Abstract
Free full text

Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily
Abstract
Production of cytokines by immune cells in response to stimuli and the binding of cytokines to specific receptors on target cells in a central feature of the immune response. The IL-12 cytokine family is particularly influential in determining the fate of T cells and is characterized by the sharing of cytokine and receptor subunits. A thorough understanding of the molecular interactions within this family will be key to the development of therapeutic inhibitors or enhancers of IL-12 family function. While the current structural and molecular data for IL-12 family members is limited, there is ample information on the structurally related IL-6 cytokine family. This review will summarize the current structural and mutagenesis data within the IL-12 family and will attempt to utilize similarities between the IL-6 and IL-12 families to understand molecular interactions between IL-12 family subunits and with receptor components.
Introduction
Cytokines are key regulators of inflammation and immunity, and modulation of their function has enormous potential for therapeutic benefit in numerous disease and autoimmune pathologies. Type-I cytokines include the IL-6 and IL-12 families, which consist of structurally related four-helix bundle proteins. Unlike members of the IL-6 family, which are secreted as single-subunit monomers, IL-12 family members form heterodimeric complexes. The IL-12 family α subunits (p19, p28, p35) are structurally homologous to IL-6 family cytokines, which include IL-6, IL-11, Leukemia Inhibitory Factor (LIF), Ciliary Neurotrophic Factor (CNTF), Neuropoetin (NP), Cardiotrophin-1 (CT-1), Cardiotrophin-like cytokine (CLC) and Oncostatin M (OSM). These alpha subunits can pair with 2 possible β subunits (p40 and Ebi3), which are structurally similar to the membrane-bound receptors for IL-6 cytokines (IL-6Rα, IL-11Rα and CNTFRα). IL-12 family β subunits lack a transmembrane domain and are thus secreted as soluble α/β heterodimers. To date, the IL-12 family consists of four cytokines with unique α/β subunit pairings: IL-12 (p35/p40), IL-23 (p19/p40), IL-27 (p28/Ebi3), and IL-35 (p35/Ebi3) (Figure 1).
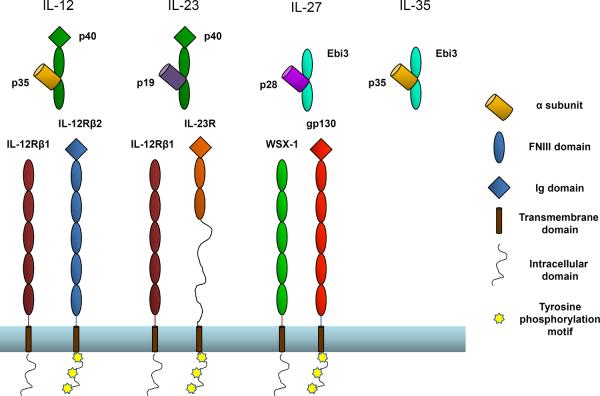
IL-12 family cytokines and their receptors
IL-12 is formed by p40 and p35 and signals through IL-12Rb1 and IL-12Rb2. IL-23 is formed from p19 and p40 and signals through IL-12Rb1 and IL-23R. IL-27 is formed by p28 and Ebi3 and signals through WSX-1 and gp130. IL-35 is formed from p35 and Ebi3. No receptor for IL-35 has been described to date. Modular representations of IL-12 family members and receptors were modified from [30].
Although structurally similar, IL-12 family members vary in function. IL-12, IL-23 and IL-27, are secreted by activated antigen presenting cells (APCs), such as dendritic cells, monocytes and macrophages. They function alone, but can often act synergistically to promote Th1 responses and interferon-γ (IFN-γ) production by T cells [1]. IL-12 promotes differentiation and proliferation of Th1 cells and can be induced by IFN-γ, but is inhibited by Th2 polarizing cytokines such as IL-4 [2]. Like IL-12, IL-23 can drive Th1 proliferation, but also promotes proliferation of memory T cells and is known to play a central role in Th17 development [3, 4]. IL-27 can drive clonal expansion of naïve CD4+ T cells by acting early in the in the Th1 response, and it can synergize with IL-12 to promote IFN-γ production by naïve CD4+ T cells [5, 6]. Recent studies have also identified anti-inflammatory and inhibitory roles for IL-27. It can synergize with IL-12 to limit IL-2 production during T. gondii infections [7]. In addition, it has been shown to block lineage commitment and prevent induction of Th17 responses, as well as to suppress inflammatory function of already differentiated Th17 cells in an EAE model [8, 9]. IL-27 can also inhibit the development of TGF-β-induced regulatory T cells [10], and it can work in conjunction with IL-6 to promote IL-10 secretion by T cells [11, 12]. The newest member of the IL-12 family, IL-35, is distinct in both expression pattern and function [13]. While all other members of this family are expressed by activated APCs, IL-35 is specifically expressed by regulatory T cells and has been shown to contribute to their suppressive capacity [13, 14]. It can also promote infectious tolerance by inducing a potent regulatory population of Foxp3− cells, iTr35, that suppress autoimmune responses in EAE and IBD models, and have been shown to contribute to the regulatory milieu at the site of tumors [15]. This induced regulatory T cell population also mediates suppression via IL-35. While the standard members of this family are heterodimers, some subunits can also function as monomers or homodimers. The IL-12 β subunit, p40, forms homodimers that can antagonize IL-12 function [16]. More recently, p28 was reported to be secreted by Ebi3− cells, and can act as an antagonist of IL-6 and IL-27 signaling [17]. To date, there are no reports of other IL-12 family subunits acting in a similar fashion. Given the propensity for this cytokine family to form novel cytokines but pairing different α and β subunits, it remains possible that other physiologically relevant combinations exist, leading to the identification of new cytokines with similar or distinct patterns of expression and function. For instance, a recent study has suggested that p28 and CLF pair to generate a cytokine heterodimer that regulates NK and T cell activity via IL-6Rα [18].
Although the functions of the IL-12 family members are diverse, all known members exert their function via receptor chains that are structurally homologous to the gp130 family of “tall” shared cytokine receptors (Figure 1). IL-12 signals through IL-12Rβ1 and IL-12Rβ2 [19]. IL-23 also uses IL-12Rβ1 and the IL-23R, which is unique to IL-23 [20]. IL-27 utilizes gp130 in combination with a novel receptor subunit WSX-1 [5, 21]. The receptor for IL-35 has not yet been described, but given the sharing of both cytokine and receptor subunits within this family, it is possible that IL-35 utilizes receptor chains and signaling components that are involved in signaling pathways of other IL-12 family members.
In this article we review the basis of subunit sharing, heterodimer formation and interaction with the receptor subunits within the IL-12 family. While there is limited structural information regarding IL-12 family members and their interactions with receptors, the structurally homologous IL-6 and gp130 systems have been extensively characterized and may provide insight to similar interactions within the IL-12 family. Given the divergent function of cytokines within this family, there are instances in which the same cytokine subunit or receptor chain can have distinct biological outcomes depending on its binding partner or receptor. A thorough understanding of these interactions at the molecular and structural level will be critical for the development of therapeutics that can block or enhance the function of specific cytokines without disrupting the function of others that may share receptors or binding partners.
General structural features of IL-6/IL-12 family cytokines and receptors
The alpha subunits of the IL-12 family cytokines (p19, p28 and p35) are characterized by a unique up-up-down-down four helix bundle conformation. This fold is also found in IL-6 and related cytokines that signal through gp130. The beta subunits of the IL-12 family (p40 and Ebi3) are homologous to the extracellular domains of alpha receptors within the IL-6 family. These receptors contain a conserved amino-terminal immunoglobulin (Ig) domain and two tandem fibronectin type III (FNIII) domains responsible for cytokine binding. In IL-6-like alpha receptors, the cytokine-binding domains are connected to the membrane by a long linker (between 50–60 amino acids), followed by a C-terminal transmembrane region and short cytoplasmic domain. The p40 and Ebi3 subunits are homologous to these receptors, although they are expressed as soluble proteins that do not contain the connecting peptide, transmembrane or cytoplasmic regions. Ebi3 also differs from other homologous proteins in that it lacks the amino-terminal Ig domain (Figure 1). These β subunits and class-I cytokine receptors contain a characteristic WSXWS motif located at the C-terminal part of the FG loop in the second FNIII domain that is thought to be involved in efficient receptor folding and in some cases cytokine recognition [22].
Signal transduction by IL-12 family cytokines is initiated through unique pairings of five receptor chains: IL-12Rβ1, IL-12Rβ2, WSX-1, IL-23R and gp130, the founding member of this group of tall cytokine receptors. While all receptor chains that interact with IL-12 family members share substantial sequence homology, only gp130 has been crystallized and its interaction with IL-6 family members has been well characterized [23–28]. Gp130 consists of an amino-terminal Ig domain followed by 5 FNIII domains, a transmembrane domain and an intracellular domain that contains five tyrosine phosphorylation motifs. The two membrane-distal FNIII domains comprise a strictly conserved cytokine-binding homology region (CHR) that can interact with the helical α subunits of IL-12 family members, or with IL-6-like helical cytokines. The remaining three membrane proximal FNIII domains do not appear to participate in cytokine binding, but have been shown to be required for signal transduction [29]. IL-6 family members CNTF and LIF also utilize the LIF receptor (LIFR), which is structurally homologous to gp130, but contains a second CHR made from two additional FNIII domains at the amino-terminus. LIFR can heterodimerize with gp130 to form the functional signaling complexes for CNTF (CNTF/CNTFRα/LIFR/gp130) and LIF (LIF/LIFR/gp130) [30].
IL-12 family receptor subunits share a modular homology similar to that of gp130 and LIFR (Figure 1). IL-12Rβ2 is the most homologous to gp130, containing the amino-terminal Ig domain, as well as the intracellular tyrosine phosphorylation motifs [19]. The IL-12Rβ1 and WSX-1 receptors also contain five tandem FNIII domains similar to gp130 and IL-12Rβ2, however they lack the N-terminal Ig-like domain, as well as some of the tyrosine motifs in the intracellular domains [31, 32]. The IL-23 receptor contains all intracellular signaling domains and an Ig domain, but is lacking the 3 membrane-proximal FNIII domains and instead contains a long peptide that connects it to the transmembrane region, similar to IL-6Rα [20].
While this review aims to focus primarily on the truly heterodimeric cytokines that comprise the IL-12 family, there is limited structural information regarding heterodimerization of subunits, and essentially no structural information on the interaction of IL-12 heterodimers with their respective receptor chains. However, both the alpha and beta subunits share strong sequence homology with the IL-6 family of cytokines and receptors, and there is a larger library of structural information regarding how the IL-6 cytokines and receptors bind to shared long- chain cytokine receptors such as gp130. Given the homology between both families of cytokines, as well as between gp130 and other long chain receptor subunits that comprise the receptors of the IL-12 family, it is insightful to use the IL-6 family, particularly IL-6, CNTF and LIF, to elucidate subunit and receptor interactions within the IL-12 family.
Interactions between the above described subunits and receptor chains generally conform to the site 1/site 2 paradigm described for the human growth hormone (hGH)/Erythropoietin (EPO) interaction [33], except that they contain an additional interaction site, termed site 3. The center of the complex is the α-helical cytokine (or the α subunit of IL-12 family members), which contacts other components of the complex through three conserved interaction sites. The interaction between the two subunits of IL-12 family members or between the α-helical cytokine and its primary receptor (i.e. IL-6/IL-6Rα) is termed “site 1”. This dimer then interacts with the CHR domain of a receptor chain via “site 2”, situated at the opposite face of the helical component. Finally, the interaction between the top face of the α-helical subunit and the Ig domain of a second receptor chain is known as “site 3” (Figure 2). In many cases sites 2 and 3 are formed by two independent receptor chains, such as in the IL-12 family, which uses paired cytokine receptors, and in the CNTF or LIF complexes, which signal through gp130 and LIFR. It is also possible for the cytokine to engage one molecule of gp130 at the CHR (site 2), and another through the site 3/Ig domain interaction in an antiparallel orientation, forming a hexameric complex, as is the case with the IL-6/IL-6Rα/gp130 interaction [26]. Studies using full-length receptor complexes (including the intracellular and transmembrane domains) have shown that there is limited flexibility between the extracellular and transmembrane regions, which may be important in propagation of ligand-induced conformational changes to the intracellular domain [30]. A recent structure of the complete ectodomain of gp130 confirms that conserved residues between D4 and D5 form an acute bend that brings the membrane proximal D6 domains close to each other, which may facilitate signaling [34].
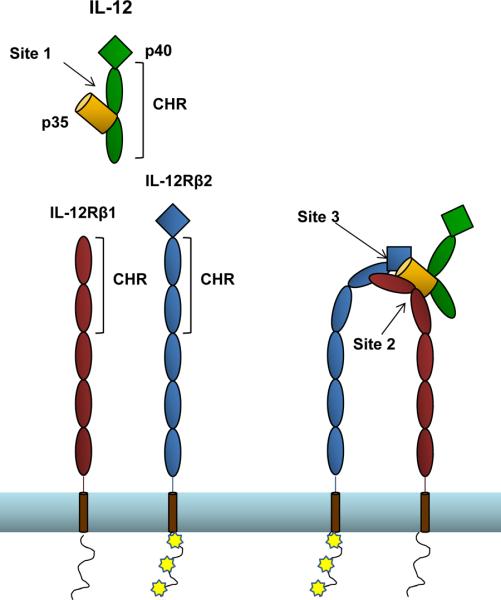
Predicted site 1, site 2 and site 3 interactions within IL-12 family complexes.
Site 1, site 2 and site 3 interactions between some IL-12 family members and their receptor complexes have been previously predicted based on IL-6 and LIF complex crystal structures [30]. Site 1 is formed by the interface between the alpha and beta subunits of the heterodimeric cytokine. The cytokine-binding homology region of a receptor subunit binds to the cytokine forming site 2. The immunoglobulin domain of a second receptor subunit interacts with the tip of the alpha subunit, forming site 3.
Site 1 interactions
The interface between the α subunits of IL-12 family members and their respective β subunit binding partners forms site 1, and is homologous to members of the IL-6 cytokines interacting with their α-receptor components. The crystal structure of IL-12, has served as a template for site 1 interactions within this family [35]. The interface between p35 and p40 is generally made up of a zipper-like series of charged interactions. The central feature of this interaction is a critical arginine residue on the p35 subunit that protrudes from the base of the D helix of p35 into a deep cavity on the p40 surface. This p40 pocket is lined with large aromatic residues and contains a negatively charged aspartic acid at its base. Mutation of the p35 arginine or the p40 aspartic acid to alanine completely abrogated the interaction between these subunits. Furthermore, the charges associated with each residue are responsible for the interaction, as substitution of either component with a conserved charge was able to partially restore the interaction. Comparison of the p40 monomer structure to that of IL-12 showed that there were some conformational adjustments of p40 to improve contacts with the central arginine residue of p35, further highlighting the importance of this interaction. Several aromatic residues that line the central pocket of p40 were also shown to be critical to the IL-12 dimer interface by mutagenesis studies, and hydrogen bonds around the periphery of the central pocket had some contribution to pairing. Another feature of IL-12 confirmed by this crystal structure is the disulfide bond between p35 and p40. While this disulfide was not absolutely required for dimer formation, it likely stabilizes the complex, as significantly reduced amounts of IL-12 were detected in the supernatants of IL-12 transfectants that lack these cysteines.
More recently, the structure of IL-23 has been solved, illustrating the molecular mechanism that the p40 subunit uses to bind to distinct α-helical cytokine components p35 and p19 [36–38]. In this structure, the p19 subunit was similar to other helical cytokines, with several of the loops connecting the helices lacking electron density, indicating they are flexible. The α-helixes of p19 are truncated when compared to those of p35. The general mode of interaction between p19 or p35 with the p40 subunit are similar, in that they interact with a central cavity on the surface of p40, as described above. The critical arginine residue described in the IL-12 structure is also conserved in p19, extending from the base of the D-helix into the crater to form a salt bridge with the same aspartic acid of p40. Hydrophobic residues surrounding the crater also play a role in this interface, and interact with alanine residues in the D-helix of p19. The interchain disulfide bond is also conserved in IL-23.
The position of p19 relative to p35 is tilted, such that even though the same p40 residues are involved in forming the interface, the complimentary p35 or p19 residues are shifted in position. Interestingly, p19 is more similar to IL-6, and only shares ~25% homology with p35. Even in the absence of sequence identity, the chemistry comprising the core hotspot of both side of the interface was conserved. More importantly, interactions surrounding the core charge interaction were quite different in the IL-12 and IL-23 structures and these differences could in theory be utilized to interfere selectively with IL-12 or IL-23 formation.
To date there are no crystal structures of the two newest members of the family, IL-27 and IL-35, however sequence analysis of homologous IL-12 and IL-6 family cytokines may shed light on these interactions. The p28 subunit is more similar in sequence to IL-6 cytokines that utilize the CNTFRα receptor than to other IL-12 family alpha subunits. The p28 subunit maintains a critical tryptophan residue in the AB loop that is conserved in CNTF, NP and CLC (W64, W85 and W94 respectively). These residues have been shown to be critical for site 1 interactions in this subgroup [39, 40]. In addition, there is an RXXXD motif near the end of the D helix that is critical for site 1 interactions and p28 contains a similar RXXXE motif [39, 40]. These observations suggest the IL-27 site I interaction may be more similar to that of CNTF, NP and CLC interaction with CNTFRα than to IL-12 and IL-23. In support of this, the Ebi3 subunit contains a conserved phenylalanine, which corresponds to a CNTFRα residue that was previously identified as a site 1 hotspot [40]. A recent model of IL-27 was generated based on known structures of cytokines in this family and suggests that the Ebi3/p28 interface mimics the interaction between CNTFRα and its cytokine partners. This interface is dominated by an aromatic cluster involving the Ebi3 phenylalanine and the p28 tryptophan and further mutagenesis studies on these residues identified them as critical for IL-27 dimer formation [41].
It is difficult to speculate on the interaction of Ebi3 and p35 that forms the newest family member, IL-35. While a crystal structure of the p35 subunit exists in the form of IL-12, there are no mutagenesis studies indicating that similar residues of p35 are involved in both interactions. It is possible that the interface consists of charge interactions involving the critical p35 arginine, as it already utilized by p35 in the formation of the IL-12 dimer and homologous arginines are also conserved across other family members. Mutation of the homologous arginine in IL-11 to glutamic acid resulted in only residual IL-11 bioactivity and this mutant was unable to bind to IL-11Rα [42]. IL-6 also contributes several charge interactions to the IL-6/IL-6Rα complex, including a homologous arginine [26]. Additionally IL-6/IL-6Rα and CNTF/CNTFRα interactions were found to be highly exothermic and entropically unfavorable, suggesting the general polar nature of these interactions [27]. Furthermore, the aspartic acid counterpart to this charge interaction is present on the Ebi3 subunit, indicating that the overall charged/polar nature of these interactions may remain conserved in IL-35.
It is also possible that the interactions of p35 and p28 with Ebi3 are quite distinct from other members of the IL-6/IL-12 families. Ebi3 is the only member of either family that is lacking the amino-terminal Ig domain. It also displays a non-canonical WSXWS motif (LSDWS in human, PSDWS in mouse). This motif has been shown to be involved in efficient receptor folding and secretion, and the tryptophan residues were especially critical [22, 43]. While these features may not have a direct impact on the Ebi3 heterodimer interface, they may affect the overall structure of the molecule, making sequence homology a less effective means of predicting site 1 interactions. Finally, Ebi3 is also lacking the homologous p40 cysteine residue that forms the inter-chain disulfide bond in IL-12 and IL-23, suggesting that cytokines involving Ebi3 may be more inherently unstable compared to their counterparts that utilize p40.
Site 2 Interactions
Although no structures exist, the site 2/site 3 usage of IL-12 family receptors can be inferred in some cases, based on the presence or absence of the amino-terminal Ig domain, which is required for site 3 interactions. For the IL-12 receptor, IL-12Rβ1 lacks an Ig domain, therefore IL-12Rβ2 must be used in the site 3 interaction, leaving IL-12Rβ1 for site 2. Similarly for IL-23 and IL-27, only one component of each receptor contains an N-terminal Ig domain (IL23R and gp130 respectively), suggesting these chains must be involved in site 3 interactions and leaving IL-12β1 and WSX-1 to form site 2 interactions with IL-23 and IL-27.
Knowledge of IL-12 family interactions with receptor components through site 2 is currently sparse as no direct crystallographic information and few mutagenesis studies exist. However, the IL-6 family has been well characterized and may provide insights into IL-12 family site 2 receptor interactions. In some IL-6 family receptor interactions, site 2 is formed by a composite of the helical cytokine and its alpha receptor. This is true for IL-6 and CNTF, which require prior engagement to IL-6Rα or CNTFRα, respectively, in order to interact with gp130 [30]. Structures of the IL-6/IL-6Rα/gp130 complex show extensive interactions between IL-6 and gp130, but also a large portion of the total buried surface area between the D3 domain of gp130 and IL-6Rα, explaining why interaction of IL-6 and gp130 requires IL-6Rα [26]. Other IL-6 family members do not require an alpha receptor subunit, and site 2 recognition can be restricted to an epitope on the cytokine alone. For example, LIF, which does not engage in a site 1 interaction with a specific receptor, interacts mainly with the D2 domain of gp130, rather than with the larger region of the elbow formed by D2–D3 like IL-6/IL-6Rα [27]. Given the heterodimeric nature of the IL-12 family, it seems reasonable to assume that site 2 is made up of a composite interface formed by specific pairings of the α/β subunits, similar to IL-6/IL-6Rα and CNTF/CNTFRα. However, some data suggests that this is not the case. The interaction between IL-12Rβ1 and IL-12 seems to be primarily with regions of the p40 subunit, rather than with p35 or a composite IL-12 site, and homodimers of p40 are known to antagonize IL-12 signaling via interaction with IL-12Rβ1 [44, 45].
Studies of site 2 interactions involving CHRs of tall cytokine receptors have centered on the founding member of this family, gp130, and several mutagenesis studies have identified residues required for cytokine binding. Mutation of aromatic gp130 residues Y190 and F191 resulted in dramatic reduction in STAT activation in response to both IL-6 and IL-11, and these mutants failed to form a ternary complex with either IL-6/IL-6Rα or IL-11/IL-11Rα. Other hydrophobic interactions were identified as important, although there were differential requirements for binding IL-6 versus IL-11 [46]. Mutation of gp130 residues K219 and L220 resulted in a 50% reduction in binding to CNTFRα, and mutation of homologous residues in LIFR reduced binding to CNTFRα by 50%, suggesting that both receptors interact with CNTFRα in a conserved fashion [47]. These initial mutagenesis studies suggested that recognition of multiple cytokines by gp130 and other tall cytokine receptors involved some conserved hotspots, but also utilize interactions that are unique to different cytokines.
Structures of the IL-6/IL-6Rα/gp130 complex [26] and gp130 interactions with LIF [27] and viral IL-6 [24] revealed that gp130 F191 contributes a large amount of surface area to site 2 in interactions with human IL-6, viral IL-6 and LIF. This central gp130 residue forms a hotspot for complex formation, while surrounding residues utilize diverse chemistries to form interactions appropriate to the numerous cytokines that signal through gp130. Consistent with this notion, the surface CHR of gp130 is amphipathic in nature, allowing it to accommodate multiple binding chemistries. In addition, thermodynamic analysis of LIF, IL-6, OSM and CNTF site 2 interactions with gp130 indentified water expulsion from the interface as a conserved driving force in all of these interactions [27]. Given the growing amount of information regarding the conserved mechanism by which gp130 interacts with multiple cytokines, it is reasonable to assume that this will also be the case for recognition of any newly discovered IL-6 or IL-12 family cytokines that require site 2 interactions with gp130.
It is possible that homologous receptor subunits within the IL-12 family will share a similar mode of recognition, given that some members (IL-12Rβ1) are already known to interact with more than one cytokine. Hydrophobic gp130 residues involved in IL-6 and IL-11 interactions are somewhat conserved across other members of the IL-12 receptor family, including IL-12Rβ2, IL23R and WSX-1. Interestingly, none of the other IL-12 family receptor subunits contain residues homologous to gp130 hotspot phenylalanine. If similar hotspots exist for other IL-12 family receptors to facilitate interaction with multiple cytokines, they are likely comprised of residues unique to each receptor chain. Conversely, the lack of this hotspot residue may imply that the mode of recognition by IL-12 family receptors is dominated by more specific interactions between cytokines and receptors and that there is less cross-reactivity in these interactions, relative to gp130.
Consistent with gp130 using an amphipathic surface to accommodate multiple binding chemistries, mutagenesis studies have identified IL-6/IL-6Rα, IL-11/IL-11Rα and CNTFRα site 2 interactions with gp130 that are unique to each interaction. Charge swap of an arginine residue situated in the predicted site 2 of IL-11 (R135E) resulted in a mutant with improved binding to gp130 [42]. In addition IL-6Rα residues H280 and D281 have been shown to be important for the site 2 interaction with gp130 [48, 49], and mutation of homologous CNTFRα residues resulted in soluble receptors with antagonist activity for CNTF, indicating a reduction in the binding of this mutant complex to site 2 of gp130 [47, 50]. While there is some conservation of these residues within IL-6 family members, none are conserved in any of the IL-12 family subunits (α or β), suggesting unique structural solutions to site 2 interactions within the IL-12 family.
Site 3 Interactions
Site 3 interactions are between the cytokine (or helical α subunit of heterodimeric cytokines) and the Ig domain of a receptor subunit. These interactions generally serve to homo- or heterodimerize receptor subunits. Comparison of several gp130 structures reveals that the gp130 Ig domain is structurally rigid and doesn't appear to use flexibility to accommodate binding to multiple cytokines. More likely the loops of the cytokine can adjust to fit to gp130. Similar to site 2, site 3 uses an amphipathic surface to accommodate multiple chemistries [24]. The amino-terminus of gp130 has been shown to interact with various cytokines and modifications to this region have been shown to disrupt signaling [51]. Structures of the IL-6/IL-6Rα/gp130 hexameric complex revealed that the gp130 amino-terminus interacts with IL-6 and buries a significant amount of surface area [26]. More recently, it was demonstrated that p28 can be secreted in the absence of Ebi3, and that soluble p28 can antagonize gp130-mediated signaling. Models of the p28/gp130 interaction also implicate the amino-terminus of gp130 in this interaction, and suggest that the enhanced hydrophobicity of p28 in the absence of a binding partner also facilitates this interaction [8]. The amino-terminal residues of gp130 that are involved in this interaction are LL(D/E)PC. While this sequence is not strictly conserved in other IL-12 family receptor chains, IL-12Rβ2 contains an aspartic acid at the same position, and IL-12Rβ2 and IL-23R both contain at least one isoleucine residue in place of the gp130 leucines. Similar chemistries in this region of the Ig domain of IL-12 family receptors may be involved in site 3 interactions throughout the IL-12 family.
Further insight into site 3 interactions comes from LIFR complexes. LIFR maintains a similar modular homology as other tall cytokine receptors, however it contains two additional FNIII domains at the amino-terminus, creating two CHR regions with the Ig domain in the middle. Active signaling complexes of LIF and CNTF/CNTFRα utilize LIFR and gp130, both of which contain Ig domains and can potentially participate in both site 2 and site 3 interactions. The crystal structure of the LIF bound to LIFR indicated that this is a site 3 interaction involving the Ig domain of LIFR positioned between two CHR regions [52]. Thermodynamic analysis of the assembly of these complexes revealed that there is no difference in the binding of CNTF/CNTFRα to gp130 in the presence or absence of the gp130 Ig domain, indicating that gp130 does not participate in site 3 interactions in this complex. Further analysis by single particle EM confirmed that the Ig domain of LIFR engages site 3, while gp130 CHR binds through site 2. The recruitment of LIFR to these complexes occurs with high affinity and these cytokines do not require homodimerization of the receptors, whereas cytokines that homodimerize gp130 (IL-6, IL-11) have weaker site 3 interactions that are likely stabilized by homodimerization [30].
Several studies have implicated large hydrophobic residues at the amino-terminus of the D helix of cytokines that bind to gp130 as being critical to formation of a functional site 3 [53–56]. This critical residue is a tryptophan in human IL-6, and a phenylalanine in cytokines that interact with LIFR (OSM, CNTF and LIF). Molecular mimicry of this interaction via a tryptophan in viral IL-6 highlights its importance, and this residue buries a large amount of surface area in the structure of viral IL-6 and gp130 [24]. This key aromatic residue is also conserved in IL-12 family members. In the IL-23 structure, a tryptophan residue is located in a position similar to that of other IL-6 related cytokines [36]. It is likely that p19 engages the Ig domain of IL-23R at this position, given that IL-12Rβ1 lacks an N-terminal Ig-like domain and cannot participate in site 3 interactions. While there is no structural information for IL-27 or p28, recent mutagenesis studies have implicated this conserved tryptophan in the IL-27/gp130 site 3 interaction. Mutation of this critical residue had no effect on p28 interaction with Ebi3 or with WSX-1, but was unable to activate the bipartite receptor and initiate signaling events via gp130 [41]. Even less is known about the potential site 3 interactions of IL-35 with its receptor(s), although the conserved aromatic residue is present in p35. Interestingly, both murine and human p35 contain a tyrosine at this position while all other homologs contain phenylalanine or tryptophan. A slight difference in the residue at this position may serve to mediate a more specific interaction between p35 and IL-12Rβ2. If this is the case, it suggests that IL-12Rβ2 may also be a component of the IL-35 receptor.
Engagement of LIFR by CNTF and LIF involves a region homologous to the IL-6/gp130 interaction. A conserved FxxK motif is shared between all cytokines that interact with LIFR (CNFT, LIF, OSM, CLC, CT-1 and murine NP). The mutation of these residues in human CNTF has been shown to abrogate the interaction between CNTF and LIFR but does not affect the interaction with either CNTFRα or gp130, indicating the involvement of these residues specifically in the site 3 interaction [57]. The complementary site on the Ig domain of LIF receptor is a phenylalanine / aspartic acid motif that forms a mirror image of the conserved FxxK motif on LIFR-binding cytokines. Combinatorial mutagenesis of these two residues completely impairs responses to LIF and CT-1 and partially impairs responses to OSM, indicating that all 3 cytokines utilize this site, although with potentially different binding affinities [58]. While none of these motifs are strictly conserved in IL-12 family cytokines or receptor chains, there is some conservation of similar mirrored motifs in p35 and gp130. P35 is the only IL-12 family α subunit that contains a YxxK motif, similar to the FxxK motif found in LIFR-binding cytokines, and these residues are conserved in both human and murine p35. While neither of the IL-12 receptor subunits contains the mirror image phenylalanine / aspartic acid motif, a similar motif is present in gp130 in which the aspartic acid is replaced by glutamic acid. This suggests that p35 may also be able to interact to some degree with gp130 via a site 3 like interaction, although there is no published evidence of this interaction to date.
Concluding Remarks
Insights into the molecular interactions between IL-12 family members and their receptor subunits have been greatly facilitated by studies of the IL-6 family. Indeed, models of the interaction of both IL-12 and IL-27 with their receptors have been generated based on available structural data and conservation of domain motifs and sequences [30]. These models maintain the site 1/site 2/site 3 interactions observed in IL-6 and gp130 family structures and presume that the organization of IL-12 family complexes will ultimately follow similar structural rules. While most evidence supports this view, some data suggests that this may not be the case. A recent crystallographic analysis of an IL-12/IL-23 neutralizing antibody determined the antibody epitope to be in the D1 domain of p40 [38], which is not predicted to be involved in site 2 or site 3 interactions, based on the current paradigm established by IL-6 structures. In addition, recently developed neutralizing antibodies against Ebi3 are capable of inhibiting IL-35-mediated signaling, but have no effect on IL-27-mediated signaling, suggesting differences in either the pairing of these heterodimeric cytokines or interactions with their respective receptors [15]. In the absence of structures of IL-12 family cytokines bound to receptor subunits, further mutagenesis studies of putative interaction sites may aid in identifying similarities and differences in how IL-12 cytokines interact with their respective receptors. This is particularly relevant for IL-35, for which there is no data in support of or against conserved interaction motifs. This insight will be critical for future development of therapeutics that selectively target specific members of the IL-6/IL-12 cytokine family.
Acknowledgements
This work was supported by the NIH (F32 AI084330 to LLJ; AI091977 to DAAV), the American Asthma Foundation - Strategic Program for Asthma Research (SPAR; 10-0128 to DAAV), an NCI Comprehensive Cancer Center Support CORE grant (CA21765 to DAAV), and the American Lebanese Syrian Associated Charities (ALSAC to DAAV).
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12026-011-8209-y
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3529148?pdf=render
Citations & impact
Impact metrics
Article citations
IL-27 attenuated macrophage injury and inflammation induced by Mycobacterium tuberculosis by activating autophagy.
In Vitro Cell Dev Biol Anim, 25 Oct 2024
Cited by: 0 articles | PMID: 39455490
Ebi3 Binding to IFN-γ and IL-10 Limits Their Function.
J Immunol, 213(8):1115-1124, 01 Oct 2024
Cited by: 0 articles | PMID: 39240167
IL-27 expression regulation and its effects on adaptive immunity against viruses.
Front Immunol, 15:1395921, 20 Jun 2024
Cited by: 0 articles | PMID: 38966644 | PMCID: PMC11222398
Review Free full text in Europe PMC
Exogenous IL-25 ameliorates airway neutrophilia via suppressing macrophage M1 polarization and the expression of IL-12 and IL-23 in asthma.
Respir Res, 24(1):260, 28 Oct 2023
Cited by: 5 articles | PMID: 37898756 | PMCID: PMC10613395
Elevated expression of interleukin-27, IL-35, and decreased IL-12 in patients with thyroid-associated ophthalmopathy.
Graefes Arch Clin Exp Ophthalmol, 261(4):1091-1100, 12 Nov 2022
Cited by: 1 article | PMID: 36370169
Go to all (64) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Modular organization of Interleukin-6 and Interleukin-11 α-receptors.
Biochimie, 119:175-182, 10 Nov 2015
Cited by: 11 articles | PMID: 26551279
IL-6/IL-12 Cytokine Receptor Shuffling of Extra- and Intracellular Domains Reveals Canonical STAT Activation via Synthetic IL-35 and IL-39 Signaling.
Sci Rep, 7(1):15172, 09 Nov 2017
Cited by: 17 articles | PMID: 29123149 | PMCID: PMC5680241
Characterization of the Interleukin (IL)-6 Inhibitor IL-6-RFP: fused receptor domains act as high affinity cytokine-binding proteins.
J Biol Chem, 282(2):1238-1248, 03 Nov 2006
Cited by: 14 articles | PMID: 17085445
Signal transduction through gp130 that is shared among the receptors for the interleukin 6 related cytokine subfamily.
Stem Cells, 12(3):262-277, 01 May 1994
Cited by: 97 articles | PMID: 8075593
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: CA21765
Grant ID: P30 CA021765
NIAID NIH HHS (3)
Grant ID: R01 AI091977
Grant ID: AI091977
Grant ID: F32 AI084330





