Abstract
Free full text
AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan
Abstract
Adenosine monophosphate-activated protein kinase (AMPK) is a crucial regulator of energy metabolic homeostasis and thus a major survival factor in a variety of metabolic stresses and also in the aging process. Metabolic syndrome is associated with a low-grade, chronic inflammation, primarily in adipose tissue. A low-level of inflammation is also present in the aging process. There are emerging results indicating that AMPK signaling can inhibit the inflammatory responses induced by the nuclear factor-κB (NF-κB) system. The NF-κB subunits are not direct phosphorylation targets of AMPK, but the inhibition of NF-κB signaling is mediated by several downstream targets of AMPK, e.g., SIRT1, PGC-1α, p53, and Forkhead box O (FoxO) factors. AMPK signaling seems to enhance energy metabolism while it can repress inflammatory responses linked to chronic stress, e.g., in nutritional overload and during the aging process. AMPK can inhibit endoplasmic reticulum and oxidative stresses which are involved in metabolic disorders and the aging process. Interestingly, many target proteins of AMPK are so-called longevity factors, e.g., SIRT1, p53, and FoxOs, which not only can increase the stress resistance and extend the lifespan of many organisms but also inhibit the inflammatory responses. The activation capacity of AMPK declines in metabolic stress and with aging which could augment the metabolic diseases and accelerate the aging process. We will review the AMPK pathways involved in the inhibition of NF-κB signaling and suppression of inflammation. We also emphasize that the capacity of AMPK to repress inflammatory responses can have a significant impact on both healthspan and lifespan.
Introduction
The AMP-activated protein kinase (AMPK) is a crucial regulator of energy metabolic homeostasis at the cellular and whole organism levels [1–3]. AMPK is a serine/threonine kinase which has been highly conserved during evolution. AMPK consists of a heterotrimeric complex including a catalytic α subunit and regulatory β and γ units. There are two isoforms of α (α1 and α2) and β (β1 and β2) and three γ subunits (γ1-3) which are differently expressed in mammalian tissues. AMPK is activated via allosteric regulation of increased AMP concentration and by the phosphorylation of α subunit (Thr172) via the upstream kinases serine/threonine kinase 11 (LKB1), Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ), and transforming growth factor-β-activated kinase 1. AMPK has a wide array of downstream substrates; these are typically energy metabolic enzymes and proteins involved in transcriptional regulation. One major role of AMPK signaling is to respond to metabolic requirements either by stimulating energy production including glucose and lipid catabolism or by inhibiting energy consuming pathways, e.g., synthesis of protein, fatty acids, and cholesterol. Impaired AMPK activity can induce insulin resistance in many tissues [3, 4]. It is not surprising that there are several observations linking deficiency in AMPK signaling to the appearance of the metabolic syndrome including obesity, diabetes, and cardiovascular diseases [2]. Currently, AMPK is an exciting research topic and the focus of intensive drug discovery projects. Several pharmacological AMPK activators have been identified, e.g., 5-aminoimidazole-4-carboxamide riboside (AICAR), metformin, statins, thiazolidinediones, and several natural plant products [2, 5, 6].
The AMPK signaling system plays a key role in cellular and organismal survival during stress by its ability to maintain metabolic homeostasis. Chronic nutrient overload and positive energy balance induce stress in adipose tissue. Accumulation of lipids in adipocytes as well as the ectopic storage of fat in liver, muscles, and pancreas stimulate the innate immunity defense leading to the secretion of proinflammatory cytokines and subsequently to recruitment of monocytes, especially into the adipose tissue [7–9]. There are several studies indicating that a low-grade, chronic inflammation has a crucial role in the development of the metabolic syndrome. Interestingly, there is emerging evidence that through its signaling network, AMPK can suppress the activation of nuclear factor-κB (NF-κB) system, a key regulator of innate immunity and inflammation. We will review the signaling pathways of AMPK which are involved in the inhibition of NF-κB signaling and the suppression of inflammatory responses. We will emphasize that the capacity of AMPK to repress the appearance of a proinflammatory phenotype can has a major impact on both healthspan and lifespan.
AMPK: repressor of inflammation
Innate and adaptive immunity are the major host defense mechanisms which not only can provoke inflammation in order to protect organism against invading pathogens but also repair tissue injuries and alert the immune system from jeopardizing cellular damage. Inflammation is a crucial survival mechanism but it can be dangerous if it becomes overwhelming or if it is converted to a chronic state. Several metabolic disorders, e.g., obesity, type 2 diabetes, and atherosclerosis, trigger immune defense mechanisms and induce chronic inflammation which in turn aggravates the symptoms of the disease [7, 10, 11]. The endoplasmic reticulum (ER) is a sensitive nutrient sensor, e.g., unfolded protein response (UPR) becomes activated in response to hyperglycemia and fatty acid overload [12]. ER stress can generate an inflammatory response via different signaling pathways [10, 13, 14]. Hotamisligil [10] has recently reviewed the role of ER stress in the induction of low-grade, chronic inflammation associated with metabolic diseases. Oxidative stress is another major stress condition present in the metabolic syndrome [15] and aging [16] which can stimulate inflammatory responses [17].
There is an extensive literature describing how the activation of AMPK can inhibit inflammatory response induced by different stimulating insults whereas a decrease in AMPK activity is associated with increased inflammation. Several studies have demonstrated that the activation of AMPK by AICAR can inhibit, e.g., acute and chronic colitis [18], autoimmune encephalomyelitis [19], inflammation in cystic fibrosis [20], and proinflammatory effects after lung injury [21]. It has also been observed that the therapeutic drug which is an AMPK agonist, metformin, can reduce the systemic inflammation by decreasing the level of C-reactive protein and interleukin-6 (IL-6) in mild metabolic syndrome [22]. Metformin can also lower plasma macrophage migration inhibitory factor concentrations in obese patients [23]. Many in vitro experiments have demonstrated that the lipopolysaccharide (LPS)-induced inflammatory response can be inhibited by activating AMPK with AICAR [18, 21, 24]. However, some recent observations imply that AICAR can also inhibit inflammatory responses via AMPK-independent pathways [25].
Several studies have revealed a close link between the reduced AMPK activity and inflammation, e.g., in adipose tissue and macrophages [26–29]. Steinberg et al. [30] observed that tumor necrosis factor-α (TNF-α) suppressed the activity of AMPK by upregulating the expression of protein phosphatase 2C, an inhibitor of AMPK signaling. The reduced AMPK activity decreased fatty acid oxidation, increased diacylglycerol accumulation, and induced insulin resistance in skeletal muscle [30]. Ko et al. [28] demonstrated that nutrient stress induced by high-fat feeding reduced cardiac AMPK activity and triggered inflammation reflected in an increased number of macrophages and upregulation of IL-6 levels in the mouse heart. In addition, they revealed that acute infusion of IL-6 profoundly decreased the activity of AMPK, reduced the myocardial glucose uptake, and triggered inflammation in the heart. They also observed that diet-induced cardiac insulin resistance and the level of inflammation were attenuated in IL-6-deficient mice [28]. However, there are observations that IL-6 knockout mice can develop mature-onset obesity [31] and display an enhanced formation of diet-induced atherosclerosis [32]. This could be linked to the reduced AMPK activity in IL-6 knockout mice [33], but there may be tissue-specific changes in diet-induced responses between heart and adipose/vascular tissues, in particular, in chronic knockout conditions. Yang et al. [27] demonstrated that inflammatory stimuli and a fatty acid rich-diet decreased the expression and activity of α1AMPK in mouse adipose tissue and macrophages and triggered TNF-α expression. The AICAR treatment could reverse the LPS and diet-induced inflammation. Sag et al. [29] observed that IL-10 and transforming growth factor-β (TGF-β), two anti-inflammatory cytokines, stimulated a rapid phosphorylation and activation of AMPK whereas a proinflammatory insult with LPS decreased the AMPK activity in mouse and human macrophages. These experiments indicate that the activity of AMPK regulates the inflammatory responses and consequently can induce insulin resistance in different tissues.
There are several physiological inducers of AMPK activity including many hormones, e.g., adiponectin and leptin [34], dietary phytochemicals, e.g. resveratrol, curcumin, and berberine [35], and physical exercise [36] (Fig. 1). Interestingly, many of these stimuli are linked to the inhibition of inflammatory responses. For instance, adiponectin has many anti-inflammatory, antiatherogenic, and antidiabetic properties [37]. Aggarwal [38] has reviewed the anti-inflammatory effects of curcumin and its capacity to reverse insulin resistance, hyperglycemia, and hyperlipidemia linked to obesity. Resveratrol is also a potent anti-inflammatory compound [39] activating AMPK-SIRT1 (silent information regulator 1) pathway (see below).
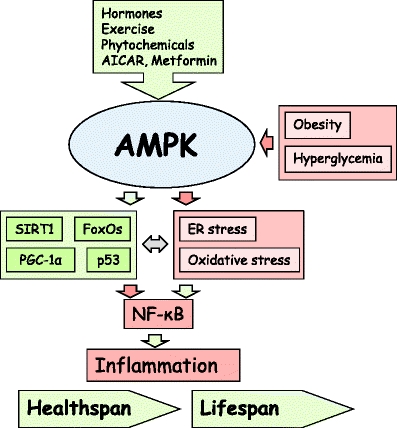
Schematic illustration of the functional connections of AMPK linked to the inhibition of NF-κB signaling and suppression of inflammation. Green arrows show the activating pathways and red arrows are the inhibitory connections. Several hormones and phytochemicals, physical exercise, and some drugs, e.g., AICAR and metformin, activate AMPK. In contrast, obesity and hyperglycemia inhibit the expression of AMPK. On the downstream, AMPK stimulates SIRT1, PGC-1α, p53, and FoxO factors which can inhibit the NF-κB signaling with different mechanisms. AMPK inhibits the appearance of ER and oxidative stresses which can trigger NF-κB signaling. NF-κB is the key inducer of inflammatory responses which affect the healthspan and lifespan
AMPK inhibits NF-κB signaling via several pathways
The NF-κB signaling system is the principal pathway which is involved in the activation of both the innate and adaptive immune systems [40]. A plethora of studies have demonstrated that the activation of AMPK signaling downregulates the function of NF-κB system [18, 27, 29, 41–43]. AMPK has several direct phosphorylation targets [1], but it seems that AMPK suppresses NF-κB signaling indirectly via its downstream mediators, e.g., SIRT1, Forkhead box O (FoxO) family, and peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α), which can subsequently repress the expression of inflammatory factors (Fig. 1). Moreover, there is an extensive literature indicating that activation of AMPK inhibits the NF-κB-mediated proinflammatory signaling whereas it can activate the NF-κB system in order to stimulate the expression of antiapoptotic proteins, e.g., Bcl-2 and survivin [44]. Cacicedo et al. [45] observed that AMPK activation inhibits NF-κB transactivation induced by TNF-α and fatty acid palmitate in endothelial cells. In addition, calcium entry into endothelial cells stimulates CaMKKβ, one of the upstream activators of AMPK [1], and subsequently, AMPK activates the NF-κB system via protein kinase Cδ and p38MAPK signaling [46]. These observations indicate that there are multiple downstream signaling pathways through which AMPK can regulate a number of different gene arrays, e.g., those regulating metabolic events, cell cycle and differentiation, and inflammatory responses.
Although AICAR and metformin are not specific AMPK activators, these compounds have been used in many inflammatory studies to demonstrate that AMPK can inhibit NF-κB signaling. However, there are a number of studies in which the AMPK subunits have been specifically inhibited by short hairpin RNA knockdown technique or expression of dominant-negative AMPK components. For instance, Huang et al. [47] demonstrated that the knockdown of α1AMPK abolished the anti-inflammatory effect of metformin. Yang et al. [27] revealed that the constitutively active α1AMPK suppressed the NF-κB signaling and fatty acid-induced inflammation in macrophages and that dominant-negative α1AMPK could reverse the inhibition. Katerelos et al. [48] have observed that the overexpression of α1AMPK reduced NF-κB signaling in aortic endothelial cells. AICAR induced similar effects as the activation of α1AMPK. Wang et al. [41] demonstrated that the NF-κB signaling was activated in the aortic endothelial cells isolated from AMPKα2 knockout mice whereas AMPK activation by AICAR and constitutively active AMPKα2 had an opposite effect.
AMPK–SIRT1–NF-κB signaling
AMPK and SIRT1 are evolutionary conserved partners which have similar functions in metabolism and cellular survival [49]. Canto et al. [50] demonstrated that AMPK can activate SIRT1 deacetylase by increasing cellular NAD+ levels. SIRT1, type III deacetylase, has several target proteins which regulate many host defense functions. Conversely, SIRT1 can stimulate the activity of LKB1, which subsequently activates AMPK [51]. This represents a positive feedback loop to enhance cellular survival during energy deficiency. Yeung et al. [52] were the first researchers who demonstrated that SIRT1 could interact with the RelA/p65 subunit of NF-κB complex and consequently deacetylates the p65 protein at lysine 310. The acetylation of p65 enhances the transactivation capacity of the NF-κB complex, and thus, SIRT1-induced deacetylation inhibits NF-κB signaling (Fig. 1). Recently, Yang et al. [53] observed that the deacetylation of lysine 310 at p65 component enhanced the Set9-mediated methylation of lysines 314 and 315 and triggered the ubiquitination and degradation of the p65 subunit of NF-κB. There are many reports that SIRT1 can repress inflammatory responses via inhibition of NF-κB signaling [27, 54, 55]. Several dietary polyphenols can activate AMPK and SIRT1, and it seems that their anti-inflammatory effects are mediated via the AMPK/SIRT1-induced p65 deacetylation [56]. We have recently reviewed the role of SIRT1–NF-κB signaling pathway in the suppression of immune responses, e.g., in human immunodeficiency virus-1 infection [57].
AMPK–NF-κB/PGC-1α signaling
PGC-1α is one of the key regulators of energy metabolism, e.g., enhancing glycolysis and mitochondrial biogenesis [58]. AMPK phosphorylates PGC-1α protein which triggers SIRT1-mediated deacetylation and activation of PGC-1α. Alvarez-Guardia et al. [59] demonstrated that PGC-1α could bind to the p65 subunit of NF-κB in human cardiac cells and mouse heart. They also observed that the activation of NF-κB signaling increased the interaction between p65 and PGC-1α which consequently reduced the expression of PGC-1α protein. This is an important observation since it may explain why activation of NF-κB system, e.g., via inflammatory cytokines, has such profound effects on metabolism promoting the appearance of metabolic disorders. Moreover, Morari et al. [60] observed that fatty acids can induce the association of PGC1α factor with the p50 component of NF-κB complex in hepatocytes. They also detected that PGC-1α and p50 could bind to the IL-10 promoter and induce the expression of IL-10 cytokine. IL-10 is the protective cytokine against diet-induced liver inflammation. These first two studies imply that the physical interaction of PGC-1α and NF-κB complexes may be situated at crossroads in the regulation of energy metabolism and inflammation although the reciprocal regulation of PGC-1α and NF-κB system still needs to be confirmed. Kim et al. [61] demonstrated that the increased expression of PGC-1α could inhibit the NF-κB activity and the proinflammatory response induced by TNF-α in human aortic smooth muscle and endothelial cells (Fig. 1). Palomer et al. [62] observed that the cardiac-specific overexpression of TNF-α in the transgenic mouse significantly downregulated PGC-1α expression and induced cardiomyopathy. There are cell culture experiments demonstrating a crucial decrease in PGC-1α expression being mediated by NF-κB and p38MAPK signaling [62].
AMPK–p53/FoxO signaling
The FoxO family and p53 are evolutionary conserved transcription factors which are involved in a variety of different functions including the regulation of energy metabolism [63, 64] and inflammation [65, 66]. Jones et al. [67] demonstrated that AMPK phosphorylated p53 protein at serine 15 a modification which could trigger AMPK-dependent cell cycle arrest. The p53 and NF-kB signaling have many antagonistic functions, e.g., p53 can inhibit NF-κB signaling [68, 69]. It is known that p53 is a potent inhibitor of glycolysis whereas it increases aerobic respiration [63]. Kawauchi et al. [70] observed that the lack of p53 protein expression clearly increased the rate of glycolysis and significantly stimulated the activity of NF-κB signaling. Increased glycolytic activity promotes the O glycosylation of IκB kinase-beta (IKKβ) at serine 733. This modification blocks the inactivating feedback phosphorylation site at IKKβ and in that way it can enhance the activity of IKKβ which accordingly triggers NF-κB signaling [71]. It seems that glycolysis is not the only connection which links p53 to the inhibition of inflammation (Fig. 1). Komarova et al. [66] have demonstrated that the functional activities of NF-κB were increased in the p53-null mice including the potentiated responses to inflammatory insults. AMPK can target the serine 15 and 20 sites in the transactivation domain of p53 protein [67, 72]. Both of these phosphorylation sites are linked to the inflammation [73] and aging processes [72] though the exact molecular mechanisms need to be clarified.
The mammalian FoxO family consists of FOXO1, FOXO3a, FOXO4, and FOXO6 transcription factors which can regulate several processes, e.g., stress resistance, glucose metabolism, and inflammation [64, 65]. In Caenorhabditis elegans, the AMPK–FoxO pathway has a crucial role in the caloric restriction induced lifespan extension [74]. Greer et al. [75] demonstrated that the mammalian AMPK can phosphorylate FOXO3a at six regulatory sites and this will activate the transcription of a specific set of genes. Currently, it is not known whether there are differences in the activation levels of separate FoxO factors, in particular, in different tissues. However, Lin et al. [76] revealed that the FOXO3a deficiency in mice induced inflammatory responses in several tissues and lymphoid proliferation. Helper T cells were hyperactivated and produced more Th1 and Th2 cytokines than their wild-type counterparts. They also observed that FOXO3 inhibited the NF-κB activity in T cells and the lack of FOXO3a generated the autoinflammatory condition in these mice. Zhou et al. [77] demonstrated that also FOXO4 was an endogenous inhibitor of NF-κB and a deficiency of FOXO4 could induce colonic inflammation and injury in mice. Interestingly, mammalian SIRT1 bound to FOXO4 and increased its transactivation capacity by NAD-dependent deacetylation [78]. These results indicate that AMPK can regulate the function of FoxO factors either by direct phosphorylation or indirectly via the SIRT1-induced activation.
AMPK inhibits ER and oxidative stresses
There are several studies indicating that the activation of AMPK represses the oxidative stress triggered by different insults, e.g., hyperglycemia and fatty acids [41, 79–81] (Fig. 1). Xie et al. [80] observed that AMPK signaling induced the expression of mitochondrial uncoupling protein-2 (UCP-2) and in that way clearly reduced the production of superoxide radicals in hyperglycemic endothelial cells. It is known that UCP-2 is able to inhibit the mitochondrial reactive oxygen species (ROS) production. Moreover, Wang et al. [41] demonstrated using different techniques that AMPKα2 is the physiological suppressor of the ROS produced via NAD(P)H oxidase. Their results support the mechanism whereby AMPK inhibits the NF-κB signaling and thus downregulates the expression of different NAD(P)H oxidase subunits and thus alleviates oxidative stress. Recently, Li et al. [79] revealed that AMPK signaling via the AMPK–FOXO3 pathway induced the expression of thioredoxin (Trx), a disulfide reductase which protects cellular proteins against cysteine oxidation. The function of Trx can be inhibited in cells by binding of the thioredoxin-interacting protein (Txnip). Oxidative stress, e.g., such as that occurring in hyperglycemia, is a potent inducer of the expression of Txnip which subsequently inhibits the activity of Trx and exposes cells to the effects of oxidative stress [81]. Interestingly, Zhou et al. [17] demonstrated that Txnip could interact with inflammasomal receptor Nod-like receptor protein 3 (NLRP3). Oxidative stress dissociated the complex between Trx and Txnip which subsequently could bind to the NLRP3 receptor and in that way activate the inflammasomal multiprotein complex. Inflammasomes cleave the pro-IL-1β and pro-IL-18 precursors to the active cytokines which can trigger the expression of a variety of inflammatory factors. Increased expression of Trx via the AMPK signaling can prevent the inflammasomal activity which is increased in many metabolic diseases.
The endoplasmic reticulum (ER) is a sensitive stress sensor in the maintenance of cellular protein quality and nutrient balance [12, 82]. Oxidative stress impairs homeostasis in ER and leads to protein misfolding in ER and calcium release into the cytoplasm. ER stress triggers a set of transducer proteins that stimulate the unfolded protein response (UPR) [82]. Recent studies have revealed that the UPR involves the activation of NF-κB system which can trigger a low-grade inflammatory response [10, 13, 14]. Several studies have reported that AMPK activity can attenuate the ER stress, e.g., in oxidized LDL-exposed aortic endothelial cells [83] and in hypoxic cardiomyocytes [84]. Dong et al. [85] demonstrated that the deletion of AMPKα2 increased ER stress in endothelial cells and caused aortic lesions in the knockout mice. They also noted that several antioxidants were able to suppress the appearance of ER stress. This indicates that ER stress is provoked by oxidative stress induced by the deletion of AMPKα2.
Significance of anti-inflammatory potency of AMPK
Current lifestyle including caloric overload and minimal level of physical exercise has created an epidemic of obesity and metabolic syndrome. A plethora of studies have revealed that nutritional excess is linked to a low-grade, metabolic inflammation, particularly in the adipose tissue, and there is clear evidence that inflammation has a key role in the progression of metabolic diseases [7–9]. AMPK is the master regulator of energy homeostasis, and it can also inhibit the NF-κB signaling and thus prevent the appearance of a proinflammatory phenotype during aging process itself and in several age-related diseases (Fig. 1). There are extensive reviews concerning the role of AMPK in type II diabetes and metabolic syndrome [86, 87], cardiovascular diseases [88, 89], and cancer [90]. All of these diseases involve the presence of inflammation, but currently, the role of AMPK in the prevention of inflammatory responses needs to be clarified.
The overload of adipose tissue with fatty acids induces a stress response in adipocytes, i.e., oxidative stress and ER stress, which can trigger inflammatory reactions via the NF-κB signaling and inflammasomal activation [10, 17]. Moreover, the increased fatty acid concentration can inhibit the AMPK activity by stimulating protein phosphatase 2A [91]. Hyperglycemia can also generate oxidative stress and activate NF-κB signaling. As described above, AMPK signaling can repress ROS production [41] and increase the expression of thioredoxin which is a potent inhibitor of inflammasomal activation [17, 79]. AMPK activity can also reduce the level of ER stress and thus suppress inflammatory responses and improve healthspan [83–85]. AMPK can also repress inflammatory responses via SIRT1 [27, 55], PGC-1α [61, 62], and FoxO [76, 77] signaling (see above). Emerging results in mouse models indicate that the activation of SIRT1 can effectively prevent the appearance of age-related pathologies [92]. In addition, the LKB1–AMPK signaling is a powerful tumor suppressor pathway, and there are therapeutic implications that AMPK agonists could be potent cancer drugs [90, 93].
AMPK activity can support the adaptive capabilities of cells by enhancing the biogenesis of mitochondria [94]. Mitochondrial dysfunction is associated with the aging process and age-related degenerative diseases. For instance, impaired mitochondria release damage-associated molecules which can stimulate systemic inflammatory response [95]. Zhang et al. [96] demonstrated that the LPS-induced systemic inflammation was linked to a fast and strong downregulation of the AMPK expression in blood leukocytes and liver. Recently, Zhou et al. [97] observed that mitochondria are the source of ROS release that stimulates inflammasomes and triggers the secretion of IL-1β and IL-18. Inflammasomes are activated in several obesity-related diseases, e.g., type II diabetes and cardiovascular diseases [98, 99]. It seems that AMPK can regulate inflammasomal activation by (1) stimulating autophagy and cleansing dysfunctional mitochondria [100] or (2) inhibiting NF-κB activation which is a priming phase in the activation of inflammasomes [101].
Exercise is a potent activator of AMPK signaling, in particular, in skeletal muscles and also in heart, liver, and adipose tissue [36]. The activation of AMPK during exercise is important for energy production, but it seems that several beneficial effects of exercise on healthspan could be induced by AMPK signaling pathways [36]. In addition, many hormones, e.g., adiponectin and leptin [34], can stimulate AMPK signaling (Fig. 1). Adiponectin, in particular, the high molecular weight isoform of the hormone, is a potent physiological activator of AMPK which inhibits NF-κB signaling and inflammatory response in endothelial cells [101]. These observations underline the significance of AMPK in prevention in vivo against inflammation. Currently, AMPK is an important drug design target in order to find specific agonists [6]. Metformin is a clinically used AMPK agonist against hyperglycemia in type II diabetes, and it is also a potent anticancer drug in certain types of cancers [102]. Metformin can inhibit NF-κB signaling and repress inflammatory responses (see above) and thus also alleviate the metabolic syndrome. Zhou et al. [87] have reviewed the current status in the drug development of small molecule AMPK activators.
Several research approaches have revealed that the aging process is associated with a low-grade inflammation [103–105]. The inflammatory phenotype is linked to the activation of NF-κB system in several tissues during aging [104–107]. Interestingly, many observations have indicated that the activation capacity of AMPK declines with aging which seems to be linked to the reduced stress resistance [108–110]. Confirming the role of AMPK in the aging process, many studies in C. elegans have demonstrated that the AAK-2, the nematode analog of AMPK, is a crucial factor involved in the lifespan extension induced by the overexpression of SIR-2.1 (analog of SIRT1) and mitochondrial manipulation [111, 112]. AAK-2 also regulates energy metabolism during the dauer state of C. elegans [112]. Recently, Selman et al. [113] observed that the extension of lifespan in ribosomal protein S6 kinase 1-deficient mice was most likely linked to the increased activation of AMPK since these mice displayed a strong overlap in the gene expression patterns with those induced by AICAR treatment. Several observations indicate that the autophagic capacity declines during aging [114] which could be caused by the reduced activation of AMPK [108–110]. Interestingly, SIRT1 is a potent activator of autophagy [115] and the activation of SIRT1, e.g., by resveratrol, could stimulate autophagic uptake of damaged mitochondria. Bauer et al. [116] demonstrated that resveratrol could enhance the healthspan and extend the lifespan of mice consuming a high-calorie diet. In conclusion, many longevity factors, e.g., SIRT1, p53, and FoxOs, are associated with the activation of AMPK signaling and subsequently can inhibit inflammation [117] but, in addition, activate autophagic housekeeping and maintain cellular energy status, and in that way, AMPK signaling is able to improve the healthspan, prevent age-related diseases, and extend the lifespan.
Concluding remarks
Many studies have demonstrated that AMPK activity can inhibit inflammatory responses in diverse types of cells and tissues. The inhibition is mediated by several transcription factors which are the downstream targets of AMPK signaling, of these, SIRT1, PGC-1α, p53, and FoxOs are the best characterized. AMPK can also repress inflammatory responses triggered by endoplasmic reticulum and oxidative stresses. These studies indicate that AMPK can regulate both the energy metabolism and inflammatory defense. Interestingly, the AMPK activity is decreased in obesity and the metabolic syndrome, and this may enhance the activation of NF-κB signaling and the appearance of a low-grade, metabolic inflammation, e.g., in the adipose tissue. Currently, it is not known how ubiquitous this connection between the AMPK activity and NF-κB inhibition will be since there are different AMPK isoforms and the regulation clearly seems to be tissue specific. The NF-κB signaling is an important metabolic regulator in the hypothalamus and pancreatic beta cells, but whether this is linked to AMPK activity in the metabolic syndrome still needs to be clarified. A low-grade inflammation is also observed during aging in several tissues. However, it is not known whether the aging process and overnutrition induce a similar proinflammatory phenotype. Emerging evidence indicates that the overexpression of AMPK can delay the aging process, and surprisingly, AMPK can enhance the signaling of several longevity factors, e.g., SIRT1, p53, and FoxOs, which are involved in the inhibition of AMPK-mediated inflammation. Moreover, the prevalence of obesity and metabolic syndrome increases with aging which could support the concept that a dysfunction of AMPK is involved in aging and the metabolic syndrome.
Acknowledgments
This study was financially supported by grants from the Academy of Finland and the University of Eastern Finland, Kuopio, Finland. The authors thank Dr. Ewen MacDonald for checking the language of the manuscript.
Disclosure of potential conflicts of interests
The authors declare no conflicts of interests related to this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Antero Salminen, Phone: +358-5057-40740, Fax: +358-1716-2048, Email: [email protected].
Juha M. T. Hyttinen, Email: [email protected].
Kai Kaarniranta, Email: [email protected].
References
 +
+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. [Europe PMC free article] [Abstract] [Google Scholar]
metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. [Europe PMC free article] [Abstract] [Google Scholar]Full text links
Read article at publisher's site: https://doi.org/10.1007/s00109-011-0748-0
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007%2Fs00109-011-0748-0.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s00109-011-0748-0
Article citations
Metformin in gestational diabetes: physiological actions and clinical applications.
Nat Rev Endocrinol, 25 Oct 2024
Cited by: 0 articles | PMID: 39455749
Review
Comparative outcomes of systemic diseases in people with type 2 diabetes, or obesity alone treated with and without GLP-1 receptor agonists: a retrospective cohort study from the Global Collaborative Network : Author list.
J Endocrinol Invest, 20 Sep 2024
Cited by: 0 articles | PMID: 39302577
Activation of AMPK/SIRT1/FOXO3a signaling by BMS-477118 (saxagliptin) mitigates chronic colitis in rats: uncovering new anti-inflammatory and antifibrotic roles.
Front Pharmacol, 15:1456058, 18 Sep 2024
Cited by: 0 articles | PMID: 39359253 | PMCID: PMC11445602
Metformin dampens the progression of cholangiofibrosis induced by thioacetamide using deep learning.
Heliyon, 10(18):e37347, 07 Sep 2024
Cited by: 0 articles | PMID: 39309781 | PMCID: PMC11416239
Mortality and major adverse cardiovascular events after glucagon-like peptide-1 receptor agonist initiation in patients with immune-mediated inflammatory diseases and type 2 diabetes: A population-based study.
PLoS One, 19(8):e0308533, 08 Aug 2024
Cited by: 0 articles | PMID: 39116084 | PMCID: PMC11309412
Go to all (439) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.
Cell Signal, 25(10):1939-1948, 11 Jun 2013
Cited by: 488 articles | PMID: 23770291
Review
AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network.
Ageing Res Rev, 11(2):230-241, 15 Dec 2011
Cited by: 410 articles | PMID: 22186033
Review
Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways.
Ageing Res Rev, 28:15-26, 06 Apr 2016
Cited by: 95 articles | PMID: 27060201
Review
AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): impact on cancer and aging.
J Mol Med (Berl), 97(8):1049-1064, 25 May 2019
Cited by: 59 articles | PMID: 31129755 | PMCID: PMC6647228
Review Free full text in Europe PMC

 1,2
1,2 



