Abstract
Free full text

Host Defense Pathways: role of redundancy and compensation in infectious disease phenotypes
Summary
Innate host defense pathways consist of microbial sensors, their signaling pathways and the anti-microbial effector mechanisms. Several classes of host defense pathways are currently known, each comprising several pattern-recognition receptors that detect different types of pathogens. These pathways interact with one another in a variety of ways that can be categorized into cooperation, complementation and compensation. Understanding the principles of these interactions is important for better understanding of host defense mechanisms, as well as for correct interpretation of immunodeficient phenotypes.
Introduction
The last decade has seen tremendous progress in the elucidation of innate immune recognition mechanisms. Several families of pattern recognition receptors have now been characterized and established to function as sensors of microbial infections (see reviews by Flavell; 2011, Akira; 2011, Reis e Sousa; 2011 and Gale; 2011 in this issue of Immunity). A common feature of these receptors is their ability to trigger signaling pathways that activate innate anti-microbial and inflammatory responses. In addition, these receptors induce a set of requisite signals for activation of T and B cells, thus coupling microbial recognition with the initiation of adaptive immune responses.
While the functions of different families of pattern recognition receptors are increasingly well characterized, their specific contributions to host defense from infections are still being defined and are often a subject of debate. This is due in part to incomplete knowledge of the host protection mechanisms, and in part due to differences in the interpretation of the existing experimental and clinical data.
Here we will discuss the functional interactions of different host defense pathways and the contribution of these interactions to host protection and susceptibility to infections.
Innate host defense pathways
The innate host defense pathways consist of microbial sensors, their signaling pathways and the effector mechanisms they induce. The effector mechanisms fall into three broad categories: inflammatory mediators, anti-microbial effectors, and signals inducing adaptive immune responses.
The best-known microbial sensors are pattern recognition receptors, including Toll-like receptors (TLRs), Nucleotide Oligomerization Domain (NOD) proteins, C-type lectin receptors (CLRs) and RIG-I-like receptors (RLRs). Microbial sensors that are not based on pattern recognition also exist, though they have not yet been extensively characterized. Upon recognition of their microbial ligands, pattern recognition receptors activate signal transduction pathways that generally converge on several key transcription factors including nuclear Factor (NF)-κB, activator protein 1 (AP1), interferon regulatory factors (IRFs), and nuclear factor of activated T cells (NFAT) (Lee and Kim, 2007). These transcription factors often function in combination with each other to turn on the expression of several classes of genes, including anti-microbial effectors; cytokines and chemokines that orchestrate inflammatory and innate immune responses; as well as genes involved in the induction of adaptive immunity. In addition, pattern recognition receptors can induce transcription-independent responses, such as degranulation, phagocytosis, chemotaxis and activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Some microbial sensors do not directly control gene expression, but rather initiate extracellular host defense responses. For example, pentraxins, mannan-binding lectin and ficolins can activate complement pathways upon binding to pathogen surfaces. Finally, certain microbial receptors, such as macrophage receptor with collagenous structure (MARCO) and macrophage mannose receptor, are involved in bacterial phagocytosis (Elomaa et al., 1995), but presumably do not activate gene expression on their own.
It is important to note that most pathogens can be detected by more than one microbial sensor. Thus, bacterial pathogens can be recognized by several TLRs, NODs, phagocytic receptors, complement system, inflammasomes and in some cases intracellular DNA sensors. Fungal pathogens can be detected by TLRs, Dectins, complement and inflammasomes. Viral pathogens can be detected by TLRs as well as intracellular RNA and DNA sensors, and in some cases, by inflammasomes (see reviews in this issue). Thus the innate immune system has a great deal of apparent redundancy at the level of pathogen detection.
Anti-microbial effector mechanisms
Once the microbial sensors become activated by pathogens, they induce a broad array of anti-microbial defense mechanisms. These defense mechanisms fall into several broad categories, depending on the pathogen class as well as the identity of the infected cell types and tissue compartments.
Viral infections lead to production of type-I IFNs (IFN-α and β), which induce expression of over two hundred anti-viral genes that can interfere with multiple stages of viral infection cycles and sensitize infected cells to killing by cytotoxic NK cells and CD8+ T cells. In addition, type I IFNs promote cytotoxic activity of NK and CD8+ T cells and induce an anti-viral state in neighboring cells (Stetson and Medzhitov, 2006). Importantly, all these responses can be induced by any of the viral pathogen sensors as their signaling pathways converge on IRF3 and/or 7 activation and type I IFN production (Honda and Taniguchi, 2006). An important difference exists between IFN induction by cell intrinsic sensors, such as RLRs and cytosolic DNA sensors, and cell extrinsic mechanisms mediated by TLRs 3, 7, 8 and 9. Cell intrinsic sensors are ubiquitous and trigger IFN-β production in infected cells, whereas the TLRs involved in viral recognition are expressed on specialized cells, such as plasmacytoid dendritic cells (pDCs), which make large amounts of IFN-α in infected tissues. The common strategy of host defense against viral pathogens is to interfere with viral replication and spread and to kill infected cells, often with the help of cytotoxic lymphocytes (NK cells and CD8+ T cells). This latter strategy is particularly useful in tissues with a high rate of renewal, such as the epithelium. The cell types that cannot be easily replaced, such as neurons and cardiomyocytes, may rely on alternative mechanisms of anti-viral defense, which remain to be fully characterized.
Detection of bacterial, fungal and protozoan pathogens by multiple receptors results in the induction of antimicrobial peptides (e.g., defensins and cathelicidins) and enzymes (e.g., iNOS, NADPH oxidase, lysozymes and proteases), as well as proteins involved in deprivation of iron (NRAMP, lactoferrin, lipocalins) and tryptophan (IDO). Macrophages and neutrophils, acute phase proteins, the complement system as well as surface epithelia producing mucins and anti-microbial peptides, all contribute to host defense against bacterial, fungal and protozoan infections. Importantly, these effector mechanisms can be induced by multiple microbial sensors (TLRs, NODs, Dectins) through the NF-κB and MAP kinase signaling pathways, or by cytokines induced downstream of these pathways. The common strategy of host defense against the majority of bacterial, fungal and protozoan pathogens is their direct killing by antimicrobial effectors and the generation of an uninhabitable microenvironment (low pH, nutrient deprivation).
Mechanisms of innate immune recognition of multi-cellular parasites are not yet understood. Pattern recognition in this case may be limited to a few parasitic products, such as chitin (Reese et al., 2007). The main mechanism of recognition may rely on detection of parasite-derived enzymatic activities, such as cysteine proteases that are secreted by parasitic worms. The defense strategy against multi-cellular parasites can vary depending on their life stage. At the larval stage they can be targeted and killed by toxic products of eosinophils (ROS and major basic protein). At the adult stage, efficient parasite killing may be limited by their size and the high potential of host tissue damage. Therefore, the main defense strategy is to reduce their entry into the host (by promoting the barrier function of mucosal epithelia), to minimize their spread throughout the host (through vasoconstriction and coagulation) and to promote their expulsion from the host (by peristalsis, mucus production, vomiting, diarrhea, epithelial ciliary movement and other mechanisms). The main targets of the immune response against adult parasites, therefore, are the host tissues in the infected compartment, particularly mucosal epithelium, smooth muscles and endothelium. These tissues are activated by inflammatory mediators induced upon parasitic infections including IL-13, histamine and bradykinin, which are also responsible for allergic reactions following chronic exposure to allergens.
The summary of host defense strategies and the examples of effector mechanisms involved are shown in Table I. Even a brief survey of antimicrobial effectors illustrates the high degree of apparent functional redundancy within each of the defense categories. Before we consider the reasons for these functional redundancies we need to discuss the causes of diversity of effector mechanisms.
Table 1
Effector Mechanism Categories
| Defense Strategy | Effector Class | Pathogen Type |
|---|---|---|
| Blocking pathogen entry into the host organism | Epithelial and mechanical barriers, IgA, AMPs, mucins, cilia-mediated expulsion | Most pathogens |
| Blocking entry into host cells | Neutralizing antibodies | Bacteria, viruses |
| Blocking pathogen spread | Coagulation, vasoconstriction, neutralizing antibodies | Most pathogens |
| Direct Killing of pathogen | AMPs, BPI, lysozymes, proteases, acidic pH (lysosomes and stomach), complement, ROS and RNS | Most pathogens |
| Direct Killing of infected host cell | IFN-α/β, NK cells, CTLs, ADCC | Viruses, intracellular bacteria, protozoa |
| Expulsion of pathogen | IgE production, release of soluble mediators (leukotrienes, prostaglandins and histamine), mucus secretion, smooth muscle cell contraction, cilia-mediated expulsion | Multicellular parasites |
| Nutrient deprivation | NRAMP, lactoferrin, lipocalin, calprotectin (Iron and Zinc), IDO (tryptophan) | Bacteria, protozoa |
Abbreviations: AMPs, antimicrobial peptides; IgA, Immunoglobulin A; BPI, Bacterial Permeability Increasing Protein; ROS, Reactive Oxygen Species; RNS, Reactive Nitrogen Species; ADCC, Antibody-dependent cellular cytotoxicity; IFN-α/β, Type I Interferons; CTL, Cytotoxic T lymphocyte; IgE, Immunoglobulin E; NRAMP, Natural resistance-associated Macrophage proteins; IDO, Indoleamine 2,3-dioxygenase.
Diversity and hierarchy of effector mechanisms
Most pathogens can be recognized by multiple microbial sensors, which in turn can induce multiple anti-microbial effector mechanisms. There are several reasons for the existence of diverse recognition and effector responses. The existence of multiple pathogen detecting pathways allows for a greater ‘coverage’ of the microbial world, while diversity and redundancy of the effectors accounts for robustness of host defenses in the face of continuous pathogen evolution. Clearly different sensors and effectors have evolved to detect and eliminate different classes of pathogens, for example, RNA viruses versus tape worms. However, there are additional evolutionary causes of the diversity of host defense mechanisms. First, different defense mechanisms operate in different tissues and body compartments. The anatomy and physiology of different tissues may dictate which defense mechanisms can and cannot be used (Matzinger and Kamala, 2011). In general, host physiology can affect the repertoire of available host defenses. For example, amphibian skin is an important organ for gas exchange and therefore it lacks a cornified keratinocyte layer. As a result, amphibians do not have an important physical barrier to pathogen entry, which in turn caused an elaboration of a highly potent antimicrobial peptide defense in the skin. This type of physiological constraints and evolutionary compensation is likely to be commonplace. Another example of how host biology may affect the diversity of immune defenses is provided by comparison of the immune response genes in Drosophila or Anopheles and the honeybee. The honeybee genome contains only about a third of the genes known to be involved in immunity in the fruit fly (The Honeybee Genome Sequencing Consortium, 2006). The honeybees have effective social immunity mechanisms, such as pathogen avoidance, social grooming and nest hygiene (Cremer and Sixt, 2009), which are absent in the non-social insects including fruit flies and mosquitoes. This may account for reduced reliance on the immune defense mechanisms that are essential in the Drosophila (Evans et al., 2006).
Another important factor accounting for the diversity of host defense pathways emerged from the field of ecological immunology. A key point here is that host defenses are associated with fitness costs. There are two types of fitness costs: evolutionary costs and maintenance costs (Sadd and Schmid-Hempel, 2009). The evolutionary costs arise when development of host defense mechanisms is negatively correlated with the development of other physiological systems. For example, evolution of anti-microbial defenses in the gut may have constrained the evolution of a more efficient digestive function. The maintenance costs arise whenever the defense mechanisms are employed and include metabolic costs and immunopathology. The metabolic costs of the immune response are well appreciated in certain host species, for example in insects and some birds. To what degree the metabolic costs constrain immune defenses in mammals is less clear. Presumably, the ability to store significant energy reserves in adipose tissues in some animal species may alleviate these constrains to some extent. The costs associated with immunopathology, on the other hand, are likely to be universal (Graham et al., 2005). Importantly, the anti-microbial effector mechanisms differ widely in terms of their fitness costs. Fitness costs are important to consider for several reasons: First, they help to explain how host defenses change over evolutionary time – the higher the cost the more likely a given host defense pathway will be lost whenever pathogen-induced selective pressure is removed or reduced, or when the defense mechanism can be replaced by other, less costly mechanisms. Second, fitness costs may underlie an important regulatory strategy of host defenses: it appears that in the course of an infection less costly defenses are induced first and only if they are insufficient, the defenses with higher fitness costs are employed. Indeed, if we consider the host defenses from this perspective, they form a spectrum in terms of their fitness costs (for example, their ability to cause immunopathology), that correlates with the order of their induction. The epithelial antimicrobial peptides and IgA secreted into the lumen of the intestine probably have the lowest fitness costs, because their ability to cause tissue damage is minimal or nonexistent. Accordingly, these effector mechanisms are known to be engaged constitutively. If their protective effects are insufficient, however, the host engages activation of resident myeloid cells that have increased microbicidal activity but also increased tissue damage potential. If these defenses are still insufficient, highly potent and damaging defenses (recruited neutrophils and inflammatory monocytes, activated Th1 and Th17 cells) are employed. Their tissue damage potential is the highest, and they are induced as the last resort. Similarly, IgM and IgG1 antibodies are more easily inducible compared to IgG2 isotypes, which, when induced, have higher potential to cause tissue damage and autoimmunity. Thus the hierarchy of defense mechanisms, as defined by their fitness costs, dictates the order of their induction during an infection, as well as the signal requirements for their induction, magnitude and duration. This logic is likely to be applicable to most defense mechanisms of innate and adaptive immunity.
It should be noted, however, that different types of fitness costs are not necessarily at the same end of the spectrum: host defenses with low tissue damage potential (e.g., secreted IgA) can have high metabolic cost (in part because they are continuously engaged), whereas reactive oxygen species production by NADPH oxidase has lower metabolic cost but higher tissue damaging potential. The net fitness cost is a complex product of different types of maintenance costs and is likely to be different in different hosts depending on environment- and host-specific physiological constraints.
The discussion above illustrates that host defense mechanisms differ in multiple ways even though all are designed to provide protection from infections. This heterogeneity is important to consider when analyzing the contribution of the individual pathways to protective immunity from infections. Analyses of immunodeficient humans or mutant animals with defects in specific pathways or effector mechanisms often give unexpected results that are not easily explained based on the current understanding of immunity. Whatever the specific reasons might be, host resistance or susceptibility to infection is a product of functional interactions between different host defense pathways, which we will discuss next.
Interactions of host defense pathways
In principle, host defense pathways can engage in three types of interactions: cooperation, complementation and compensation (Figure 1).

There are three different types of host pathway interactions: cooperation, complementation and compensation. A. Cooperating pathways induce the same effector mechanism more efficiently when engaged simultaneously. B. Complementing pathways induce distinct effector mechanisms (EM1 and EM2), which complement each other to form one functional unit. C. Compensation between two pathways occurs when one pathway is deficient and the other intact. Compensation can take place at the level of sensors (panel C top), or at the level of effectors (panel C, bottom). P – pathogens, A and B – microbial sensors, EM – effector mechanisms.
Cooperation can be defined as the type of interaction where two (or more) individual pathways optimally induce the same effector mechanism when both pathways are activated (Figure 1A). An example of cooperation is the induction of optimal TNF-α production by macrophages when both TLR2 and Dectin-1 are engaged (Reis e Sousa, 2011, this issue). Another example is the cooperation between bactericidal permeability increasing protein (BPI) and defensins, which together have a synergistic bactericidal effect. For quantitative effects, cooperation often results in synergy.
Complementation is a type of interaction where individual pathways activate distinct effector mechanisms, which complement each other to form a functional unit of defense (Figure 1B). An example of complementation is the interaction between the antibodies and phagocytes: both effectors can function independently, but can complement each other to form a functional unit of antibody-mediated phagocytosis.
Compensation is a type of functional interactions that occurs when one of the host defense pathways is inactivated (for example, due to a mutation) while the other pathway(s) inducible by the same infection remain intact. Compensation can be of two types depending on whether it occurs at the level of sensors or effectors (Figure 1C). Thus, two sensors can compensate for each other if they can both activate the same effector mechanism (Figure 1C top). Alternatively, two sensors may activate distinct effector responses, but if these responses are individually sufficient to have a given effect (such as host protection) they will compensate for each other (Figure 1C bottom). There are many examples of the first type of compensation because multiple sensors can activate the same effector responses: TLR4 and NODs can induce the same antibacterial peptides; TLR7 and RIG-I can both activate the type-I IFN response, and so on. An example of the second type of compensation could be activation of NADPH oxidase and iNOS by distinct sensors, provided that activation of either enzyme is sufficient to protect against a given pathogen.
Unlike cooperation and complementation, compensation only becomes apparent when one of the host defense pathways is inactivated by mutations or by the pathogen (for example, as a result of immune evasion strategy). Understanding the rules of compensation is important for correct interpretation of infectious disease outcomes, as illustrated in the next section.
Immune compensation and susceptibility to infections
When a particular host defense pathway is disabled by mutations, as happens in experimental animals and immunodeficient patients, infection susceptibility will depend on whether the defect can be compensated by the remaining pathways (Figure 2). If pathogen Px can be detected by two sensors, A and B, that can both activate a protective effector response, inactivation of pathway A (due to mutation or by pathogen evasion mechanisms) can be compensated by pathway B and the host will remain resistant to pathogen Px (Figure 2A). However, the same host can be susceptible to pathogen Py that is detected by pathway A only (Figure 2B). A clinical outcome of immunodeficiency in pathway A would be susceptibility to pathogen Py but not Px and the incorrect conclusion would be that pathway A plays a role in defense against pathogen Py but not Px. The correct interpretation of the same clinical observation, however, is that pathway B can compensate for a defect in A in response to Px but not Py because Py does not activate pathway B.
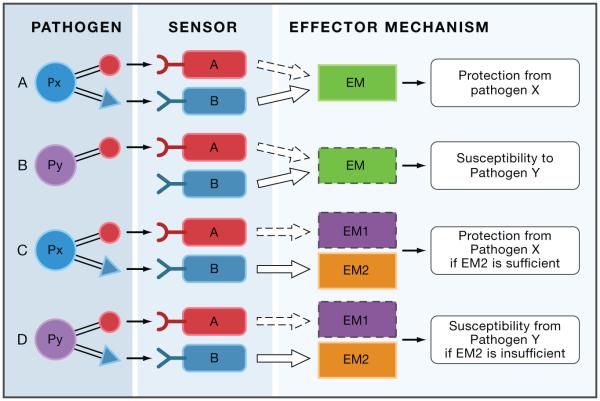
Defect in pathways A can be compensated by pathway B in the case of pathogen Px (Panel A), but not in the case of pathogen Py (panel B). Defect in EM1 can be compensated by EM2 if EM2 is sufficient to provide protection against pathogen Px (panel C). If EM2 is not sufficient to protect against Py, then EM2 will not compensate for EM1 deficiency (panel D). Solid lines: intact pathways, dashed lines: inactive pathways. Pathway deficiency can result from either mutations, or due to pathogen evasion.
In the second type of compensation, pathogen Px is detected by two pathways, A and B, that activate distinct effector mechanisms: EM1 and EM2, respectively. When pathway A is inactivated, the host will be protected from pathogen Px if EM2 is sufficient for protection (Figure 2C) and susceptible to pathogen Py if EM2 is insufficient (Figure 2D). Patients with pathway A mutation would be protected from pathogen Px and susceptible to pathogen Py. This clinical presentation may lead to an incorrect conclusion that sensor A is irrelevant for protection from Px when in fact a defect in A is compensated by EM2. If Px can evade recognition by sensor B, however (Figure 2C), the outcome will be that sensor A is critical for protection from Px.
More generally, immune evasion from a given host defense pathway can obscure the role of this pathway in pathogen detection, even though that pathway must have played an important role in defense against that pathogen, otherwise it would not have evolved to avoid that pathway in the first place.
Clearly not all combinations of host defense pathways can compensate for each other. Also, there are likely to be different degrees of compensation. Presumably, defense mechanisms that belong to the same category (Table 1) can compensate for each other most efficiently. Compensation also depends on characteristics of the pathogens, such as the combination of pathogen-associated molecular patterns (PAMPs) they produce and their evasion strategies. These host and pathogen specific properties collectively define the rules of immunological compensation that determine infectious disease susceptibility in immunodeficient humans and mutant animals.
Similar considerations apply to the analysis of innate control of adaptive immunity. If more than one innate sensing pathway can lead to activation of the adaptive immune response to a given pathogen (or immunization conditions), then none of them would be required when more than one pathway can be activated by a given pathogen or adjuvant. This can lead to an incorrect conclusion that specific innate sensing pathways are not required for activation of adaptive immunity (Gavin et al., 2006), though a trivial explanation is the engagement of compensating innate immune pathways that operate under distinct experimental conditions. One example of this point is a compensatory antibody response to commensal bacteria in the absence of TLR-mediated defenses (Slack et al., 2009).
Consequences of immune compensation
As discussed above, defects in specific host defense pathways can be compensated by intact pathways if they meet certain criteria. This is generally reflected in the infection susceptibility spectrum observed in immunodeficient humans and mutant animals. However, it should be noted that even in the presence of compensating pathways that may provide protection, there are often deleterious consequences of immunodeficiency. They may or may not have clinical manifestation and therefore may sometimes go unnoticed. Paradoxically, one common consequence of immunodeficiency can be immunopathology. The reason for that can be illustrated as follows: if there are two pathways (A and B in Figure 3a) that can be activated in response to a given pathogen, inactivation of one of these pathways (pathway A in Figure 3b) due to immunodeficiency will be compensated by the intact pathway B. However, the intact pathway has to be hyper-activated to provide protection in the absence of pathway A. This is because pathway B has to ‘work alone’ when pathway A is deficient, and because pathway A deficiency can result in (at least transient) increase in pathogen burden compared to the normal situation when both pathways are intact. Thus, a decrease in AMPs production by mucosal epithelia may result in increased mucus production, which can affect respiratory or digestive function. Similarly, a defect in innate effector mechanisms can result in a compensatory increase in the adaptive immune response (as long as it can be activated by an intact alternative innate sensing pathway), which can also lead to immunopathology. In some cases this results in subclinical symptoms, but at the extreme the enhanced activation of the compensatory adaptive immune response can lead to autoimmunity. Indeed, it is well documented that immunodeficiencies (with the obvious exception of SCIDs) are commonly associated with autoimmune diseases (Arkwright et al., 2002). In experimental animals this phenomenon is well recognized in the studies of colitis, which can be caused by defective immune responses (Izcue et al., 2009).
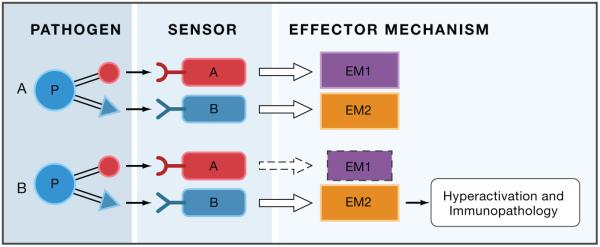
If more than one pathway is induced by a given infection, they can provide optimal protection with minimal immunopathology, because they do not have to be induced to a maximal level (panel A). If one pathway is deficient, the intact pathway will have to be induced to a higher level, thus increasing the potential for tissue damage.
It should be noted that a compensatory increase in pathway B, when pathway A is disabled, can lead to an incorrect interpretation that the mutated gene from pathway A plays a negative regulatory role in pathway B. Finally, the compensatory enhancement of pathway B can be caused not only by genetic immunodeficiency in pathway A, but also by evasion of genetically intact pathway A by a pathogen.
The immunopathological consequences of hyperactivation of host defenses in the face of immunodeficiency also highlight another reason why there are many seemingly redundant effector mechanisms: different effector mechanisms activated by a given infection can function efficiently in combination without any individual pathway having to be activated to the maximal level that will have the highest potential for immunopathology. For example, if a given infection is handled by mucus production, AMPs, IgA and CTLs, each of these mechanisms can work at the optimal range without going into ‘overdrive’. If mucus production or CTLs were the only available defense mechanisms, each would have to be activated to a much greater extent and consequently might cause an unacceptable level of immunopathology. Thus, distribution of protective mechanisms amongst different effector responses, allows for a more efficient defense with minimal immunopathology. In addition to immunopathology, other types of fitness costs may be incurred by compensatory hyper-activation of defense mechanisms, such as negative effects on metabolism or optimal tissue functions. From the evolutionary standpoint the most important fitness cost is the negative effect on reproductive success. Even a slight decrease in reproductive fitness can have a dramatic effect on the evolutionary scale. However, these effects are rarely considered in the studies of infections in experimental animals or immunodeficient humans. Therefore, conclusions that any particular host defense mechanism is ‘redundant’ are questionable in the absence of complete knowledge of their effects outside the infectious disease itself.
Redundancy in host defenses
Redundancy is a notion that is often invoked in the literature, when an expected phenotype is not observed in the absence of a specific gene. It is sometimes implicitly appreciated that redundancy is conditional on both the environment and the readout. For example, the left arm is redundant for a chess player, but not for a piano player. Similarly, many genes may be redundant in an animal facility but not in the wild. Some host defenses may be redundant with modern sanitation and health care but not without them. Finally, some genes may be redundant for human or animal survival from infections, but non-redundant for their reproductive fitness. The ability of host defenses to compensate for each other also often leads to conclusions of redundancy, even though there are almost invariably some fitness costs that are not properly evaluated.
The evolution of redundancy has been a subject of debate for decades, as simple logic would suggest that true redundancy would be evolutionarily unstable because there would be no selective pressure to maintain it. Nowak and colleagues have provided a theoretical analysis of redundancy in gene functions and described several scenarios where redundancy can be evolutionarily stable (Nowak et al., 1997). One of these scenarios can be adapted for the discussion of redundancy in host defenses (Figure 4): let’s consider a pathogen Px that can be detected by two microbial sensors, A and B, that can both activate effector mechanism EM1, which in turn can provide protection from Px. In this scenario, B would be redundant when A is intact. However, if B has other functions, such as activation of EM2, this additional function will maintain B if there is a selective pressure to preserve EM2. Thus, A and B are redundant upon infection with pathogen Px that can be detected by both A and B, but not during an infection with the pathogen Py that can only be detected by the sensor B. Whether sensor B is essential or redundant would also depend on which pathogen Px or Py is more common (see below). Clearly, the most common pathogens are the components of normal microbiota – the opportunistic pathogens that cause overt infection only in immunocompromised humans and animals. Thus, a key issue concerning the role of different host defense pathways in protection from infections is the exposure rate to different types of pathogens, an issue that is not commonly considered in the analysis of immunodeficiencies.

‘Redundancy’ in host defense pathways is conditional on the nature of infection
Pathways B is ‘redundant’ when the host is exposed to pathogen Px, but non-redundant when the host is exposed to pathogen Py. Pathways A and B are redundant with regards to the common function (activation of EM1), but non-redundant with regards to activation of EM2. Thus, conclusion of ‘redundancy’ can be affected by both the exposure rates of Px and Py, and by the ‘read-out’ (whether EM2 activation is measured or not).
Immunodeficiency and pathogen exposure
The clinical outcome of immunodeficiency depends not only on the function of the host defense pathway that is eliminated, but also on the exposure rate to different pathogens and their immune evasion strategies. For any given host organism, all the potential pathogens form a wide spectrum of exposure rates. On one end of the spectrum are the opportunistic pathogens, where exposure rate is 100 percent. On the other end of the spectrum are ‘accidental pathogens’ where exposure rate can be close to zero, especially if they are endemic. For example, most humans are exposed to Streptococcus pneumonia and Staphylococcus aureus, which are components of a normal microflora in the skin and the throat, but our exposure rate to the Ebola virus is effectively zero. Most other pathogens fall somewhere in between on the spectrum. The differences in exposure rates can significantly affect our interpretation of the infection susceptibility in immunodeficiencies. As discussed above, mutation in pathway A (Figure 2A) can be compensated by pathway B for pathogen Px, but not for pathogen Py. If Px is common and Py is rare, one could conclude that pathway A is redundant, because majority of patients will be exposed to Px and not to Py. If Py is more common, however, then the conclusion could be that pathway A is essential. In this case, whether pathway A is redundant or essential is a function of exposure rates to Px versus Py. The exposure rates to Px and Py could be very different in different environments, at different stages of human evolution, and can depend on modern environmental factors, such as availability of sanitation, vaccines and antibiotics.
Because opportunistic pathogens have the highest exposure rates, they are the most common causes of infectious diseases in immunodeficient people. However, the normal function of the defective pathway cannot be correctly inferred based on most common pathogens alone, despite the fact that they are most relevant clinically. We generally do not know what the susceptibility would be to rare pathogens, and which pathogens may have been common during different stages of evolution.
In addition to exposure rate, the evasion strategies used by different pathogens can also affect the clinical outcomes of immunodeficiency. One well-documented example of this phenomenon is deficiency in the terminal complement components, which commonly results in susceptibility to Neisseria spp. N. meningitidis and N. gonorrhoeae are opportunistic pathogens, which is one factor accounting for the high incidence of infections in complement immunodeficiency. However, complement is clearly an important host defense mechanism that plays a role in many other infections as well. Why then is terminal complement deficiency associated with such a narrow spectrum of infections? The reason is likely that other defense mechanisms can compensate for terminal complement defects in the case of other common pathogens, but not in the case of Neisseria spp. Why do not other defense mechanisms compensate for complement defects in the case of Neisseria? The reason most likely is because Neisseria has potent immune evasion mechanisms against all other relevant host defense pathways (Lo et al., 2009), making them ineffective in compensating for complement defects.
Thus pathogen exposure rates, virulence and immune evasion mechanisms can all affect the outcome of immunodeficient states.
Perspectives
Understanding the role of different host defense pathways in protection from infections is often complicated and there is debate on some fundamental issues, such as the role of different innate sensing pathways in host defense and control of adaptive immunity. The underlying problem is that immunity and infectious diseases are studied in three fields that use different language and mind-sets: basic immunology, human immunology and ecological immunology. Most of the available fundamental knowledge is generated by experiments on model organisms (such as inbred mice and fruit flies), where carefully controlled mechanistic studies are possible. Studies of human infectious diseases, including studies of human immunodeficiencies, provide valuable information that is relevant to human health. Finally, studies in ecological immunology examine biology of infections in the natural context and reveal evolutionary processes that account for disease resistance and susceptibility. Each of these approaches has important limitations: Studies in model animals, while well controlled, generally rely on a limited set of read-outs (e.g., survival and/or pathogen burden) and are performed in the unnatural environment of animal facilities. Furthermore, unique aspects of biology of model animals may preclude generalizations to other species. Analysis of human infectious diseases and immunodeficiencies is necessarily descriptive and cannot be controlled in the same way as studies in model organisms. Human studies may also have sample biases: First, the human subjects available for clinical studies may not be representative of the entire human population because of unequal access to medical care, sanitation and hygiene; and second, even patients accessible for analyses may not be homogenous in terms of their genetics, nutrition, microbiota, etc. For example, if a given immunodeficiency results in 50 percent mortality from infections, it is generally not known whether the survivors (or non-survivors) share another mutation or polymorphism (or a symbiont) that may account for their survival or mortality in the presence of the immunodeficiency in question. The modern human environment is also unnatural from the evolutionary standpoint: abnormally high population density on the one hand, and access to antibiotics, vaccination, sanitation, and hygiene products on the other hand, are not exactly natural for human species in a sense that much of human biology was shaped by an entirely different environment. Finally, ecological immunology has many logistic limitations of the field studies and is usually performed on species that can be easily observed in their natural environment. Ecological immunology provides a unique perspective on evolutionary processes that shape the immune system, but unfortunately most ‘mainstream’ immunologists are not familiar with this field.
Thus all three subfields of study of infectious diseases have their strengths and natural limitations. The future challenge is to incorporate the knowledge from all three areas into a more complete understanding of immunity and infectious diseases.
Acknowledgments
The works in RM’s laboratory is supported by the Howard Hughes Medical Institute and grants from the NIH (R37 AI046688, AI055502, DK071754). SN is supported by the NIH 055502-07S1 Diversity Supplement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–2702. [Abstract] [Google Scholar]
- Cremer S, Sixt M. Analogies in the evolution of individual and social immunity. Philos Trans R Soc Lond B Biol Sci. 2009;364:129–142. [Europe PMC free article] [Abstract] [Google Scholar]
- Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. [Abstract] [Google Scholar]
- Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, Kanost M, Thompson GJ, Zou Z, Hultmark D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol. 2006;15:645–656. [Europe PMC free article] [Abstract] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham A, Allen J, Read A. Evolutionary Causes and Consequences of Immunopathology. Annual Review of Ecology, Evolution and Systematics. 2005;36:373–397. [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. [Abstract] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. [Abstract] [Google Scholar]
- Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. [Abstract] [Google Scholar]
- Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis. 2009;9:418–427. [Abstract] [Google Scholar]
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. [Abstract] [Google Scholar]
- Nowak MA, Boerlijst MC, Cooke J, Smith JM. Evolution of genetic redundancy. Nature. 1997;388:167–171. [Abstract] [Google Scholar]
- Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. [Europe PMC free article] [Abstract] [Google Scholar]
- Sadd BM, Schmid-Hempel P. Principles of ecological immunology. Evolutionary Applications. 2009;2:113–121. [Europe PMC free article] [Abstract] [Google Scholar]
- Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. [Europe PMC free article] [Abstract] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. [Abstract] [Google Scholar]
- The Honeybee Genome Sequencing Consortium Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.immuni.2011.05.009
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1074761311001932/pdf
Citations & impact
Impact metrics
Article citations
Dual spatial host-bacterial gene expression in Mycobacterium abscessus respiratory infections.
Commun Biol, 7(1):1287, 09 Oct 2024
Cited by: 0 articles | PMID: 39384974 | PMCID: PMC11479615
Insights into the genetic theory of infectious diseases.
Tunis Med, 102(9):521-528, 05 Sep 2024
Cited by: 0 articles | PMID: 39287343 | PMCID: PMC11459253
Review Free full text in Europe PMC
Exploring the causal effect of omega-3 polyunsaturated fatty acid levels on the risk of type 1 diabetes: a Mendelian randomization study.
Front Genet, 15:1353081, 08 Jul 2024
Cited by: 0 articles | PMID: 39040994 | PMCID: PMC11260775
Coordinated nasal mucosa-mediated immunity accelerates recovery from COVID-19.
ERJ Open Res, 10(3):919-2023, 13 May 2024
Cited by: 0 articles | PMID: 38746861 | PMCID: PMC11089385
C-C motif chemokine receptor 2 and 7 synergistically control inflammatory monocyte recruitment but the infecting virus dictates monocyte function in the brain.
Commun Biol, 7(1):494, 24 Apr 2024
Cited by: 1 article | PMID: 38658802 | PMCID: PMC11043336
Go to all (110) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Detection of Microbial Infections Through Innate Immune Sensing of Nucleic Acids.
Annu Rev Microbiol, 72:447-478, 01 Sep 2018
Cited by: 204 articles | PMID: 30200854
Review
[The role of pattern-recognizing receptors in anti-infectious immunity].
Vestn Ross Akad Med Nauk, (10):47-54, 01 Jan 2011
Cited by: 1 article | PMID: 22168039
Review
Activation of toll-like receptors by microbial lipoproteins: role in host defense.
J Allergy Clin Immunol, 108(4 suppl):S104-6, 01 Oct 2001
Cited by: 7 articles | PMID: 11586275
Review
Infectious diseases not immune to genome-wide association.
Nat Genet, 42(9):731-732, 01 Sep 2010
Cited by: 17 articles | PMID: 20802473
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NIAID NIH HHS (7)
Grant ID: AI055502
Grant ID: R01 AI055502-07S1
Grant ID: R01 AI055502
Grant ID: R01 AI046688
Grant ID: R01 AI046688-05
Grant ID: R01 AI055502-07
Grant ID: R37 AI046688
NIDDK NIH HHS (3)
Grant ID: DK071754
Grant ID: R01 DK071754-05
Grant ID: R01 DK071754
National Institutes of Health (3)
Grant ID: DK071754
Grant ID: AI055502
Grant ID: R37 AI046688
PHS HHS (1)
Grant ID: NIH 055502-07S1





