Abstract
Unlabelled
Mechanisms of brain edema in acute liver failure (ALF) are not completely understood. We recently demonstrated that matrix metalloproteinase 9 (MMP-9) induces significant alterations to occludin in brain endothelial cells in vitro and in brains of mice with experimental ALF (Hepatology 2009;50:1914). In this study we show that MMP-9-induced transactivation of epidermal growth factor receptor (EGFR) and p38 MAPK/NFκB (mitogen-activated protein kinase/nuclear factor-kappa B) signals participate in regulating brain endothelial occludin level. Mouse brain endothelial bEnd3 cells were exposed to MMP-9 or p38 MAPK up-regulation in the presence and absence of EGFR inhibitor, p38 MAPK inhibitor, NFκB inhibitor, and/or appropriate small interfering RNA. Reverse-transcription polymerase chain reaction (RT-PCR) and western blotting were used for messenger RNA and protein expression analyses. Immunohistochemical staining and confocal microscopy were used to demonstrate cellular EGFR activation. Intraperitoneal azoxymethane was use to induce ALF in mice. Brains of comatose ALF mice were processed for histological and biochemical analyses. When bEnd3 cells were exposed to MMP-9, EGFR was significantly transactivated, followed by p38 MAPK activation, I-kappa B alpha (IκBα) degradation, NFκB activation, and suppression of occludin synthesis and expression. Similar EGFR activation and p38 MAPK/NFκB activation were found in the brains of ALF mice, and these changes were attenuated with GM6001 treatment.Conclusion
EGFR activation with p38 MAPK/NFκB signaling contributes to the regulation of tight junction integrity in ALF. EGFR activation may thus play an important role in vasogenic brain edema in ALF.Free full text

Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure
Abstract
Mechanisms of brain edema in acute liver failure (ALF) are not completely understood. We recently demonstrated that matrix metalloproteinase 9 (MMP-9) induces significant alterations to occludin in brain endothelial cells in vitro and in brains of mice with experimental ALF (Hepatology 50:1914, 2009). In this study, we show that MMP-9-induced transactivation of epidermal growth factor receptor (EGFR) and p38MAPK/NFκB signals participate in regulating brain endothelial occludin level. Mouse brain endothelial bEnd3 cells were exposed to MMP-9 or p38 MAPK upregulation in the presence and absence of EGFR inhibitor, p38 MAPK inhibitor, NFκB inhibitor, and/or appropriate small interfering RNA. RT-PCR and western blotting were used for mRNA and protein expression analyses. Immunohistochemical staining and confocal microscopy were used to demonstrate cellular EGFR activation. Intraperitoneal azoxymethane was use to induce ALF in mice. Brains of comatose ALF mice were processed for histological and biochemical analyses. When bEnd3 cells were exposed to MMP-9, EGFR was significantly transactivated, followed by p38 MAPK activation, IκBα degradation, NFκB activation, and suppression of occludin synthesis and expression. Similar EGFR activation and p38 MAPK/NFκB activation were found in the brains of ALF mice, and these changes were attenuated with GM6001 treatment.
Conclusion
EGFR activation with p38 MAPK/NFκB signaling contributes to the regulation of tight junction integrity in ALF. EGFR activation may thus play an important role in vasogenic brain edema in ALF.
Tight junction (TJ) proteins regulate paracellular permeability in various organs, particularly in the brain neurovascular unit (1). In brain capillaries, the endothelial cell (EC) spreads itself over the entire capillary basal lamina with the two plasmalemmal surfaces aligning to form the TJ. The TJ proteins, including occludin, claudin-5, junctional associated molecules, and their intracellular accessory factors zona occludins (ZO), seal the paracellular space. Collectively, the EC and its TJ, basal lamina, and associated astrocyte end-feet form the blood-brain barrier (BBB) that tightly regulates what enters and exits the neurovascular unit of the brain (2).
TJ proteins, particularly occludin and claudin-5, are important in the paracellular barrier function of the BBB, and their roles have been described in various pathological conditions (3-5). Occludin is a tetraspan membrane protein with two extracellular loops within the TJ and with the amino- and carboxy-terminal chains in the cytoplasm. The C-terminal domain binds to ZO-1 and -2 which serves as the link between occludin and the cytoskeleton (6). Claudin-5 has a similar distribution. Although the barrier function is typically compromised by structural breakdown of the BBB, recent evidence has suggested that subtle alterations in either occludin or claudin-5—without obvious structural changes—can result in selective permeability to small molecules (7-9). These findings support the concept that vasogenic edema might result from a subtle modification in TJ composition (10).
In acute liver failure (ALF), brain edema is lethal and remains a major determinant of patient survival (11, 12). However, the exact alterations in BBB integrity that lead to brain edema in ALF are not known. In 2006, we reported that specific monoclonal antibodies against matrix metalloproteinase-9 (MMP-9) attenuate brain extravasation and edema in ALF mice (13). We recently showed that MMP-9 significantly alters the TJ proteins, particularly occludin, in brain ECs in vitro and in brains of mice that have experimentally induced ALF (5). However, the signal transductions associated with MMP-9 that mediate the alterations in occludin remain unknown.
The role of epidermal growth factor receptor (EGFR) in cancer development and treatment is well known (14-16). EGFR belongs to the ErbB family of receptor tyrosine kinases. Upon ligand stimulation, they dimerize, and dimerization is then followed by receptor internalization and autophosphorylation of the intracytoplasmic EGFR tyrosine kinase domains, which serve as binding sites for recruiting signal transducers and activators of the intracellular signal transduction cascade. Recently EGFR was implicated in the regulation of cellular barrier function (17). EGFR has also been shown to participate in microvascular injury in diabetes (18), lung injury (19, 20), and intestinal permeability (21, 22).
Ligation of EGFR activates the mitogen-activated protein kinase (MAPK) cascades (23, 24). MAPKs have been implicated in endothelial paracellular permeability associated with oxidative insults (25-27) and with alterations of TJ proteins (28); thus, MAPKs are important in maintaining cellular and TJ integrity (29). Previously, we observed that p38 MAPK is upregulated in isolated microvessels from the brains of ALF mice (30). However, the role of p38 MAPK and EGFR in BBB permeability in ALF has not been explored.
In this study, we investigated the role of p38MAPK/NFκB signaling after EGFR transactivation by MMP-9 in altering the TJ element occludin in brain ECs in vitro and in brains of mice with ALF.
Materials and Methods
The mouse brain endothelial cell line, bEnd3, was purchased from American Type Culture Collection (CRL-2299, Manassas, VA). We purchased transfection-ready GFP-tagged human MMP-9 cDNA (RG202872) and p38 MAPK mouse cDNA (MC200120) from OriGene (Rockville, MD); anti-phospho p38 MAPK (sc9211), anti-p38 MAPK (sc9212), anti-MMP-9, anti EGFR and anti-phospho-Tyr EGFR antibodies (sc-13520) from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti-claudin (Zy34-1600), anti-occludin (Zy71-1500), and anti-ZO-1 (Zy40-2300) from Invitrogen-Zymed Laboratories (Carlsbad, CA); and anti-ZO-2 from BD Transduction Laboratories (San Jose, CA). We obtained non-targeted SignalSilence® control siRNA (cat 6568), and p38 MAPK siRNA (cat 6564), EGFR siRNA (cat 6481) from Cell Signal Tech (Danvers, MA); anti-IκBα (cat I0505) and p38 MAPK inhibitor SB203580 (cat S8307), MEK1/2 inhibitor PD 98059 (cat p215) from Sigma-Aldrich; NFκB inhibitor, MMP inhibitor GM6001, and EGFR inhibitor AG1478 from Calbiochem (San Diego, CA).
RNA extraction and RT-PCR analysis
Total RNA was extracted from bEnd3 cells using Purelink RNA Mini Kit (12183-018A, Invitrogen). RNA was transcribed into single-stranded DNA by SuperScript III First Strand reverse transcriptase (18080–051, Invitrogen). The yielded cDNA was used as a template. PCRs for MMP-9, occludin, ZO-1, ZO-2, and claudin-5 were performed using Taq PCR Master Mix kit (201443 Qiagen).
The primers were, for MMP-9, 5′-AGACGACATAGACGGCATCC-3′ (sense) and 5′-GCCCTGGATCTCAGCAATAG-3′ (anti-sense); for occludin (220 bp), 5′-CACACTTGCTTGGGACAGAGG-3′ (sense) and 5′-TGAGCCGTACATAGATCCAGGAGC-3′ 9 (anti-sense); for ZO-1 (290 bp), 5′-AGGCGCAGCTCCACGGGCTTCAGGAACTTG-3′ (sense) and 5′-CAGAAGCAGAAGTAGGGAGAGGTGCCGATC-3′ (anti-sense); for claudin-5 (200 bp), 5′-GCTGGCGCTGGTGGCACTCTTTGT (sense) and 5′-GGCGAACCAGCAGAGCGGCAC-3′ (anti-sense); and for ZO-2 (240 bp), 5′-TCAAACCCCTCATCCGCTGCTGGTA-3′ (sense) and 5′-AGTGTTCCGTTTCAATGTCTCTTTTAC-3′ (anti-sense). PCR conditions were 40 cycles at 94°C for 30 sec, 60°C for 30 sec, and 72°C for 1 min, and a final cycle of 72°C for 1 min. The 1.8% agarose gel and MultiDoc-It™ digital imaging System (UVP, LLC, Upland, CA) were used.
Overexpressing MMP-9 and p38 MAPK in bEnd3 cells
The bEnd3 cells were grown to confluence on 100-cm2 culture plates in Dulbecco's modified Eagle's medium with 4.5 g/L glucose, 3.7 g/L sodium bicarbonate, 4 mM glutamine, 10% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin. Cells were transfected with GFP-tagged MMP-9 and p38 MAPK cDNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Cells transfected with expression vector served as controls. Transfection efficiency was monitored by fluorescence microscopy.
Preparation of cell extracts and western blotting analysis
Cells were washed with PBS and collected into 1 ml of lysate buffer (50 mM Tris-HCl pH 7.3, 150 mM NaCl, 3 mM MgCl, 1 mM DTT, 1 mM EDTA, 1mM EGTA, 1.0 % Triton X-100), and supplemented with protease and phosphatase inhibitors. The extraction supernatant was collected, and 30 μg of protein from each sample was resolved on 4-20% SDS-PAGE gels, transferred, and immunoblotted onto nitrocellulose membrane. Anti-claudin-5, anti-occludin, anti-ZO-1, anti-ZO-2, anti-phospho p38 MAPK, anti-p38 MAPK, anti-IκBα, and anti-GAPDH antibodies were used as primary antibodies.
Immunoprecipitation
The cells were lysed with ice cold immunoprecipitation buffer (50 mM HEPES, pH 7.5, 50 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 mM sodium pyrophosphate, 1 mM Na3VO4, 30 mM 2-(p-nitrophenyl) phosphate, 100 mM NaF, 10% glycerol, 1.5 mM MgCl2, and protease inhibitor cocktail). Lysates were centrifuged at 14,000 g for 5 min, and the supernatant was immunoprecipitated with anti-EGFR and Dynabeads M-280 magnetic protein bead separation system (Invitrogen) overnight at 4°C.
Brains from mice with ALF
ALF was induced with an intraperitoneal (ip) injection with azoxymethane (AOM) (Sigma-Aldrich) as described in our previous report (13). Control mice received saline. At 12 hours after AOM injection, ALF mice received 2 mg/kg of GM6001 or vehicle via ip injection. Control mice received vehicle. Heating pad was used to maintain body temperature at 37°C. The use of animals was institutionally approved in accordance with The National Institute of Health Guide for the Care and Use of Laboratory Animals. The progression of hepatic encephalopathy was determined clinically (13). At the comatose stages, the study mice were killed, and their brains were removed. Hemibrains were homogenized at 150 mg tissue/mL of lysate buffer and processed for analysis.
Results
Occludin alterations in bEnd3 cells exposed to MMP-9 are mediated by p38 MAPK
We recently reported that MMP-9 disrupts TJ proteins in mouse brain EC in vitro and in brains of mice with ALF (5). We also observed a significant increase in p38 MAPK activation in ALF mice (30). Thus, we investigated whether p38 MAPK contributes to occludin alterations in bEnd3 cells exposed to MMP-9. Similar to the methods used in our previous report (5), we overexpressed MMP-9 in bEnd3 cells. We observed that MMP-9 significantly upregulated p38 MAPK phosphorylation, i.e., activation, as well as decreased the amount of occludin and its associate ZO-1 (Figure 1A). The decrease of occludin and ZO-1 was reversed by the p38 MAPK-specific inhibitor SB203580 but not by the p42/44 MAPK inhibitor PD98059 (not shown). In contrast, alterations in other TJ proteins (claudin-5 and ZO-2) were not reversed by SB20358 (not shown). To further corroborate the effect of p38 MAPK, we inhibited p38 MAPK expression by using siRNA. We found that blocking p38 MAPK upregulation effectively mitigated the reduction of occludin and ZO-1 (Figure 1 B and C). These results showed that p38 MAPK is important for occludin regulation.
Upregulating p38 MAPK leads to IκBα degradation, NFκB activation, and occludin decrease in bEnd3 cells
To investigate the effect of p38 MAPK on the transcription and expression of occludin, we transfected bEnd3 cells with p38 MAPK cDNA for 18 hours then treated the cells with or without SB203580 at 1 μM for 3 hours. Using RT-PCR, we found that overexpressing p38 MAPK resulted in significant suppression of mRNA levels of occludin and ZO-1 but not claudin-5 and ZO-2 (Figure 2 A and C). The effect was reversed with SB203580. Importantly, upregulating p38 MAPK did not change the MMP-9 mRNA level in the brain EC (Figure 2A and C).



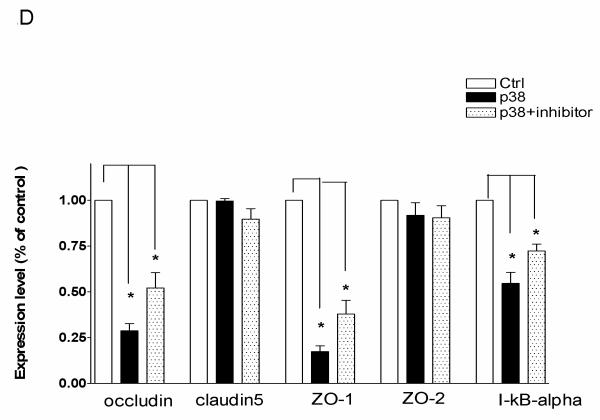

(A) RT-PCR analysis of MMP-9, occludin, claudin-5, ZO-1, and ZO-2 in bEnd3 cells in which p38 MAPK was upregulated in the presence or absence of SB203580. (B) Western blot analysis of occludin, claudin-5, ZO-1, ZO-2, and IκBα in bEnd3 cells in which p38 MAPK was upregulated in the presence or absence of SB203580. (C) Densitometric measurements for (A). (D) Densitometric measurements for (B). (E) Western blot analysis of occludin, ZO-1, and IκBα in bEnd3 cells in which p38 MAPK was upregulated in the presence or absence of the inhibitor of NFκB activation; GAPDH served as control.. *P<0.01.
With western blotting, we found that occludin and ZO-1 were significantly reduced by p38 MAPK upregulation, and the reduction was partly restored with the p38 MAPK inhibitor SB203580 (Figure 2 B and D). In contrast, claudin-5 and ZO-2 were not affected.
Because p38 MAPK is associated with IκBα degradation and NFκB activation (31, 32), we investigated the status of IκBα protein and NFκB in bEnd3 cells in which p38 MAPK cDNA was overexpressed. The bEnd3 cells were transfected with p38 MAPK cDNA then treated with 10 nM of NFκB activation inhibitor. We found that p38 MAPK upregulation reduced IκBα, occludin, and ZO-1 levels. Importantly, we demonstrated that p38 MAPK activation induces IκBα degradation, which was reversed by treatment with the p38 MAPK inhibitor SB203580 (Figure 2E). Moreover, we found that after administering NFκB inhibitor, the degradation of IκBα became enhanced in p38 MAPK-upregulated cells, and the decrease of occludin and ZO-1 was reversed (Figure 2E). Overall, our results indicate that p38 MAPK induces the degradation of IκBα, which leads to the release of NFκB activation that regulates occludin and ZO-1 expression in mouse brain EC cells.
MMP-9-induced EGFR transactivation leads to p38 MAPK signaling and occludin alterations
MMP-9 has been shown to transactivate EGFR (33, 34). Ligation of EGFR results in the activation of MAPK cascades (23) and potentially in modulating TJ proteins (35). We thus speculated that EGFR activation might be important in MMP-9-induced alteration of occludin.
We first confirmed whether MMP-9 could transactivate EGFR in bEnd3 cells. As shown in Figure 3A and B, when bEnd3 cells were exposed to MMP-9, EGFR was phosphorylated (p-TyrEGFR), i.e., activated, as determined by western blotting. We pretreated the bEnd3 cells with GM6001 (an MMP-9 inhibitor) then overexpressed MMP-9; we found that MMP-9 inhibition attenuated the EGFR activation. Using confocal microscopy, we corroborated the findings that activated EGFR was upregulated in the bEnd3 cells and that EGFR activation was prevented with GM6001 (Figure 3C). These findings confirmed that EGFR is transactivated by MMP-9 in bEnd3 cells.
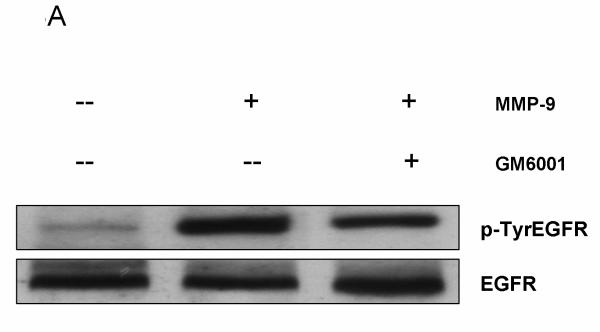

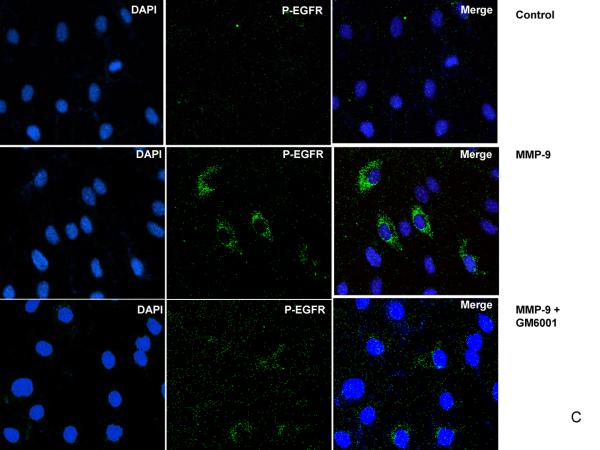
EGFR activation in bEnd3 cells that were treated with human recombinant MMP-9 (100 ng/ml for 6 hours) in the presence or absence of the MMP-9 inhibitor GM6001 (100nM). (A) Cell lysates were immunoprecipitated with anti-EGFR antibody and analyzed by immunoblotting with anti-p-TyrEGFR and anti-EGFR antibodies. (B) Densitometric analysis of the p-TyrEGFR, **P<0.01. (C) For confocal microscopy, bEnd3 cells were fixed and immunostained for p-TyrEGFR (green color) and for nuclei with DAPI (blue).
We then determined whether EGFR would directly influence the p38 MAPK activation with subsequent occludin alterations. As shown in Figure 4, the specific EGFR inhibitor AG1478 significantly reduced the p38 MAPK activation and occludin loss in a dose-dependent manner. Importantly, p38 MAPK activation and suppression of occludin were similarly blocked by EGFR siRNA (Figure 4). Overall, EGFR inhibition with AG1478 or EGFR deletion with siRNA blocked p38 MAPK phosphorylation and restored occludin in brain EC.
Activated EGFR, p38 MAPK activation, IκBα reduction, and NFκB activation are associated with occludin alterations in the brains of ALF mice
Previously we demonstrated that in ALF mice, occludin was significantly perturbed (5). In the present study, we assessed the role of EGFR activation and its associated p38 MAPK/NFκB signaling in brains of ALF mice. We showed by western blotting that occludin was significantly altered in the brains of ALF mice and the alteration was restored with GM6001 treatment. These results are consistent with our previous report (5). Importantly, we observed EGFR activation along with p38 MAPK activation and IκBα degradation in the brains of ALF mice (Figure 5 A and B). With confocal microscopy, we substantiated that significant EGFR activation occurred in brains of ALF mice and that EGFR activation was attenuated with GM6001 treatment (Figure 5C). In contrast, brains of normal control mice showed no EGFR activation.
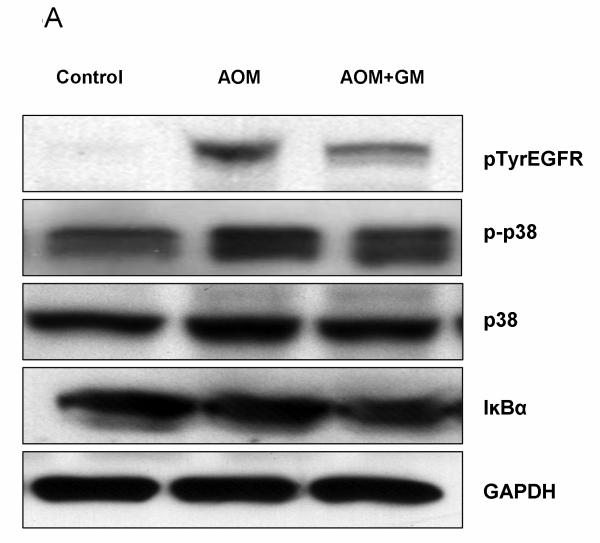

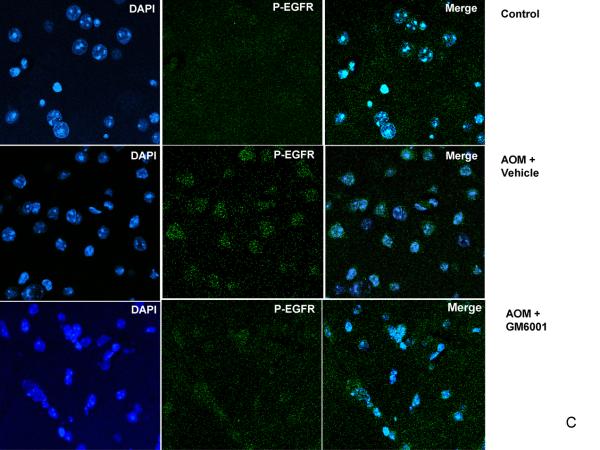
EGFR activation and p38 MAPK/NFκB activation in brains of ALF mice. (A) Western blot of brain samples from control mice, and AOM-induced ALF mice treated with vehicle or with GM6001. (B) Densitometric quantification of p38 MAPK, IκBα, and p-TyrEGFR expression in the brains of control mice and ALF mice treated with and without GM6001. Values are expressed as the mean ± SEM with t-test. (N=3,*P<0.05). (C) Confocal microscopic evaluation of the brains of control versus ALF mice that were treated with vehicle or GM6001. Activated EGFR is seen as green and the nucleus as blue.
We observed spontaneous hypothermia in AOM-induced ALF mice (Figure 6A). With heat support, the body temperature of AOM mice was maintained at normothermia. Treatment with GM6001 did not alter the body temperature of the study mice (Figure 6B). As shown in Figures 6 C and D, we observed that the occludin alteration in AOM-induced ALF mice was independent of body temperature and was reversed with GM6001. In addition, to investigate whether the occludin alteration occurs in other model of ALF, we employed a well-established model using tumor necrosis factor-alpha (TNF) and D-galactosamine (Gal) (36). We found that occludin was decreased in brains of the Gal/TNF-induced ALF mice and that the occludin alteration was reversed with GM6001 treatment and was independent of body temperature (Figure 6 E and F).

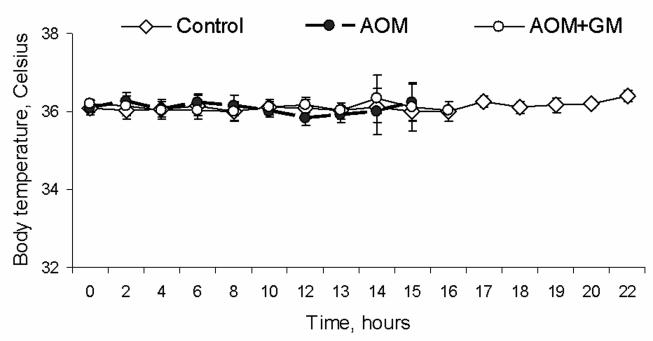


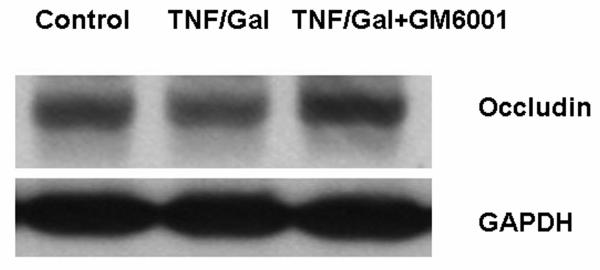

(A) Body temperature of AOM-induced mice showed a spontaneous hypothermia without (w/o) heating pad. Heating support provided a steady normothermia in the control and ALF mice. (B) Treatment with GM6001 did not alter the body temperature of the study mice. The control, AOM, and AOM+GM6001 mice were maintained at 37°C (N=8 each group). There was no difference in body temperature among study groups. (C) In the AOM-induced ALF mice with normothermia, occludin was observed to decrease in the AOM mice and was reversed to normal level with GM6001. (D) Histogram of occludin alteration in the control, AOM, and AOM-induced ALF mice treated with GM6001 (N=8 in each group; ** p <0.001). (E) In mice that had ALF induced with D-galactosamine and TNF at normothermia, occludin was observed to decrease in the ALF mice and was reversed to normal level with GM6001. (F) Histogram of occludin alteration in the control, Gal/TNF, and Gal/TNF-induced ALF mice treated with GM6001 (N=9 in each group; ** p <0.001).
These results from AOM-induced ALF mice are consistent with the findings in vitro, suggesting that MMP-9 induced EGFR transactivation and that p38 MAPK/NFkB signaling plays an important role in regulating BBB TJ proteins in ALF. Collectively our findings suggest that in addition to its direct proteolytic action (5), MMP-9 influences the TJ protein occludin in an indirect way through the following series of steps: first by transactivating EGFR on the bEnd3 cellular surface, second by upregulating p38 MAPK, third by IκBα degradation and NFκB activation, and finally by suppressing occludin expression. This model is summarized in Figure 7.


(A) Representation of a neurovascular unit consisting of a capillary endothelial cell and its tight junction, basal lamina, and astrocytic endfoot in a normal brain. (B) Neurovascular unit in brain of a subject with ALF showing a subtle alteration in the TJ protein occludin with EGFR being transactivated and consequent p38 MAPK/NFκB activation. Perturbations in endothelial cellular plasmalemma are suggested by the interrupted lines, and swollen astrocytic endfoot by size expansion. Figure key: EGFR, epidermal growth factor receptor; p-p38, phospho-p38 MAPK; MMP-9, matrix metalloproteinase-9; NFκB, nuclear factor-kappa B; IκBα, I-kappa B alpha; JAM, junction associated molecules.
Discussion
In this study, we have shown that MMP-9 transactivates EGFR in brain microvascular ECs with subsequent p38 MAPK/NFkB signaling, resulting in suppressed transcription/translation and protein expression of the TJ protein occludin. These effects were attenuated with specific inhibition of EGFR in brain ECs in vitro. Moreover, we observed EGFR activation, p38 MAPK activation, and the loss of occludin in brains of mice with experimentally induced ALF. Together these results suggest that EGFR plays a role in activating the pathobiology of brain injury in ALF.
Brain edema in ALF is unique. It occurs in the comatose stages of encephalopathy in ALF. The onset of encephalopathy in ALF patients presages impending brain edema and a lethal course of the disease. Once liver failure is resolved, either by liver transplantation or by spontaneous recovery of the injured liver, the brain edema is resolved. However, if the brain edema is inadequately controlled, it will ultimately lead to herniation and brain death. Even with significant brain edema, both light and electron microscopic evaluations of these brains reveal that the BBB and its TJs remain relatively intact. However, there are certain subtle changes, including fine perturbations at the endothelial cellular plasma membrane and thickening of the basal lamina (37). In the absence of obvious structural breakdown of the BBB, the prominent and consistent swelling of astrocytic foot processes has led to the dominant theory that cytotoxic mechanisms cause brain edema in ALF (38). Although vasogenic elements have been implicated (39, 40), evidence for vasogenic edema in ALF has been lacking.
Increasing evidence has suggested that even with a relatively intact BBB, subtle alterations in TJ composition can result in highly selective permeability to small molecules, such as water and ammonia. In mice that are selectively deficient in claudin-5, a component of TJ proteins, the BBB and TJ appear intact under electron microscopic examination. However, these mice have increased permeability to molecules that are less than 800 Da (8). Similarly, when occludin at the TJ is targeted with a specific peptide or is modified by proteolysis, TJ permeability is significantly increased without any obvious structural change (7, 9, 41). Collectively these data indicate that altered permeability of the BBB can result from very subtle changes in the BBB and/or TJ composition.
We recently observed that there are significant biochemical alterations in occludin, claudin-5, and ZO-1 in brains of mice with experimentally induced ALF (5) and that these changes were attenuated with when MMP-9 was inhibited (5, 13). We observed similar findings when murine brain EC were exposed to MMP-9 in vitro (5). However, TJs make up only a small part of the brain capillary surface area. The endothelial cellular plasmalemma interacts with MMP-9 or other inciting factors within the capillary circulation to a greater extent than the TJs. Therefore, we speculated that endothelial cellular interactions with MMP-9 via surface receptors might play a major role in regulating the BBB integrity in ALF.
EGFR is well known for its role in cancer invasion and metastasis (15). EGFR is essential in epithelial cellular integrity in response to injury (17). Recently, EGFR was shown to participate in altered microvascular permeability in intestinal disorders (21, 22), lung injury (19, 20), and diabetic vascular damage (18).
In this study, we investigated the role of EGFR activation and associated signaling in occludin regulation. We found that MMP-9 transactivates EGFR in brain ECs, which activates p38 MAPK, decreases I.Ba, and leads to the activation of NF.B with subsequent suppression of the transcription and translation of occludin at the TJs. In a mouse model of ALF that recapitulates the human form of ALF, we observed similar effects of EGFR activation and signaling on changes in occludin expression (13, 42). These results suggest that EGFR activation and p38 MAPK/NFkB signaling play important roles in regulating the TJ integrity in ALF.
The effects of MMP-9 on BBB permeability in ALF might thus involve more than one pathway. Direct degradation of the extracellular components of occludin and other TJ proteins is an important element. However, because the TJ architecture does not change in ALF, the exposure of occludin is limited. It follows that MMP-9's direct action on occludin, being most apical and closest to the capillary lumen, would be restricted (5). Because TJs make up a small portion of the BBB, quantitatively it might appear that MMP-9's effects on the EC surface could be more important. Although we do not know which path, i.e., transcellular or paracellular, is the more important regulator or contributor to the overall BBB dysfunction in ALF, the results from this study broaden the impact of MMP-9 on BBB integrity. Furthermore, EGFR might mediate a transcellular transport, and its cascade of intracellular signals could serve to fine tune the overall BBB integrity. Fine regulation of the TJ composition via the EGFR cascade may represent a subtle modulation of the BBB in ALF.
In this study we limited our focus to EGFR transactivation with MMP-9. However, MMP-2 and other MMPs, TNFα, and IL-1 may contribute to the overall disease process (43, 44). It should be noted that occludin alteration was not observed in AOM-treated mice in recent report (45). The observed difference remains to be investigated. In contrast, occludin is shown to significantly altered in mice with ALF that is induced with D-galactosamine and liposaccharide (43). Similarly, we observed significant occludin perturbations in the brains of mice that had Gal/TNF-induced ALF suggesting that occludin alteration is independent of induction agents. Moreover, a similar occludin alteration has also been shown in rats with portocaval shunt and hepatic artery ligation and the occludin reduction was not reversed with hypothermia consistent with our observation that occludin regulation is independent of hypothermia (4). Collectively, these observations suggest that occludin may be the common link in the brain injury associated with ALF.
Because both vasogenic and cytotoxic mechanisms are implicated in the pathogenesis of brain edema in ALF, which mechanism precedes and which is more important in the onset of edema formation remain unresolved. Earlier evidence suggested that increased permeability to the small molecules precedes encephalopathy and edema (47). However, the cytotoxic pathway could be the leading event. It is most likely that both vasogenic and cytotoxic mechanisms are involved. Further study is required to elucidate the extent and order of involvement of the vasogenic and cytotoxic mechanisms in ALF.
In conclusion, we have shown that EGFR activation with p38MAPK/NF.B signal transduction contributes to the regulation of BBB TJ integrity in ALF. These findings not only provide evidence for vasogenic mechanisms in the pathogenesis of brain edema, but also provide a potential target for therapeutic measures to achieve effective control of the development and progression of brain edema in ALF.
Acknowledgement
The authors thank Kathleen Norton and Lisa Maroski for their editorial assistance.
Grant support: The work was supported to JHN by the Deason Foundation, Sandra and Eugene Davenport, Mayo Clinic CD CRT-II, AHA 0655589B, and R01NS051646-01A2.
Abbreviations
| EGFR | epidermal growth factor receptor |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| NFκB | nuclear factor-kappa B |
| IκBα | I-kappa B alpha |
| TJ | tight junction |
| ALF | acute liver failure |
| BBB | blood-brain barrier |
| EC | endothelial cell |
| RT-PCR | reverse transcription-polymerase chain reaction |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/hep.24161
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/hep.24161
Citations & impact
Impact metrics
Citations of article over time
Article citations
RIPK1 inhibitor ameliorates pulmonary injury by modulating the function of neutrophils and vascular endothelial cells.
Cell Death Discov, 10(1):152, 23 Mar 2024
Cited by: 1 article | PMID: 38521771 | PMCID: PMC10960796
The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies.
Int J Mol Sci, 25(11):5601, 21 May 2024
Cited by: 1 article | PMID: 38891789 | PMCID: PMC11172262
Review Free full text in Europe PMC
Swertia cincta Burkill alleviates LPS/D-GalN-induced acute liver failure by modulating apoptosis and oxidative stress signaling pathways.
Aging (Albany NY), 15(12):5887-5916, 27 Jun 2023
Cited by: 2 articles | PMID: 37379130 | PMCID: PMC10333062
New Insight Into Mechanisms of Hepatic Encephalopathy: An Integrative Analysis Approach to Identify Molecular Markers and Therapeutic Targets.
Bioinform Biol Insights, 17:11779322231155068, 17 Feb 2023
Cited by: 1 article | PMID: 36814683 | PMCID: PMC9940182
The Cerebral Effect of Ammonia in Brain Aging: Blood-Brain Barrier Breakdown, Mitochondrial Dysfunction, and Neuroinflammation.
J Clin Med, 10(13):2773, 24 Jun 2021
Cited by: 10 articles | PMID: 34202669 | PMCID: PMC8268635
Review Free full text in Europe PMC
Go to all (29) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure.
Hepatology, 50(6):1914-1923, 01 Dec 2009
Cited by: 104 articles | PMID: 19821483 | PMCID: PMC2925168
C5a/C5aR pathway is essential for up-regulating SphK1 expression through p38-MAPK activation in acute liver failure.
World J Gastroenterol, 22(46):10148-10157, 01 Dec 2016
Cited by: 14 articles | PMID: 28028363 | PMCID: PMC5155174
IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta , NF-kappaB, and AP-1 activation.
Am J Physiol Heart Circ Physiol, 293(6):H3356-65, 05 Oct 2007
Cited by: 152 articles | PMID: 17921324
Involvement of MAPKs in endostatin-mediated regulation of blood-retinal barrier function.
Curr Eye Res, 31(12):1033-1045, 01 Dec 2006
Cited by: 20 articles | PMID: 17169842
Funding
Funders who supported this work.
NIDDK NIH HHS (3)
Grant ID: R21 DK064361-01
Grant ID: R21 DK064361
Grant ID: R21 DK064361-02
NINDS NIH HHS (4)
Grant ID: R01 NS051646-01A2
Grant ID: R01 NS051646-02
Grant ID: R01 NS051646
Grant ID: R01 NS051646-03







