Abstract
Free full text

ANGIOGENIC GROWTH FACTORS ARE NEW AND ESSENTIAL PLAYERS IN THE SUSTAINED RELAXIN VASODILATORY PATHWAY IN RODENTS AND HUMANS
Abstract
Relaxin is emerging as an important vasodilator of pregnancy, and is being tested for afterload reduction in acute heart failure. However, the mechanisms underlying relaxin-induced vasodilation are incompletely understood. The aims of this study were to establish a new in vitro model for relaxin-induced vasodilation, and to use this approach as well as chronically instrumented, conscious rats to investigate the role of angiogenic growth factors in the relaxin vasodilatory pathway. Incubation of rat and mouse small renal arteries with recombinant human H2 relaxin for 3hr in vitro attenuated myogenic constriction, which was blocked by inhibitors of gelatinases, the endothelin B receptor and nitric oxide synthase. These findings corroborate ex vivo observations in arteries isolated from relaxin-infused nonpregnant and midterm pregnant rats, thereby validating the new experimental approach and enabling study of human arteries. Incubation of small human subcutaneous arteries with relaxin for 3hr in vitro also attenuated myogenic constriction through the same molecular intermediates. Vascular endothelial growth factor receptor inhibitor SU5416, three different vascular endothelial growth factor and two different placental growth factor neutralizing antibodies prevented relaxin from attenuating myogenic constriction in rat and mouse small renal, and human subcutaneous arteries. SU5416 administration also prevented relaxin-induced renal vasodilation and hyperfiltration in chronically instrumented, conscious rats. Small renal arteries isolated from these rats demonstrated increased MMP-2 activity in the relaxin-infused group, which was not prevented by SU5416. We conclude that there is concordance of relaxin vasodilatory mechanisms in rats, mice and humans, and angiogenic growth factors are novel and essential intermediates.
Introduction
Relaxin is a 6kDa protein hormone secreted by the corpus luteum that circulates in the blood during the luteal phase of the menstrual cycle and in pregnancy (1). Evidence is also emerging for a local relaxin ligand-receptor system in arteries (2). Relaxin is a potent vasodilator in many animal species including humans (reviewed in 3–5). The hormone has also been implicated in the vasodilatory phenomena of pregnancy (6–9). Recent findings suggest that the vasodilatory responses to relaxin are mediated by its major receptor, the relaxin/insulin-like family peptide receptor 1, RXFP1 (6). An emerging concept is that the molecular mechanisms of relaxin-induced vasodilation depend on the duration of hormone exposure; i.e., rapid (within minutes) and sustained (hours to days). The rapid vasodilatory responses to relaxin are transduced by endothelial Gαi/o protein coupling to phosphotidylinositol-3 kinase/Akt (protein kinase B)-dependent phosphorylation and activation of nitric oxide synthase (NOS), and unexpectedly, this response is amplified by inhibition of vascular endothelial growth factor (VEGF) receptor tyrosine kinase (RTK) activity (7). Sustained vasodilatory responses to relaxin critically depend on increases in arterial gelatinase activity, either matrix metalloproteinase (MMP)-9 or -2 depending on whether the duration of hormone exposure is on the order of hours or days, respectively (3–5). The gelatinases, in turn, hydrolyze big endothelin (ET) at a gly-leu bond to form ET1–32, which activates the endothelial ETB receptor/nitric oxide (NO) vasodilatory pathway (3–5); see Working Model in Figure 7).
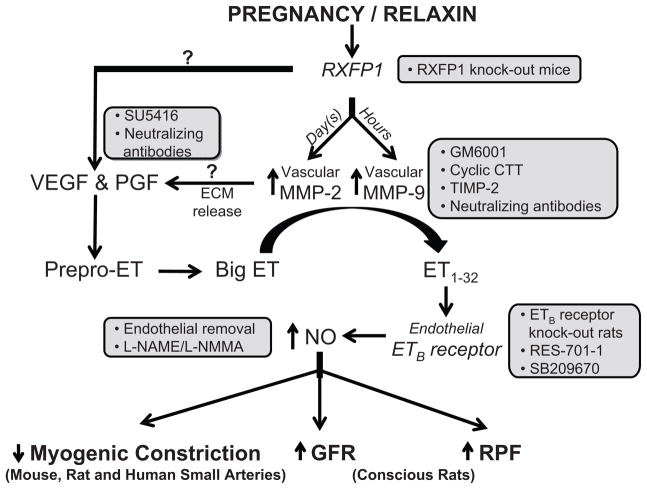
The precise localization of VEGF and PGF in the relaxin vasodilatory pathway is currently unknown (?); relaxin may increase expression of angiogenic growth factor(s) in the arterial wall and/or release them from the extracellular matrix via MMP-9 or -2. Inhibitors of pregnancy- and/or relaxin-induced vasodilation are shown in the boxes. ET, endothelin; MMP, matrix metalloproteinase; ECM, extracellular matrix; RBF, renal blood flow; GFR, glomerular filtration rate; RXFP, relaxin/insulin-like family peptide receptors; SU5416, vascular endothelial growth factor receptor tyrosine kinase inhibitor; GM6001, a general MMP inhibitor; cyclic CTT, a specific peptide inhibitor of MMP-2; TIMP-2, tissue inhibitor of matrix metalloproteinase; RES-701-1, a specific ETB receptor antagonist; SB209670, a mixed ETA and ETB receptor antagonist; L-NAME, nitro-L-arginine methyl ester; L-NMMA, NG-monomethyl-L-arginine. Note that RXFP2 knockout (in mice), STT (control peptide for cyclic CTT), heat inactivated TIMP-2, BQ-123 (a specific ETA receptor antagonist), phosphoramidon (an inhibitor of the classical endothelin converting enzyme), D-NAME and isotype-matched IgGs (controls for neutralizing antibodies) did not affect the sustained vasodilatory responses to relaxin. See text for details and supporting references.
Relaxin stimulates VEGF synthesis in several types of fibroblasts, endometrial cells and macrophages (8–11). Most (12–14), but not all (15) investigators reported that VEGF increases MMP-2 secretion and activity in cultured human endothelial cells. In addition, VEGF and placental growth factor (PGF) upregulate MMP-9, but not MMP-2 mRNA, protein and activity in cultured human aortic smooth muscle cells, suggesting an intermediary role for VEGF-R1 (16,17). Collectively, these findings motivate part of the current work, which is to investigate whether VEGF plays a role in the sustained vasodilatory response to relaxin, and if so, to determine whether it is upstream of the arterial gelatinase(s). In light of the current trial of recombinant human relaxin (rhRLX) in the treatment of acute heart failure (5,18) and its potential therapeutic use in preeclampsia (19,20), both of which capitalize on the hormone’s unique spectrum of vascular effects, revelation of the mechanisms underlying relaxin’s vasodilatory actions is crucial, as it should facilitate these as well as other future clinical applications.
We first established and extensively validated the use of isolated small arteries in vitro for investigation of the mechanisms of sustained relaxin-induced vasodilation (i.e., we incubated small arteries with rhRLX in vitro and then assessed myogenic constriction). This new experimental model is equally robust, but considerably more convenient than chronic administration of relaxin to rats (21–24). It also set the stage for translating our investigations in rodents to human arteries. We then tested whether pre-incubation with the VEGF RTK inhibitor, SU5416, or with specific VEGF and PGF neutralizing antibodies prevents relaxin from attenuating myogenic constriction in rat and mouse small renal as well as human subcutaneous arteries. In order to establish in vivo relevance, we next investigated whether the VEGF receptor antagonist, SU5416, prevents rhRLX-induced increases in glomerular filtration rate (GFR) and effective renal plasma flow (ERPF), and decreases in effective renal vascular resistance (ERVR) in chronically instrumented, conscious rats. Finally, to determine whether SU5416 prevents the increase in arterial gelatinase activity induced by relaxin, a pivotal step in the relaxin vasodilatory pathway (22), we measured MMP-2 activity in SRA isolated from the chronically instrumented, conscious rats that were studied for renal function.
Methods
A full description of procedures may be found in the Online Supplement. Please see http://hyper.ahajournals.org.
Myogenic behavior of isolated arteries treated with rhRLX in vitro
The effect of relaxin on myogenic constriction (defined here as the change in arterial diameter after increasing intraluminal pressure from 60 to 80 mmHg) was assessed in isolated mouse and rat small renal, and human subcutaneous arteries as previously described for arteries isolated from gravid or rhRLX-infused rodents (2,21,22,25–27). Human arteries were obtained from subcutaneous fat of patients undergoing elective surgery at the Magee Womens Hospital, University of Pittsburgh under the approval of the University of Pittsburgh Institutional Review Board. To determine whether in vitro treatment with rhRLX would attenuate myogenic constriction, arteries were incubated with 30 ng/mL rhRLX or its vehicle (20 mmol/L sodium acetate, pH 5 diluted identically as the stock rhRLX) for 3hr. To test the effect of NOS, ETB receptor and MMP blockade on rhRLX-mediated reduction of myogenic constriction, the respective inhibitors or their vehicle controls were added to the bath and the arteries incubated for a further 30 minutes before assessment of myogenic constriction. SU5416 and VEGF- and PGF-neutralizing antibodies were added 30 minutes before the 3hr rhRLX incubation. Note that isotype control antibodies were instilled into the lumen at the same concentration as the neutralizing antibodies, and the latter were documented to be specific (Supplemental Table S1). In order to account for inherent variability in arterial diameter as illustrated in Supplemental Figures S1 & S2, the results are expressed as % change in diameter from baseline at 60 mmHg (i.e., no change in diameter indicates relatively robust myogenic constriction, whereas an increase in diameter reflects attenuation of myogenic constriction).
Renal function studies in conscious rats
Rats were chronically instrumented as previously described for assessment of renal function and mean arterial pressure (MAP) (28,29). The experimental protocol followed the timeline illustrated in Figure 1. Renal function and MAP were assessed in the conscious state as previously reported (28,29).

The experiment was designed to test the hypothesis that angiogenic growth factor receptor(s) are involved in relaxin-induced increases in effective renal plasma flow (ERPF) and glomerular filtration rate (GFR) in conscious female rats. Mean arterial pressure (MAP) was also assessed. Note that osmotic pumps containing either recombinant human relaxin (rhRLX) or dilute sodium acetate vehicle were primed prior to implantation, so that infusion commenced immediately.
Zymographic analysis of small renal arteries (SRA)
After the final renal function experiment, SRA were isolated and snap frozen for assessment of gelatinase activity by zymography, which was performed as previously described with minor modifications (22,26,30). The Institutional Animal Care and Use Committee approved all procedures involving animals.
Results
Validation of a new experimental model for investigating mechanisms of sustained relaxin-induced vasodilation
SRA isolated from rats or mice that were administered rhRLX for 5 days showed attenuated myogenic constriction (21,22,26). In the present work, treatment of isolated rat and mouse small renal, and human subcutaneous arteries with 30 ng/ml rhRLX for 3 hr in vitro also reduced myogenic constriction (Table 1). A full dose response for rhRLX revealed a threshold of ~ 6 ng/ml in rat SRA (Supplemental Figure S3). Among other members of the insulin-relaxin family of structurally related hormones, relaxin-3, but not Insl-3, attenuated myogenic constriction of rat SRA (Supplemental Figure S4). Addition of GM6001 (1 μmol/L), RES-701-1 (10 μmol/L) or L-NMMA (100 μmol/L) instead of their respective vehicles to paired arteries from the same animal or patient for 30 min after 3 hr rhRLX treatment (and in the continuing presence of rhRLX) abolished the effect of rhRLX and restored myogenic constriction (Table 1 & Supplemental Figure S4A). Incubation of rat SRA with a specific MMP-9, but not MMP-2 neutralizing antibody (Ab) also prevented rhRLX from attenuating myogenic constriction (% change in diameter from 60 to 80 mmHg in the presence of MMP-9 Ab: 1.6 ± 0.3 [n=6 rats]; MMP-2 Ab: 8.7 ± 0.9 [n=3 rats]; control mouse IgG1k: 8.8 ± 0.9 [n=4 rats]; P<0.0001 MMP-9 vs other treatments by ANOVA with LSD post-hoc tests). Finally, in essential control experiments, the vehicle for rhRLX (20mM sodium acetate pH 5.0 diluted identically to the stock rhRLX) did not prevent the normal myogenic constriction response of isolated rat small renal and human subcutaneous arteries to a 20 mmHg increase in intraluminal pressure, either in the presence of inhibitors or their respective vehicles (Supplemental Table S2). Thus, treatment of small arteries with rhRLX in vitro inhibits myogenic constriction by the same molecular intermediates as previously described for small arteries isolated from rhRLX-treated animals and studied ex vivo.
Table 1
In most experiments, rat and mouse small renal, and human subcutaneous arteries were isolated and incubated with 30 ng/ml rhRLX for 3 hr in vitro. Inhibitor or vehicle was then added to the bath for an additional 30 min (in the continuing presence of rhRLX), after which myogenic reactivity was measured. The exception is for nitric oxide synthase inhibition with L-NMMA in mouse small renal arteries, in which the arteries were dissected from mice administered 1 μg/h rhRLX by osmotic pump in vivo for 5 days. Myogenic reactivity is expressed as % change in internal diameter from 60 to 80 mmHg (mean ± SEM). See Expanded Methods for details.
| Rat small renal arteries | Mouse small renal arteries | Human subcutaneous arteries | ||||
|---|---|---|---|---|---|---|
| rhRLX + inhibitor | rhRLX + vehicle | rhRLX + inhibitor | rhRLX + vehicle | rhRLX + inhibitor | rhRLX + vehicle | |
| GM6001 | −0.5 ± 0.9 (n=5 rats) † | 9.1 ± 0.9 (n=6 rats) | 0.3 ± 0.9 (n=3 mice) * | 5.2 ± 0.7 (n=3 mice) | 1.5 ± 0.5 (n=4 patients) * | 6.7 ± 1.2 (n=3 patients) |
| RES-701-1 | 1.6 ± 1.3 (n=6 rats) * | 10.3 ± 2.0 (n=6 rats) | 2.1 ± 1.1 (n=2 mice) | 7.8 ± 1.3 (n=2 mice) | 0.5 ± 0.9 (n=3 patients) | 5.4 ± 0.7 (n=2 patients) |
| L-NMMA | −2.3 ± 1.3 (n=6 rats) * | 5.8 ± 0.8 (n=6 rats) | 1.2 ± 1.0 (n=3mice) * | 5.7 ± 1.2 (n=3 mice) | -3.2 ± 1.1 (n=4 patients) * | 5.8 ± 1.1 (n=4 patients) |
Role of angiogenic growth factors in the sustained relaxin vasodilatory pathway: arterial myogenic constriction
Pre-treatment of rat small renal and human subcutaneous arteries with the VEGF RTK inhibitor SU5416 (1μmol/L) for 30 min prevented rhRLX (30 ng/ml; 3 hr) from inhibiting myogenic constriction (P<0.005 and P=0.05 vs dilute dimethylsulfoxide (DMSO) vehicle control, respectively; Figure 2A & B). Thus, myogenic constriction was reduced by rhRLX in the presence of dilute DMSO alone, but was preserved when arteries were pre-treated with SU5416. Surprisingly, incubation of arteries with SU5416 for 30 min after 3 hr treatment with, and in the continuing presence of rhRLX, did not prevent relaxin-induced attenuation of myogenic constriction (P=NS; Figure 2C).

(A) Rat small renal and (B) human subcutaneous arteries were pretreated for 30 min with SU5416 (1 μmol/L) or dilute dimethylsulfoxide (DMSO) vehicle and incubated with recombinant human relaxin (rhRLX; 30 ng/ml) for 3 hr. (C) Additional rat small renal arteries were first treated with rhRLX for 3 hr, then SU5416 or DMSO was added to the bath for 30 min in the continuing presence of rhRLX before assessing myogenic constriction (P=NS). *P=0.05, †P<0.005 SU5416 vs DMSO. The initial diameters (μm) were: (A) SU5416 225.1±24.9, DMSO 210.0±14.0; (B) SU5416 281.7±26.3, DMSO 201.7±64.6, and (C) rhRLX/SU5416 245.4±30.5, rhRLX/DMSO 245.5±16.3 (all P=NS by Wilcoxon signed-ranks or Krusal-Wallis test).
Consistent with the effect of SU5416, pre-treatment of isolated rat and mouse small renal, and human subcutaneous arteries with three different VEGF-neutralizing antibodies (1 or 3 μg/ml instilled internally) prevented rhRLX from reducing myogenic constriction (P<0.01, <0.001 and <0.05 vs. goat IgG, respectively; Figure 3A–C). Unexpectedly, a specific PGF-neutralizing antibody (1.0 μg/ml) also blocked the response to rhRLX in isolated mouse and rat SRA (P<0.01 and P=0.05 vs. rat IgG2A, respectively; Figure 3D & E). Using another PGF neutralizing antibody (10 μg/ml), a similar trend for human subcutaneous arteries was clear, although small sample size precluded statistical analysis (Figure 3F). Note that isotype control antibodies were instilled into the lumen at the same concentration as the neutralizing antibodies, and the latter were documented to be specific (Supplemental Table S1).

(A) Mouse and (B) rat small renal, and (C) human subcutaneous arteries were pretreated for 30 minutes with specific VEGF-neutralizing or goat IgG isotype control antibodies (1 – 3 μg/ml instilled internally) and incubated with recombinant human relaxin (rhRLX; 30 ng/ml) for 3 hr. (D) Mouse and (E) rat small renal, and (F) human subcutaneous arteries were pretreated for 30 min with specific PGF-neutralizing or rat IgG2A & mouse IgG1 isotype control antibodies (0.1 – 10 μg/ml; instilled internally) and incubated with rhRLX (30 ng/ml) for 3 hr. For additional antibody details and concentrations, see Supplemental Table 2. *P<0.05 †P<0.01 ‡P<0.001 isotype control vs VEGF or PGF antibody (Ab). The initial diameters (μm) were: (A) VEGF Ab 178.1±16.6, Goat IgG 172.3±20.6; (B) VEGF Ab 245.2±15.1, Goat IgG 247.9±14.1; (C) VEGF Ab 178.1±36.4, Goat IgG 276.5±78.5; (D) PGF Ab 162.4±21.2, Rat IgG2A 143.6±8.4; (E) PGF Ab 265.7±44.8 Rat IgG2A 264.3±18.3 (all P=NS by Krusal-Wallis test) and (F) PGF Ab 492.0±73.0 (n=2), Mouse IgG1 534 (n=1).
We had anticipated that PGF neutralizing antibodies would serve as additional negative controls along with the isotype control antibodies. To minimize the possibility that the effect of PGF-neutralizing antibodies was due to non-specific inhibition of VEGF (despite 0% cross constriction reported by the manufacturer; Supplemental Table S1), we also tested a 10-fold lower concentration of PGF Ab in rat SRA and obtained identical results (% change in diameter with control rat IgG2A/rhRLX: 8.0 ± 1.6 [n=4 rats]; 0.1 μg/ml PGF Ab/rhRLX: −0.4 ± 0.6 [n=5 rats], P=0.014). Importantly, treatment of isolated rat SRA with VEGF or PGF neutralizing antibodies or SU5416 in this experimental paradigm produced little, if any change in the dilatory response to methacholine, suggesting that endothelial function (and hence, ability of the arteries to dilate in response to rhRLX, which ultimately occurs via endothelial NO) was not compromised (Figure 4). Finally, the vehicle for rhRLX alone had no effect on myogenic constriction of rat SRA in the presence of either VEGF- or PGF-neutralizing or control antibodies (Supplemental Table S2).

After assessment of myogenic constriction in isolated rat small renal arteries pre-incubated with (A) SU5416 or dilute DMSO vehicle, (B) PGF or (C) VEGF neutralizing or control antibodies, and then incubated with rhRLX (30 ng/ml) for 3 hr, internal pressure was returned to 60 mmHg and arteries were re-equilibrated in fresh buffer for 15 min. Arteries were then constricted to 50% of their initial diameter with phenylephrine, and methacholine was added at either a maximal dose (1×10−6M; A) or in dose-response fashion (B & C). Responses to methacholine are expressed as % relaxation from the phenylephrine-constricted value. Endothelial function was either not compromised in the case of SU5416, slightly enhanced by PGF blockade (P=0.0127), or slightly decreased by VEGF immunoneutralization (P<0.001). P values were obtained by t-test (A) or two way ANOVA (P values in brackets) with LSD post-hoc tests (B & C). *P < 0.05 vs control antibody.
Role of angiogenic growth factors in the sustained relaxin vasodilatory pathway: renal function in chronically instrumented, conscious rats
We previously demonstrated that relaxin-induced renal vasodilation and hyperfiltration in chronically instrumented, conscious rats are mediated by arterial gelatinase, the ETB receptor and NOS (22–24); i.e., the same molecular intermediates that mediate relaxin-induced attenuation of myogenic constriction (21,22,26,30) and vide supra). Because our data so far suggested that VEGF and PGF are also involved in mediating reduction of myogenic constriction by relaxin in rat, mouse and human arteries in vitro, we next tested whether these angiogenic growth factors play a role in relaxin-induced renal vasodilation and hyperfiltration in vivo. To this end, we administered SU5416 (20 mg/kg/d) or its vehicle (DMSO) s.c 24 hr prior to and then daily during chronic administration of rhRLX (4 μg/h) or its vehicle for 3–5 days in conscious, chronically instrumented rats (see Methods and Figure 1). The circulating concentrations of rhRLX reached in rats administered the hormone were similar between SU5416- and DMSO-treated animals (33.4 ± 4.9 vs. 36.9 ± 3.6 ng/ml, respectively; P=0.28 by unpaired t-test). These levels are comparable to midterm pregnancy in rats when gestational renal vasodilation and hyperfiltration peak in this species (1,28).
In the presence of DMSO (the vehicle for SU5416), rhRLX significantly increased GFR and ERPF, and reduced ERVR after 4–6 hr and 3–5 days of infusion compared to baseline (Figure 5A–Cleft panels, all P<0.001 by one way ANOVA) as previously reported (23,31). In contrast, SU5416 administration not only prevented the increases in GFR and ERPF, and the decrease in ERVR observed after 4–6 hr and 3–5 days of rhRLX infusion, but significantly reduced ERPF and enhanced ERVR (Figure 5A–C left panels, all P<0.05 vs baseline). rhRLX had no significant effect on MAP in either DMSO- or SU5416-treated rats (Figure 5D left panel). Infusion of the vehicle for rhRLX also had no significant effect on GFR, ERPF, ERVR or MAP at any time point in the presence of either DMSO or SU5416 (Figure 5A–D right panels).

Treatment with SU5416 (20 mg/kg/d s.c.) blocked the increases in (A) glomerular filtration rate and (B) effective renal plasma flow, and decreases in (C) effective renal vascular resistance induced by recombinant human relaxin (rhRLX; 4 μg/h) infusion for 4–6 hr and 3–5 days without affecting (D) mean arterial pressure (left panels). Control animals received vehicle (20 mM sodium acetate, pH 5) instead of rhRLX (right panels). *P<0.05 vs baseline, †P<0.05 vs SU5416 group by two way ANOVA with LSD post-hoc tests.
Relationship between angiogenic growth factors and MMP-2 in the sustained relaxin vasodilatory pathway
We then investigated gelatinase (MMP-2) activity in SRA harvested from the rats after the last measurements of renal function and MAP. We reasoned that if angiogenic growth factors are proximal to gelatinases in the sustained relaxin vasodilatory pathway as hypothesized, then SU5416 should block relaxin-induced increases in arterial gelatinase activity. Therefore, MMP-2 activity should be lower in SRA from SU5416-treated rats. As we previously reported (22,26,30), rhRLX increased both pro and active MMP-2 activities compared to its vehicle in SRA from rats that received DMSO, the vehicle for SU5416 (Figure 6A). Expressed as the densitometric ratios of rhRLX:vehicle (whereby a ratio of 1.0 would indicate no difference), the average elevation in total MMP-2 (i.e., both pro and active MMP-2) activities induced by rhRLX was 2.31 ± 0.53 (P=0.028 vs 1.0 by one sample t-test). However, there was no consistent pattern of change in pro MMP-2 activity (active MMP-2 band intensity was too low to be quantified for this comparison) between pairs of rhRLX-infused rats (n=9) that were administered DMSO or SU5416 (ratio SU5416: DMSO = 1.24 ± 0.26; P=0.39, Figure 6B).
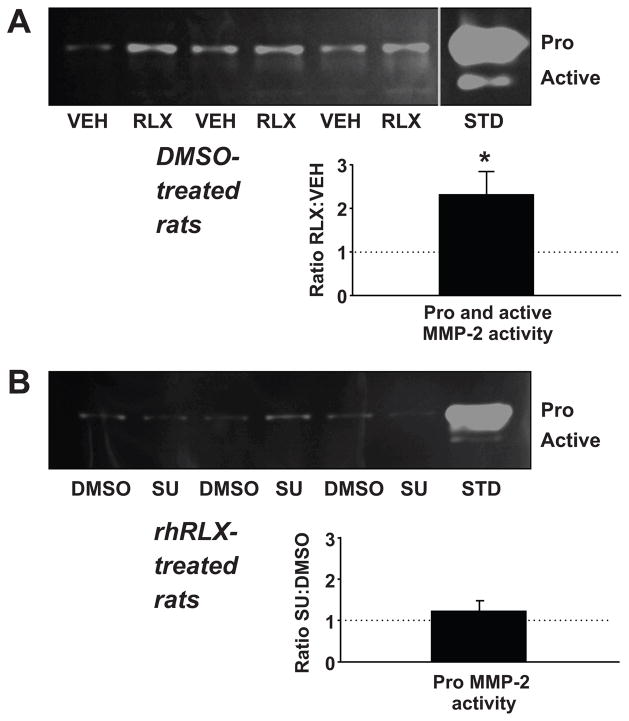
After the last measurement of MAP and renal function (see Figure 1), rats were euthanized and small renal arteries harvested. Upper panels in (A) and (B) depict representative zymograms. The densitometric ratios of rhRLX:vehicle and SU5416:DMSO are shown in the lower panels (n = 7–9 rats in each of the 4 treatment groups). (A) Recombinant human relaxin (RLX; 4 μg/hr) administered in vivo increased pro + active MMP-2 activity compared to sodium acetate vehicle (VEH) in the small renal arteries of rats receiving daily injections of DMSO. *P=0.028 vs 1.0 by one-sample t-test. (B) Pro MMP-2 activity was not consistently different in the small renal arteries of rats treated with rhRLX + SU5416 (SU) compared to rhRLX + DMSO. (Note that active MMP-2 band intensity was too low to be quantified for this comparison.) STD: MMP-2 standard.
Discussion
A major finding of the present work was that treatment of SRA isolated from rats and mice with rhRLX in vitro attenuates myogenic constriction, thus mimicking the functional behavior of SRA isolated from rats and mice administered rhRLX by osmotic pump, and then studied ex vivo (6,21,22,26,30). Relaxin-3 also attenuates myogenic constriction, most likely through the RXFP1 receptor (although a role for the alternative relaxin-3 receptor RXFP3 was not excluded). In contrast, Insl-3, which signals through the RXFP2 receptor, was inactive alone, and also did not prevent rhRLX from attenuating myogenic constriction. This reduction of arterial myogenic constriction by rhRLX corresponds to the renal vasodilation observed in conscious midterm pregnant and rhRLX-infused nonpregnant rats (22–24,28,29,32).
Importantly, we further showed the essential role of arterial gelatinase(s), the endothelial ETB receptor and NOS in rhRLX-induced attenuation of myogenic constriction in vitro. The involvement of these mediators was previously shown for rhRLX- or pregnancy-induced renal vasodilation, and for reduction of myogenic constriction of SRA harvested from rhRLX-infused nonpregnant rats or from gravid rats (reviewed in 3–5,20). Also consistent with our previous observation that attenuation of rat SRA myogenic constriction is mediated exclusively by MMP-9 after short-term (4–6 hr) rhRLX infusion (26), we observed a specific dependency on arterial MMP-9 for reduction of myogenic constriction after 3 hr rhRLX incubation in vitro. In summary, the present studies demonstrate that rhRLX attenuates myogenic constriction of isolated SRA in vitro in an identical manner to SRA isolated from gravid rats or non-pregnant rats administered rhRLX, and by the same molecular intermediates, thus legitimizing the use of isolated SRA in vitro to further explore the molecular mechanisms underlying relaxin’s sustained vasodilatory effect. This new experimental model is as robust, yet considerably more convenient than earlier approaches, because it obviates the need to administer rhRLX in vivo. Therefore, it may accelerate discovery of additional novel relaxin vasodilatory mechanisms. It also provided the opportunity to investigate the effects of relaxin on myogenic constriction in human arteries for the first time.
As such, the discovery that incubation of small subcutaneous arteries from humans with rhRLX for 3 hr in vitro attenuates myogenic constriction, again corresponding to rhRLX-induced renal vasodilation in men and women (33), was another major finding of this study. Attenuation of myogenic constriction in human subcutaneous arteries in vitro was restored to baseline after blockade of MMPs, the ETB receptor and NOS, analogous to rodent arteries (vide supra). Taken together, these findings in rat, mouse and human small arteries in vitro suggest considerable species conservation of relaxin-induced reduction of myogenic constriction and the underlying molecular mechanisms.
The third major finding is conceptually novel, insofar as we revealed strong evidence for the role of VEGF, and unexpectedly PGF as well, in the sustained relaxin vasodilatory pathway, using the in vitro myogenic constriction bioassay established herein (vide supra). We discovered that pre-incubation with the VEGF RTK inhibitor, SU5416, blocked attenuation of myogenic constriction by rhRLX in rat and mouse small renal, and human subcutaneous arteries. However, SU5416 may not be completely specific for VEGF RTK and could interfere with signal transduction of other growth factor receptors (34). Rather than using another pharmacological inhibitor that may lack specificity, we took an immunological approach instead to confirm this finding. Three different VEGF neutralizing antibodies prevented rhRLX-induced reduction of myogenic constriction in isolated rat and mouse small renal, and human subcutaneous arteries, analogous to the results using SU5416.
To demonstrate a unique role for VEGF, we tested two different PGF neutralizing antibodies. Unexpectedly, the PGF antibodies were equally effective in blocking attenuation of myogenic constriction by rhRLX in isolated small arteries. PGF and VEGF have <45% homology and all of the antibodies are documented to be specific (Supplemental Table S1). Nevertheless, we tested a 10-fold lower concentration of the anti-rat/mouse PGF antibody (0.1 μg/ml), in order to minimize any chance of any cross-reactivity with VEGF. Despite the lower concentration, blockade of rhRLX’s effect on isolated rat SRA persisted. Thus, vascular PGF serves as an important intermediate in the sustained relaxin vasodilatory pathway, along with VEGF, gelatinase, endothelin B receptor and NOS. On the one hand, PGF was previously reported to rapidly vasodilate arteries within minutes (35,36), but on the other, 3 weeks of treatment with a PGF neutralizing antibody did not affect MAP in healthy nonpregnant mice (37). PGF neutralizing antibodies also had a negligible effect on maternal body weight gain and fetal outcome in mouse pregnancy (37). Further investigation is needed to reconcile these potentially incongruous findings. Importantly, VEGF and PGF neutralizing antibodies, but not SU5416 only slightly affected the vasodilatory response to methacholine. These results suggest that blockade of relaxin-induced attenuation of myogenic constriction, which ultimately depends on endothelial nitric oxide, was not simply due to non-specific endothelial dysfunction caused by withdrawal of angiogenic growth factors.
Surprisingly, we observed that only pre-, and not post-rhRLX treatment with SU5416 blocked reduction of myogenic constriction by relaxin. One interpretation of this is that angiogenic growth factors are situated upstream of the endothelial ETB receptor and NOS in the sustained relaxin vasodilatory cascade. That is, treatment of arteries with SU5416 for 30 min after 3 hr incubation with rhRLX (and in the continuing presence of rhRLX) is ineffective in restoring myogenic constriction because distal mechanisms activated during the 3 hr rhRLX incubation period are stable, rendering ongoing VEGF RTK activitation unnecessary. In future studies, we will investigate whether myogenic constriction might be restored during longer periods of SU5416 treatment.
Another important facet of this work is that we extended our observation of a critical role for both VEGF and PGF in rhRLX-induced attenuation of myogenic constriction in vitro by testing whether SU5416 prevents rhRLX-induced renal vasodilation and hyperfiltration in chronically instrumented, conscious rats. We previously reported that the latter were dependent on increased arterial gelatinase activity, and activation of the endothelial ETB receptor and NOS (22–24). In the present study, we measured renal function both 4–6 hr and 3–5 days after starting rhRLX infusion, because we also previously showed non-overlapping roles for arterial MMP-9 and -2 in the sustained relaxin vasodilatory pathway at these two time points, respectively (22,26,30). Irrespective of the time point, however, relaxin-induced increases in GFR and ERPF, and decrease in ERVR were prevented by VEGF RTK blockade with SU5416. Interestingly, the combination of SU5416 and rhRLX actually resulted in ERPF and ERVR that were significantly below and above baseline, respectively (i.e., as measured before administration of either SU5416 or rhRLX). One potential explanation for this observation is that relaxin simultaneously activates both vasodilatory and counter-regulatory vasoconstrictor system(s), the latter becoming unmasked when relaxin’s vasodilatory pathway is blocked with SU5416. However, which counter-regulatory vasoconstrictor system(s) may be concurrently activated by relaxin, and by what mechanisms, are presently unknown.
Interestingly, we did not observe either hypertension or albuminuria after 3–5 days of SU5416 administration, perhaps because 3–5 days is insufficient time to produce hypertension and albuminuria at least with the dose used (20 mg/kg/d s.c.), and more chronic exposure is required. Since we were interested in whether SU5416 would inhibit “physiological” renal vasodilation elicited by relaxin, we believe it was actually to our advantage to circumvent, albeit serendipitously, these “pathological” effects of SU5416.
Consistent with our previous work, rhRLX administration over several days increased both pro and active MMP-2 activity in SRA. Thus, the critical role for arterial gelatinases in sustained relaxin vasodilatory responses is supported by both functional and biochemical evidence (22,30), and this study). However, in contrast to our hypothesis, the increase in MMP-2 induced by rhRLX was not prevented by SU5416. One possible explanation is that the effect of angiogenic growth factors on sustained relaxin-induced vasodilation is indirect and permissive. Another is that VEGF and PGF may be downstream of MMP-2, such that inhibition of these growth factors does not affect the increase in MMP-2 mediated by relaxin. For example, MMP-2 or -9 may release VEGF and/or PGF from the extracellular matrix (38,39) which, in turn, are critical to the relaxin vasodilatory pathway by stimulating expression of prepro-ET (40,41) as depicted in our working model (Figure 7).
Concordance of results from both in vitro and in vivo approaches strongly support a role for angiogenic growth factors in the sustained relaxin vasodilatory pathway, but the precise molecular details require further elucidation. Whether relaxin directly affects the expression of VEGF and PGF, their receptors or soluble receptors, and which VEGF receptor(s) mediates relaxin-induced reduction of SRA myogenic constriction are currently under study. As PGF binds specifically to VEGF-R1 and not VEGF-R2, and VEGF binds to both receptors, it is tempting to speculate that VEGF-R1 is particularly important (42). It is also possible that one or both of the angiogenic growth factor co-receptors, neuropilins 1 and 2, are involved (42). Furthermore, there is considerable potential for cross talk among the molecular mediators of relaxin vasodilation in arteries. For example, it has been shown that endothelin can promote VEGF expression in rat aortic smooth muscle cells (40). The net effect of this positive feedback would be potentiation and propagation of the vasodilatory signal in arteries, which may be important during gestation.
Perspectives
A potential limitation of this study is that we did not evaluate myogenic constriction over a range of increasing intraluminal pressures, nor did we evaluate myogenic dilation with decreasing pressure. However, we previously reported the effect of rhRLX administration for 5 days on myogenic constriction of both rat small mesenteric and renal arteries using a broad range of pressures (20 – 120 mmHg). Myogenic constriction was markedly attenuated by rhRLX virtually throughout the pressure range for both artery types, and in the small renal arteries, this effect was blocked by nitric oxide synthase inhibition (21).
Extrapolating from the current work, arterial-derived angiogenic growth factors may be critical to pregnancy-induced renal and systemic vasodilation. This idea is supported by previous studies demonstrating the crucial role of circulating relaxin in maternal vasodilation and increasing global arterial compliance during midterm pregnancy in rats (25,43). Considering the marked increases in serum concentrations of placental-derived PGF particularly during the second half of pregnancy (44), circulating PGF might synergistically drive maternal vasodilation in late pregnancy at least partly through the same vasodilatory effectors utilized by relaxin (i.e., the endothelial ETB receptor and NO). In support of this suggestion, preliminary findings in late-pregnant rats indicate that the increase and decrease in global arterial compliance and systemic vascular resistance, respectively, are only partly abolished by immunoneutralization of circulating relaxin (K. P. Conrad, unpublished observations, 2008).
Currently, there are no preventative measures, specific therapies or cures for preeclampsia other than delivery. Thus, there is considerable urgency to evaluate prophylactic, therapeutic or curative strategies for preeclampsia that emerge from promising preclinical research. To this end, both our current and previous studies of relaxin suggest a therapeutic approach that warrants testing (20). That is, the vasodilatory properties of administered relaxin should improve arterial function, organ perfusion, and hence, disease manifestations. From a mechanistic point of view, the hormone might exert salutary effects in preeclampsia by increasing arterial-derived VEGF and PGF activity, thereby offsetting the deficiency of these angiogenic growth factors in the vascular wall secondary to elevated circulating VEGF-R1 (35).
Acknowledgments
The authors thank the Department of Pathology, Health Sciences Tissue Bank of the University of Pittsburgh Medical Center for assistance with human tissue procurement. The authors gratefully acknowledge Wei Hou PhD, Assistant Professor, Department of Health Outcomes Policy, University of Florida College of Medicine for assistance with statistical analysis. Portions of this work were presented in abstract form: Novak J et al., FASEB J. 21:A1371, 2007; McGuane JT et al., XVI International Society for the Study of Hypertension in Pregnancy, Washington DC, 2008; Danielson LA et al., Reprod Sci. 11: 357A, 2010. The human relaxin-3 and Insl-3 peptides were synthesized by Akhter Hossain and Suode Zhang and kindly provided by John D. Wade (Howard Florey Institute, University of Melbourne).
Sources of funding: This work was supported by NIH RO1 DK063321, RO1 HL067937, R21 HL093605, AHA Grant-in-Aid 0855090E, and an AHA Postdoctoral Fellowship (to JTM).
Footnotes
Author contributions: Jonathan T. McGuane: gelatin zymography, data analysis and interpretation, drafting and revising of the manuscript; Leslie A. Danielson: measurement of renal function in conscious rats, inulin and para-aminohippurate assays, data analysis and interpretation; Julianna E. Debrah: functional studies of isolated small arteries, data analysis; J. Peter Rubin: acquisition of human subcutaneous fat; Jacqueline Novak: functional studies of isolated small arteries, data analysis; Kirk P. Conrad: chronic instrumentation of rats for measurement of renal function, data analysis and interpretation, drafting and revising of the manuscript, conception and design of experiments.
Disclosures: Dr. Conrad holds patents for relaxin. The other authors report no conflicts of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/hypertensionaha.110.165027
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/HYPERTENSIONAHA.110.165027
Citations & impact
Impact metrics
Article citations
Uterine Vascular Control Preconception and During Pregnancy.
Compr Physiol, 11(3):1871-1893, 01 Jun 2021
Cited by: 13 articles | PMID: 34061977 | PMCID: PMC8485361
Peripartum cardiomyopathy.
Heart Fail Rev, 26(4):781-797, 13 Jan 2021
Cited by: 5 articles | PMID: 33438106
Review
Sympathetic nervous system control of vascular function and blood pressure during pregnancy and preeclampsia.
J Hypertens, 37(3):476-487, 01 Mar 2019
Cited by: 20 articles | PMID: 30160658 | PMCID: PMC6355368
Review Free full text in Europe PMC
Effects of human relaxin-2 (serelaxin) on hypoxic pulmonary vasoconstriction during acute hypoxia in a sheep model.
Hypoxia (Auckl), 6:11-22, 22 May 2018
Cited by: 3 articles | PMID: 29862306 | PMCID: PMC5968803
Go to all (53) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries.
Circ Res, 93(12):1249-1257, 30 Oct 2003
Cited by: 86 articles | PMID: 14593002
Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats.
Am J Physiol Regul Integr Comp Physiol, 283(2):R349-55, 01 Aug 2002
Cited by: 70 articles | PMID: 12121847
Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide.
Endocrinology, 152(7):2786-2796, 10 May 2011
Cited by: 67 articles | PMID: 21558316 | PMCID: PMC3115605
Effects of relaxin on arterial dilation, remodeling, and mechanical properties.
Curr Hypertens Rep, 13(6):409-420, 01 Dec 2011
Cited by: 53 articles | PMID: 21971830
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (4)
Grant ID: R01 HL067937-01
Grant ID: R21 HL093605
Grant ID: R21 HL093605-02
Grant ID: R01 HL067937
NIDDK NIH HHS (2)
Grant ID: R01 DK063321
Grant ID: R01 DK063321-10





