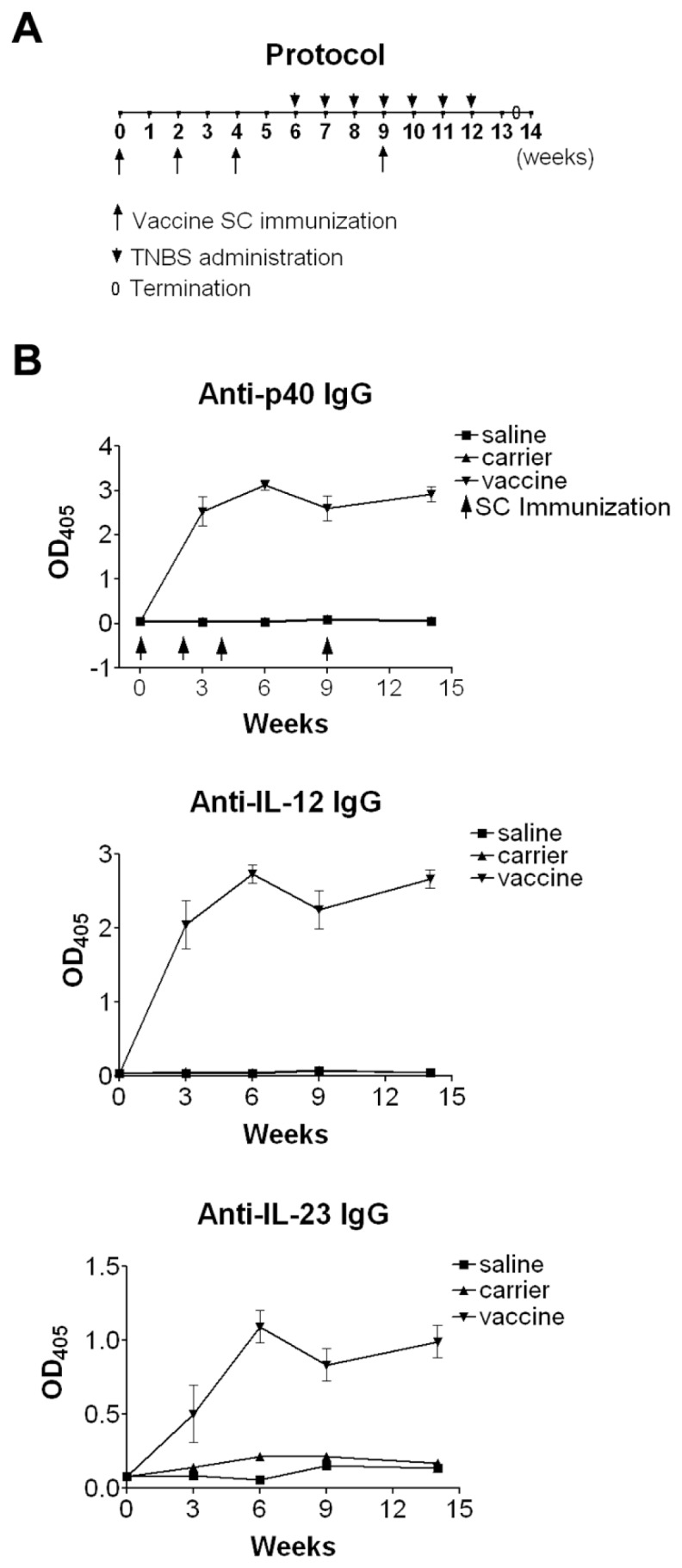
Immunization with the p40 vaccine induces specific IgG antibodies to p40, IL-12 and IL-23. (A) Protocol. Mice were subcutaneously primed with 100 μg/ 200 μL of vaccine or carrier or 200 μL of saline, and boosted at wks 2, 4 and 9 with 25 μg/200 μL of vaccine or carrier or 200 μL saline. Chronic colitis was induced by seven weekly administrations of TNBS. (B) Serum specific IgG antibodies. Sera were obtained at the indicated weeks and diluted 1:200 for determination of specific IgG levels by ELISA, in which p40, IL-12, or IL-23 (0.25 μg/mL) were coated on the plates, separately. Data represent one of at least two repeated experiments.
Two days (for acute colitis) or 10 days (for chronic colitis) after the last TNBS delivery, mice were euthanized. Colons, mesenteric lymph nodes and blood samples were collected and processed according to different assays. The evaluations were repeated once, and similar results were found.
Body Weight
Mouse body weight was monitored daily in acute colitis (before the delivery of TNBS on the delivery day and in the morning of remaining days) and weekly in chronic colitis before each delivery of TNBS.
Histological Examination
Ten percent buffered formalin fixed and paraffin-embedded colon sections were cut and stained with H&E or Masson trichrome. Histological scoring was evaluated by a pathologist blinded to the source of treatment based on the method previously described (28). During each histological examination, three different parameters were estimated: severity of inflammation, depth of injury and crypt damage. All values were added to a sum, in which the maximum possible score was 10.
Soluble Collagen Assay
For quantitative measurement of collagen, a marker of chronic inflammation, colons were homogenized in 0.5 mol/L acetic acid containing 1 mg of pepsin (at a concentration of 10 mg of tissue/5 mL of acetic acid solution). The resulting mixture was then incubated and stirred for 24 h at 4°C. Total soluble collagen content of the mixture was determined with a Sircol Collagen Assay Kit (Biocolor Ltd, Westbury, NY, USA) (27). Acid soluble type I collagen supplied with the kit was used to generate a standard curve.
Myleroperoxidase (MPO) Activity Assay
MPO activity, an index of neutrophilic infiltration, was assayed using the method described previously (29). The colon tissue (100 mg tissue/mL) was homogenized in potassium phosphate buffer (50 mmol/L, pH 6.0,) containing 5% hexadecyltrimethylammpnium bromide, and then subjected to three cycles of freezing and thawing. After centrifuging for 15 min at 18,300g at 4°C, the supernatant was transferred to a 96-well plate (7 μL per well, triplicate each sample). The enzyme reaction was carried out by adding 200 μL of phosphate buffer (50 mmol/L, pH 6.0) containing 0.167 mg/mL o-dianisodine hydrochloride (Sigma-Aldrich) and 0.0005% H2O2. The kinetics of absorbance changes at 460 nm was measured at 0, 30 and 60 min in a microtiter reader. The MPO activity is presented as MPO units per gram of tissue.
Measurements of Antibodies and Cytokines by ELISA
Frozen colonic samples were homogenized mechanically in buffer containing 1 mol/L Tris-HCl, 3 mol/L NaCl, and 10% Triton supplemented with pro-tease cocktail (Sigma-Aldrich). Samples were then frozen (−70°C) and thawed (37°C) 3×, followed by centrifugation at 18,300g for 30 min at 4°C. Supernatants were frozen at −70°C until assay (13).
Serum p40-, IL-12- and IL-23-specific IgG levels were assayed by using ELISA techniques established in our laboratory (30,31). The results obtained from each sample were expressed using optical density at 405 nm (OD405). The results obtained from sera pooled from each group were expressed using “titer,” the reciprocal of the highest dilution in which the OD405 was twice that of the corresponding control serum when its OD405 was 0.10. Cytokine concentrations in colon tissues were measured by ELISA according to the manufacturer’s instructions. IL-12p40, IL-17, IFN-γ, IL-13 and TNF-β were purchased from BD Biosciences (Mississauga, ON, Canada); IL-23 and IL-10 were from eBioscience (San Diego, CA, USA); TGFβ1 was from R&D Systems (Burlington, ON, Canada).
Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Briefly, total RNA was extracted from colons by Trizol (Invitrogen, Burlington, ON, Canada) according to the manufacturer’s instruction. Complementary DNA was synthesized using iScript cDNA synthesis kit (Bio-Rad, Mississauga, ON, Canada). Primer sequence was as follows: 5′-GGCAAATTCAACGGCACAGT-3′ and 5′-AGATGGTGATGGGCTTCCC-3′ for GAPDH; 5′-ACTTCGCAGAGATGT CCAGTCA-3′ and 5′-TGGCAAAGCG TCCCCTC-3′ for Bcl-2; 5′-AACATCTGGA ACCACGGGCACTAT-3′ and 5′-GCATT GCTTGAGGCTGCGTATGAT-3′ for Foxp3; 5′-GTGACGGCAAACATGACTTCAG-3′ and 5′-GCCATCGGGCATCTGGTA-3′ for iNOS; 5′-TTCCGAATTCACTGGAGCCT CGAA-3′ and 5′-TGCACCTCAGGGAAG AATCTGGAA-3′ for TNF; 5′-CTCAC CTGTGACACGCCTGA-3′ and 5′-CAGGA CACTGAATACTTCTC-3′ for IL-12/IL-23p40; 5′-TGAATTCCCTGGGTGAGAAG CTGA-3′ and 5′-TGGCCTTGTAGACAC CTTGGTCTT-3′ for IL-10. Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) was assayed in special optical tubes of 96-well microtiter plates with ABI PRISM 7700 Sequence Detector Systems (Applied Biosystems, Foster City, CA, USA). The reaction mixture consisted of SYBR Green PCR master mix (Applied Biosystems). PCR retain conditions were 50°C for 2 min, 95°C for 10 min and then 40 cycles at 95°C for 15 s, 60°C for 15 s and 72°C for 35 s, followed by a dissociation stage. Gene expression was calculated relative to the housekeeping gene GAPDH.
Intracellular Staining for Identification of Th1 and Th17 Cells
To examine the percentages of Th1 and Th17 cells, single-cell suspensions containing 1 × 106 mesenteric lymph node (MLN) cells were cultured and stimulated for 7 h with 50 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin (Sigma-Aldrich), with brefeldin A added for the last 4 h of culture. Cells were harvested, washed and stained with anti-CD4 (eBioscience). Surface-stained cells were fixed (2% paraformaldehyde) and resuspended in permeabilization buffer (0.5% saponin). This was followed by staining for intracellular IL-17 or IFN-γ (eBioscience). Cells were analyzed using a FACSCalibur (BD Biosciences).
Statistical Analysis
Values were expressed as mean ± SD. Differences between experimental groups were assessed by one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test (GraphPad Software, San Diego, CA, USA). P values <0.05 were considered statistically significant.
RESULTS
Vaccine Induces High Levels of Specific IgG Antibodies Against p40, IL-12, and IL-23, and Improves Intestinal Inflammation in Acute Colitis
After three immunizations, the p40 peptide-based vaccine induced significantly high levels of specific antibodies against p40, IL-12 and IL-23. The titers of the serum pooled from vaccinated mice were 150,000 for p40-specific IgG, 80,000 for IL-12-specific IgG and 10,000 for IL-23-specific IgG. This was similar to our previous results (26).
After mice developed high levels of antibodies against p40, IL-12 and IL-23, they were intrarectally challenged with TNBS twice to induce acute colitis (see Figure 1A). As shown in Figure 1B, after each TNBS administration, mice of saline and carrier groups show obvious body weight loss, but vaccine-treated mice have significantly improved body weight loss (P < 0.05) and their body weight is quickly recovered to the level of normal mice on days 3–7.
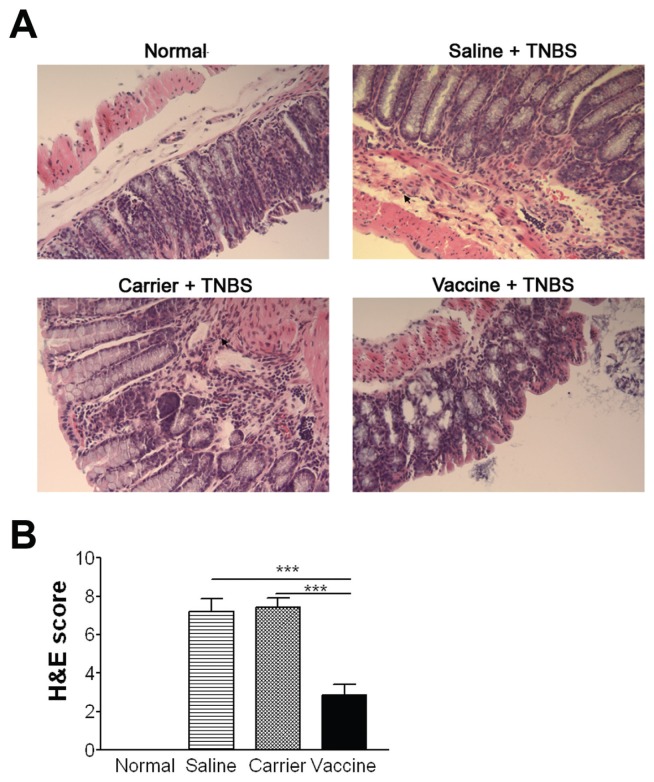
Histological analysis revealed that untreated mice (saline and carrier groups) exhibited severe thickening of the muscularis, a marked mucosal inflammatory cell infiltrate, many focal lesions characterized by epithelial cell sloughing, increased neutrophilic infiltrate, and complete loss of crypt architecture, resulting in high scores (Figure 1C, D). In contrast, colons from vaccine-treated mice showed much less inflammation than controls (P < 0.05), indicating that vaccine treatment could ameliorate intestinal inflammation significantly.
The effectiveness of vaccination, seen in histological analysis, was further confirmed quantitatively by the results obtained from the measurement of MPO activity, an enzyme specific to granulocyte lysosomes, and, therefore, directly correlated with the number of granulocytes. MPO activity was increased about fourfold in carrier and saline groups compared with normal mice. In contrast, there was a significant decrease in the MPO activity in vaccine-treated mice (P < 0.05) that was close to normal mice (Figure 1E).
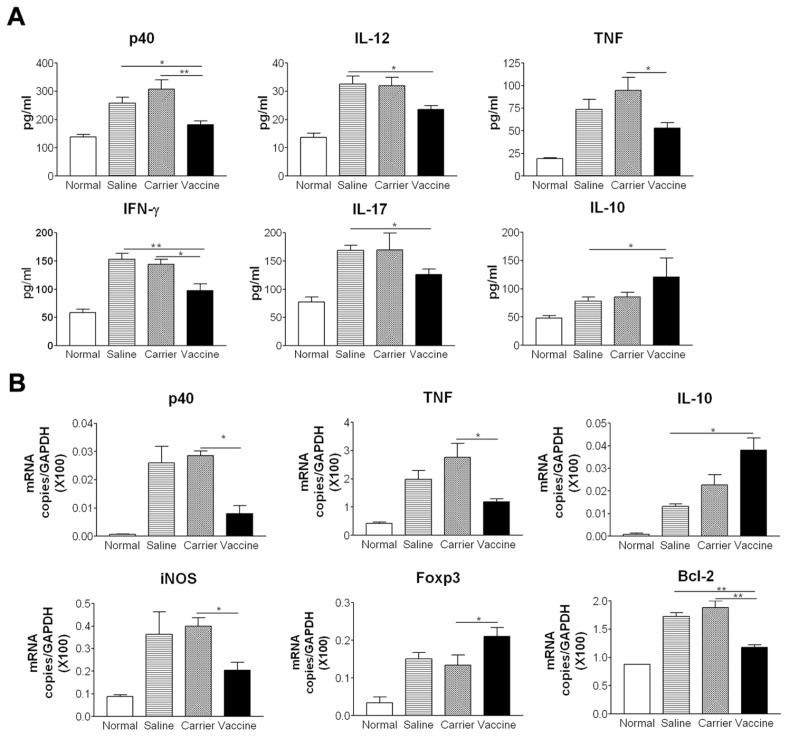
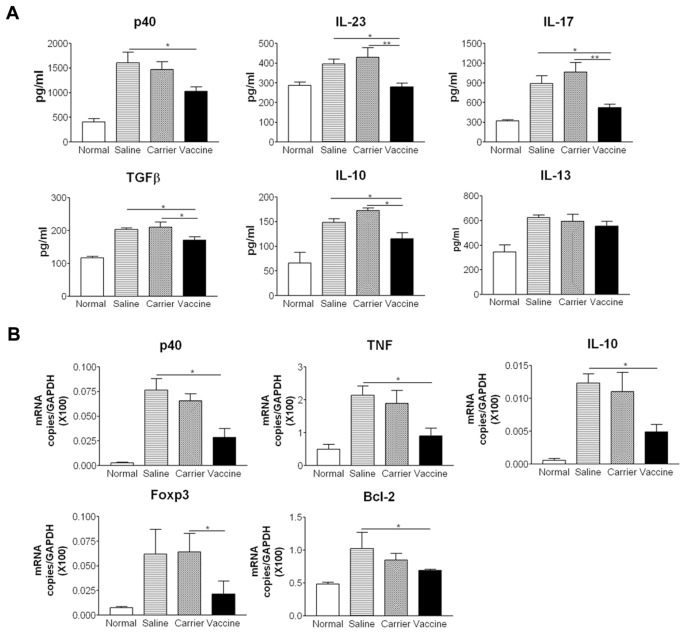
We examined cytokine expression levels in colon tissue by ELISA. The results showed that vaccine treatment suppressed the protein levels of p40, IL-12, IFN-γ, IL-17 and TNF, but increased IL-10 production compared with saline and carrier groups (P < 0.05) (Figure 3A). IL-23 levels were not elevated significantly in any colitis group (data not shown). We further examined cytokine and proinflammatory molecules by using real time RT-PCR. Vaccine treatment also resulted in a decrease in the mRNA expression levels of p40, TNF, proinflammatory molecules inducible nitric oxide synthase (iNOS) and anti-apoptotic molecules Bcl-2 (Figure 3B). Consistent with the increased protein levels of IL-10 measured by ELISA, the mRNA levels of IL-10 and Foxp3 were increased in the vaccine-treated group. Taken together, these data indicate that the p40 vaccine ameliorates intestinal inflammation in TNBS-induced acute colitis by inhibiting Th1 cytokines and inflammatory molecule expression and increasing IL-10 production.
Vaccine Induces Specific IgG Antibodies and Improves Body Weight Loss in Chronic Colitis
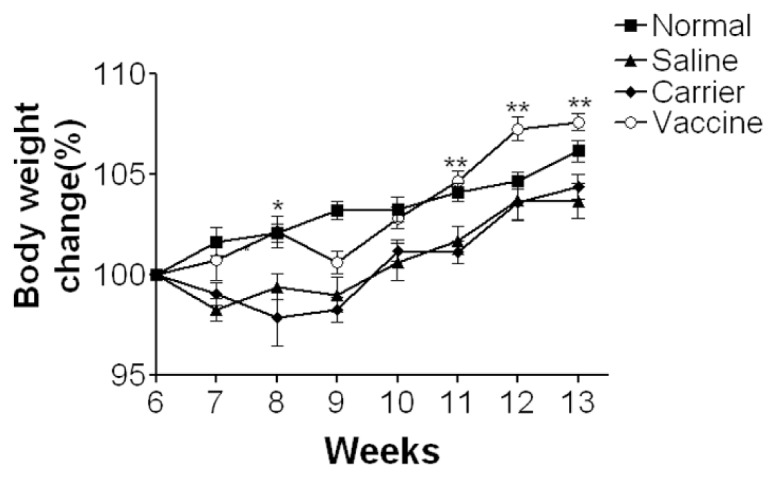
Since IBD is a chronic disease, it would be more important and relevant to evaluate this vaccine strategy in a chronic colitis model, so we further explored the effects of the vaccine in TNBS-induced chronic model. As shown in Figure 2B, after two immunizations, the vaccine induces significantly high levels of specific antibodies against p40, IL-12 and IL-23, which reach a plateau after three immunizations and levels remain high in the entire experiment. These antibodies were undetectable in carrier and saline groups (P < 0.001). The titers of the serum pooled from vaccinated mice were 200,000 for p40- specific IgG, 150,000 for IL-12-specific IgG and 20,000 for IL-23-specific IgG, which were slightly higher than those in acute colitis. This may be owing to the use of different batches of mice in the two experiments as immune responses are individually different. After mice developed high levels of antibodies, they were intrarectally challenged with TNBS seven times to induce a chronic colitis (see Figure 2A). Consistent with the findings in acute colitis, in this chronic colitis model mice in saline and carrier groups showed obvious body weight loss after the first administration of TNBS that lasted from wk 6 to wk 9, and then gradually regained weight and recovered from other signs of chronic illness despite continued TNBS administrations after wk 10 (Figure 4). In contrast, vaccine-immunized mice showed no obvious body weight loss compared with saline and carrier groups (P < 0.01), and their body weight changes were similar to those of normal healthy mice and even higher than normal mice between wk 12 and wk 13. These results indicated that the vaccine could lessen body weight loss in mice with TNBS-induced chronic colitis.
Vaccine Suppresses Intestinal Inflammation in Chronic Colitis
In addition to the improvement of body weight loss, vaccine treatment also ameliorated other manifestations of chronic colitis. Histological analysis revealed that colons from saline and carrier groups exhibited obvious inflammation, including inflammatory cell infiltration, goblet cell reduction and distorted architecture (Figure 5A). In contrast, colon tissues of vaccine-treated mice showed much less inflammation than controls. The vaccine-induced improvement of colon inflammation was further confirmed by semiquantitative analysis in which vaccine-treated mice had significantly lower inflammatory scores than saline and carrier groups (P < 0.001) (Figure 5B).
Vaccine Inhibits Fibrosis in Chronic Colitis
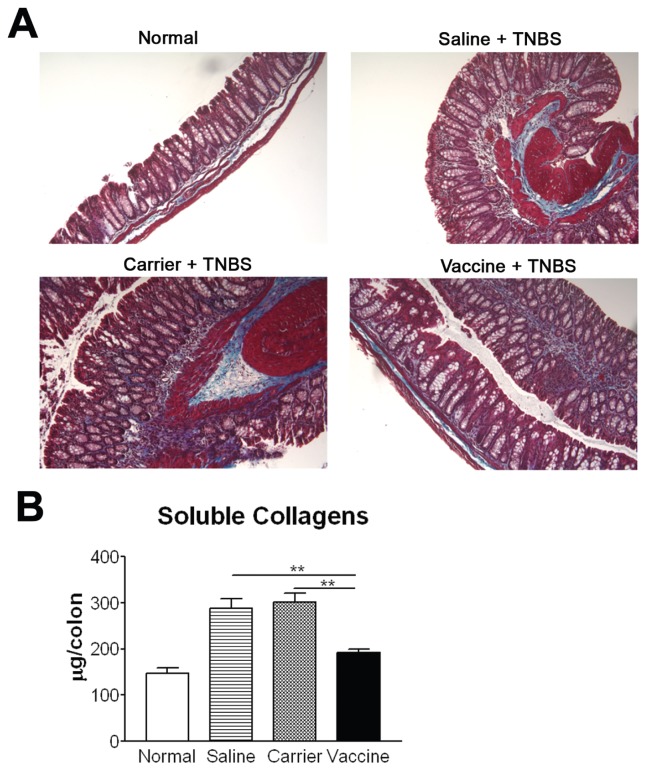
As fibrosis is an important indicator for chronic inflammation, colon collagen deposition was evaluated. As shown in Figure 6A, compared with the normal group, the amount of collagen is increased in the subepithelium, in deeper layers of the colonic lamina propria, and in the muscular layer in saline and carrier groups. No significant increase in collagen deposition was observed in vaccine-immunized mice. The reduction in collagen deposition was further corroborated by the Sircol collagen assay that detects soluble collagen quantitatively. The amount of soluble collagen in vaccine-treated mice was significantly lower than that in saline and carrier groups, and was reduced almost to the normal level (P < 0.01) (Figure 6B), confirming that vaccine immunization could decrease fibrosis in chronic colitis.
Expression Levels of Cytokines in Chronic Colitis
Studies have provided evidence that the production of some proinflammatory cytokines (such as IL-23, IL-17 and TNF) and a profibrotic cytokine (TGFβ1), are increased in colon tissue of chronic colitis. As indicated in Figure 7, after administrations of TNBS, the protein levels of p40, IL-23, IL-17 and TGFβ1 in colon tissue of saline and carrier groups were significantly higher than those in normal control (Figure 7A). As expected, vaccine-treated mice had a decrease in p40, IL-23, IL-17 and TGFβ1 levels compared with carrier and saline groups (P < 0.05). The decreased p40 and TNF levels in the vaccine-treated group were further confirmed by the measurement of mRNA expression (Figure 7B). However, in contrast to the finding in acute colitis that IL-10 was increased in vaccinated mice, IL-10 levels were decreased in vaccinated mice in chronic colitis. The decreased IL-10 was supported by reduced IL-10 mRNA expression in colon tissue, compared with control groups. The mRNA expression levels of Bcl-2 and Foxp3 also were downregulated in the vaccine-treated group. The IFN-γ levels in the normal control group were comparative with other groups (data not shown), which was similar to the results reported by Fichtner-Feigl et al. (27). We also detected the expression of IL-13, a typical Th2 cytokine, in the colon tissue. Our results showed that after TNBS challenge, IL-13 expression was increased significantly in saline, carrier and vaccine groups when compared with normal control; however, no significant differences were found between these three groups.
Vaccine Decreases the Percentages of Th1 and Th17 in MLNs in Chronic Colitis
Studies have shown that IL-12 promotes the differentiation of Th1 cells and that IL-23 stabilizes the proliferation of Th17 cells. Vaccination, by blocking IL-12 and IL-23, may inhibit the differentiation of Th1 and Th17 cells. As shown in Figure 8, in both acute (A) and chronic (B) colitis, the percentages of CD4+IFN-γ +Th1 and CD4+IL-17+Th17 cells in MLNs, detected by flow cytometry analysis, tend to be higher in the saline and carrier groups compared with normal mice. In contrast, the vaccine-treated group had lower percentages (close to that of normal mice) than saline and carrier groups. Although the differences didn’t reach statistical significance, the trends were supported by the results obtained from ELISA and RT-PCR (see Figures 3, ,7).7). If the numbers of test samples were to be increased, a statistically significant difference may be reached. Furthermore, the mean percentage of Th1 cells was higher and that of Th17 cells was lower in acute colitis than those in chronic colitis. These data indicate that predominant Th1 cell responses may initiate the acute intestinal inflammation, while Th17 cell responses perpetuate the chronic intestinal inflammation, which are in agreement with previous findings (27). Taken together, vaccine treatment tends to decrease the percentage of Th1 and Th17 cells in MLN.
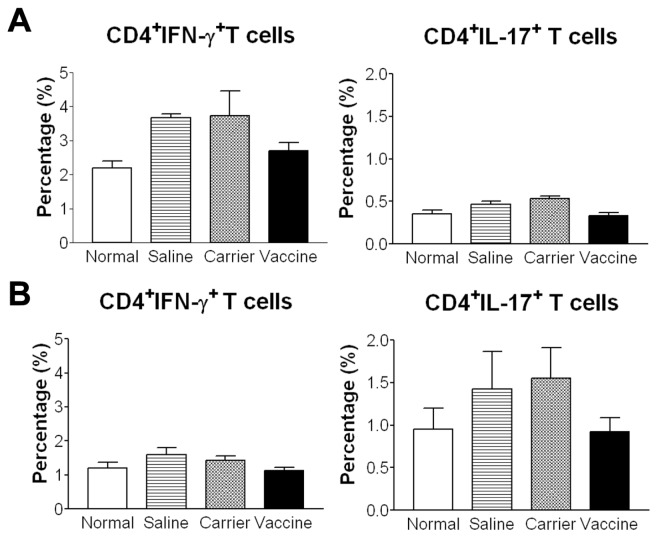
Vaccine treatment tends to reduce the percentages of Th1 and Th17 cells in MLNs. After stimulation with PMA and inomycin, lymphocytes from MLNs were stained with anti-CD4, anti-IFN-γ and anti-IL-17 to detect the percentages of CD4+IFN-γ+Th1 and CD4+IL-17+Th17 cells by flow cytometry. (A) Acute colitis. (B) Chronic colitis. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
We have previously developed a mouse IL-12/IL-23 p40 peptide-based virus-like particle vaccine that induced high titered and relative long-lasting antibodies to p40, IL-12 and IL-23, and the antisera could inhibit IL-12-induced interferon-γ (26) and IL-23-induced IL-17 production of splenocytes in vitro (data not shown). In this study, we systemically evaluated the in vivo effectiveness of the vaccine and the immune mechanisms involved in both TNBS-induced acute and chronic colitis models.
To date, to overcome B cell tolerance, vaccines against excessive endogenous cytokines are developed mainly by either inserting a foreign peptide into the intact cytokine molecule (24) or linking the intact cytokine to a heterogeneous carrier such as ovalbumin (25). Antibodies raised by these vaccines act against multiple antigen determinants of the target cytokine. As such, their use may be hindered by undesirable crossreactions with other self-proteins containing similar epitopes. This is of particular concern when this strategy is used in humans. Cytokine peptide-based vaccines are currently under development; however, these vaccines use inappropriate carrier proteins such as keyhole limpet hemocyanin (32). As these vaccines are not virus-like particles, their antigenicity is low, requiring strong adjuvants, such as complete Freund adjuvant, to elicit high titers of antibodies to the target cytokine. Virus-like particles induce potent B-cell responses even in the absence of adjuvants (30,31), and as carrier proteins, they are preferable to other proteins. More recently, cytokine peptide-based virus-like particle vaccines are being investigated by chemically conjugating synthesized cytokine peptides to bacteriophage Qβ virus-like particles (33). Compared with the vaccines that are currently being investigated, the p40 vaccine used in the present study has unique advantages: (1) it uses a small peptide, avoiding possible cross-reaction with other self proteins; (2) it is highly antigenic, eliciting long-lasting specific antibodies without the use of an adjuvant owing to its virus-like particle feature; (3) it is a recombinant protein, making it easier to prepare and to control the quality of the vaccine than those by chemical coupling (33); (4) the safety of the vaccine carrier HBcAg has been confirmed in a phase I clinical trial for a malaria vaccine used in humans (34), making this vaccine even more attractive for human use. For these reasons, the vaccine developed in the present study exhibits great advantages over other cytokine vaccine strategies.
In the TNBS-induced chronic colitis model, the initial Th1 response subsides after three weekly TNBS challenges and, then, is supplanted by a Th17 response after 4 to 5 weeks (27). This pattern of immune response is confirmed in the present study in which CD4+IFN-γ+ T cells, not CD4+IL-17+ T cells, tended to increase in the acute colitis, while CD4+IL-17+ T cells, not CD4+IFN-γ+ T cells, were increased in chronic colitis (see Figure 8). Although the differences didn’t reach statistical significance, the trends were supported by the results obtained from ELISA and RT-PCR. In acute colitis, Th1 cytokine expression (IFN-γ, IL-12, TNF) was increased significantly after TNBS challenge (see Figure 3); in chronic colitis, Th17 cytokine expression (IL-17, IL-23, TGFβ1) was increased significantly (see Figure 7). As the p40 vaccine effectively targets both IL-12 and IL-23, it exerts its antiinflammatory effects during the entire course of chronic colitis. We speculate that this vaccine, by downregulating IL-12, is able to halt the differentiation of naïve T cells into Th1 cells, leading to a reduction in IFN-γ and TNF. As IL-12 also has been shown to inhibit Fas-mediated cell death of CD4+ T cells through the inhibition of caspase-3 activity and the upregulation of the anti-apoptotic molecule Bcl-2 (35), blocking IL-12 resulted in a decreased mRNA expression of Bcl-2 in colon tissue (see Figures 3, ,7),7), which indicated an increase of T-cell apoptosis indirectly. In addition, the vaccine, by blocking IL-23, may hinder the differentiation of naïve T cells into Th17 cells. Through these immune mechanisms, vaccine treatment indeed decreases the percentages of Th1 and Th17 cells in the MLN after TNBS challenges, returning them almost to the levels in normal mice. Although the decrease is a trend, the significantly reduced Th1 and Th17 cytokine levels in colon tissue support the trend of the decreased percentages of Th1 and Th17 cells. In the future, we will use flow cytometry analysis or immunohistological staining to quantitate the frequency and apoptosis of Th1/Th17 in the colon tissue.
In the acute colitis model, the IL-17 level in colon tissue was elevated significantly (see Figure 3) which is in agreement with previous reports (36–38). However, the percentage of Th17 in MLN cells was not increased significantly (Figure 8A right). Although IL-17 is described as a Th17 cell-secreted cytokine, much of the IL-17 released during an inflammatory response is produced by innate immune cells which include γδ T cells, CD11b+ cells, macrophages and neutrophils, etc. (39). Therefore, the significantly increased IL-17 levels in colon tissue might be produced by local innate immune cells, not the T cells in the mesenteric lymph nodes. Indeed, Fitzpatrick et al. has reported that TNBS administration resulted in increased staining cells for IL-17 and that the IL-17+ stained cells at the top of the muscularis mucosa appeared to be macro phages (37). In the future, we will use immuno-histological staining to identify IL-17 producing cells in colon tissue, which may be more relevant than the analysis of Th17 cells in MLN cells.
Studies have demonstrated that the main sources of IL-10 production are regulatory T cells (Treg) and CD11b+ myeloid cells (40,41) and that IL-10 acts on Treg cells to maintain Foxp3 expression (41). In the present study, colon IL-10 levels were measured by both protein and mRNA expression. As Treg are one of the important IL-10 producing cells, IL-10 levels may reflect the function of Treg. Our results showed that in acute colitis, IL-10 levels, measured two days after TNBS challenge, were higher in the vaccine-treated group than in the control groups (see Figure 3), which coincides with results reported by Stallmach et al.(7). Recently, IL-23 has been found to restrain the activity of Treg to drive T cell-dependent colitis (42), and the homodimer of p40 is found to suppress the expression of CD25 and Foxp3 (43). We speculate that, by blocking p40 and IL-23, Treg might be activated or recruited rapidly into the local colon tissue to control inflammation in the acute phase of colitis. Therefore, p40 vaccine treatment might ameliorate colitis through improving the function and/or numbers of Treg in colon tissue in acute colitis. This is supported by the increased mRNA expression in vaccinated mice. In chronic colitis, levels of IL-10 and Foxp3, measured 10 days after the last TNBS challenge, were significantly lower in vaccine-treated mice than those in saline and carrier controls (see Figure 7). Because vaccine-treated mice had significantly reduced colon inflammation at this time point, accompanied by significantly lower inflammatory cytokines such as p40, IL-23 and IL-17, Tregs might not be required to exert their antiinflammatory effects. This is supported by clinical observations that IL-10 expression in lamina propria mononuclear cells of patients with Crohn’s disease had a tendency to decrease after treatment with monoclonal antibodies against IL-12/IL-23p40 (19). In future studies, the frequency of CD4+CD25+Foxp3+ Treg in colon tissue will be investigated to confirm this finding.
Vaccine treatment reduced histological inflammation and MPO activity. This may be through the inhibition of IL-12. IL-12 has been demonstrated to be chemotactic for neutrophils and NK cells through induction of synthesis of platelet-activating factor (44,45). It also has been reported that IL-12 is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture (46). Therefore, vaccine-induced specific antibodies blocked the biophysical functions of IL-12, leading to less migration and activation of neutrophils to the local colon tissue and, thus, ameliorating intestinal inflammation.
Our results showed that the colon inflammatory scores in the chronic colitis were slightly lower than those in the acute colitis. This is different from other reports where colon inflammatory scores are significantly higher in the chronic phase than those in the acute phase (36,37). The reason may be due to the following factors: 1) Different detection times. In our acute colitis, mice were euthanized 2 days after the second TNBS delivery; while in the chronic colitis, mice were euthanized 10 days after the last TNBS delivery. In our experience, after a TNBS challenge, the body weight shows an obvious loss in the first 2 days, and then gradually recovers during the next 4 days. At day 7, the body weight is close to that of baseline, accompanied with a decreased colon inflammation. 2) Different TNBS doses. In our acute colitis, mice were intrarectally challenged with TNBS twice (1.5 mg and 2.0 mg, respectively), and in the chronic colitis, the mice were challenged with increasing doses of TNBS (1.0–2.5 mg) over 7 weeks. 3) Different experimental designs. In our study, the acute and chronic experiments were performed separately at different times with different mouse batches. This makes the data less compatible between acute and chronic colitis, when compared with other reports (36,37). In future studies, we will combine the acute and chronic experiments as one experiment for kinetic observations.
Our present study also indicated that vaccine treatment could ameliorate fibrosis observed in chronic colitis. This inhibition may be through the downregulation of p40, TGFβ1 and IL-17 levels. Studies have shown that IL-12/IL-23p40 is overproduced during the establishment of an experimental fibrotic process (47,48), TGFβ1, involved in most of the processes of wound repair, is identified as a major profibrotic factor, and IL-17 has profibrotic functions in multiple forms of fibrosis (49–51). In the present study, vaccine treatment decreased the expression of IL-12/IL-23p40, IL-17 and TGFβ1 in colon tissue (see Figure 7), and, therefore, ameliorated intestinal fibrosis.
A potential concern with cytokine vaccines is that the level of the target cytokine may be reduced to a level below baseline, thus impeding the normal functions of the cytokine. First, as seen in our previous studies, the target cytokine levels are downregulated only to a level that is lower than the disease condition but still above the normal level (30,31). Second, vaccine-induced antibodies are capable of neutralizing the excessive target cytokine within the extracellular compartment, not the cytokines inside of the immunological synapse where normal cytokine processes occur (22). Third, the antibody levels that persist for about 4 months are reversible and can be adjusted by the frequency of immunization as the immunogenicity of cytokine vaccines is much less than that of microbe vaccines (22). Therefore, the vaccine strategy may be safe when used with careful monitoring. The influence of this vaccine on the susceptibility to intracellular bacteria infections in mice is currently under investigation in our group.
In summary, we have demonstrated that the IL-12/IL-23p40 peptide-based vaccine that induces specific antibodies to IL-12 and IL-23 is capable of ameliorating experimental colitis significantly, as seen by improved body weight loss and a decrease of colon inflammation and collagen deposition, accompanied with reduced expressions of Th1 and Th17 cytokines and other inflammatory mediators in colon tissues. By using an active immunization strategy, the p40 vaccine, we offer an innovative and long-lasting immune inhibitory approach for the treatment of IBD. In addition, this strategy can serve as a research tool in our understanding of the pathogenesis of IBD, as it provides a persistent blockade of a pathogenic cytokine rather than the temporary blockage using mAbs or the permanent deletion using the gene knockout method.
ACKNOWLEDGMENTS
This research was supported by grants from the Canadian Institutes of Health Research (ROP-92387 to Z Peng) and conducted using facilities of the Manitoba Institute of Child Health, Inc. Q Guan is the recipient of the CIHR Frederick Banting and Charles Best Canada Graduate Scholarship. CN Bernstein is supported in part by a Research Scientist Award of the Crohn’s and Colitis Foundation of Canada and the Bingham Chair in Gastroenterology.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
Articles from Molecular Medicine are provided here courtesy of The Feinstein Institute for Medical Research at North Shore LIJ
Full text links
Read article at publisher's site: https://doi.org/10.2119/molmed.2010.00252
Read article for free, from open access legal sources, via Unpaywall:
https://molmed.biomedcentral.com/counter/pdf/10.2119/molmed.2010.00252
Citations & impact
Impact metrics
Article citations
Multi-omics and multi-stages integration identified a novel variant associated with silicosis risk.
Arch Toxicol, 98(9):2907-2918, 29 May 2024
Cited by: 0 articles | PMID: 38811393
TH17 cell: a double-edged sword in the development of inflammatory bowel disease.
Therap Adv Gastroenterol, 17:17562848241230896, 21 Feb 2024
Cited by: 0 articles | PMID: 38390028 | PMCID: PMC10883129
Review Free full text in Europe PMC
Vaccines for immune tolerance against autoimmune disease.
Adv Drug Deliv Rev, 203:115140, 18 Nov 2023
Cited by: 2 articles | PMID: 37980949
Review
Dynamic alterations in metabolomics and transcriptomics associated with intestinal fibrosis in a 2,4,6-trinitrobenzene sulfonic acid-induced murine model.
J Transl Med, 21(1):554, 18 Aug 2023
Cited by: 2 articles | PMID: 37592304 | PMCID: PMC10436422
Cellular and Molecular Mechanisms of Intestinal Fibrosis.
Gut Liver, 17(3):360-374, 10 Mar 2023
Cited by: 8 articles | PMID: 36896620 | PMCID: PMC10191785
Review Free full text in Europe PMC
Go to all (31) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Employing an IL-23 p19 vaccine to block IL-23 ameliorates chronic murine colitis.
Immunotherapy, 5(12):1313-1322, 01 Dec 2013
Cited by: 7 articles | PMID: 24283842
Development of recombinant vaccines against IL-12/IL-23 p40 and in vivo evaluation of their effects in the downregulation of intestinal inflammation in murine colitis.
Vaccine, 27(50):7096-7104, 26 Sep 2009
Cited by: 20 articles | PMID: 19786142
Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis.
Inflamm Bowel Dis, 16(6):1040-1050, 01 Jun 2010
Cited by: 34 articles | PMID: 19924805
[Diosmetin regulates intestinal immune balance by inhibiting PI3K/AKT signaling to relieve 2, 4, 6-trinitrobenzene sulfonic acid-induced Crohn's disease-like colitis in mice].
Nan Fang Yi Ke Da Xue Xue Bao, 43(3):474-482, 01 Mar 2023
Cited by: 1 article | PMID: 37087594 | PMCID: PMC10122747
Funding
Funders who supported this work.
Canadian Institutes of Health Research (1)
Grant ID: ROP-92387