Abstract
Free full text

Allele loss on chromosome 16 associated with progression of human hepatocellular carcinoma.
Abstract
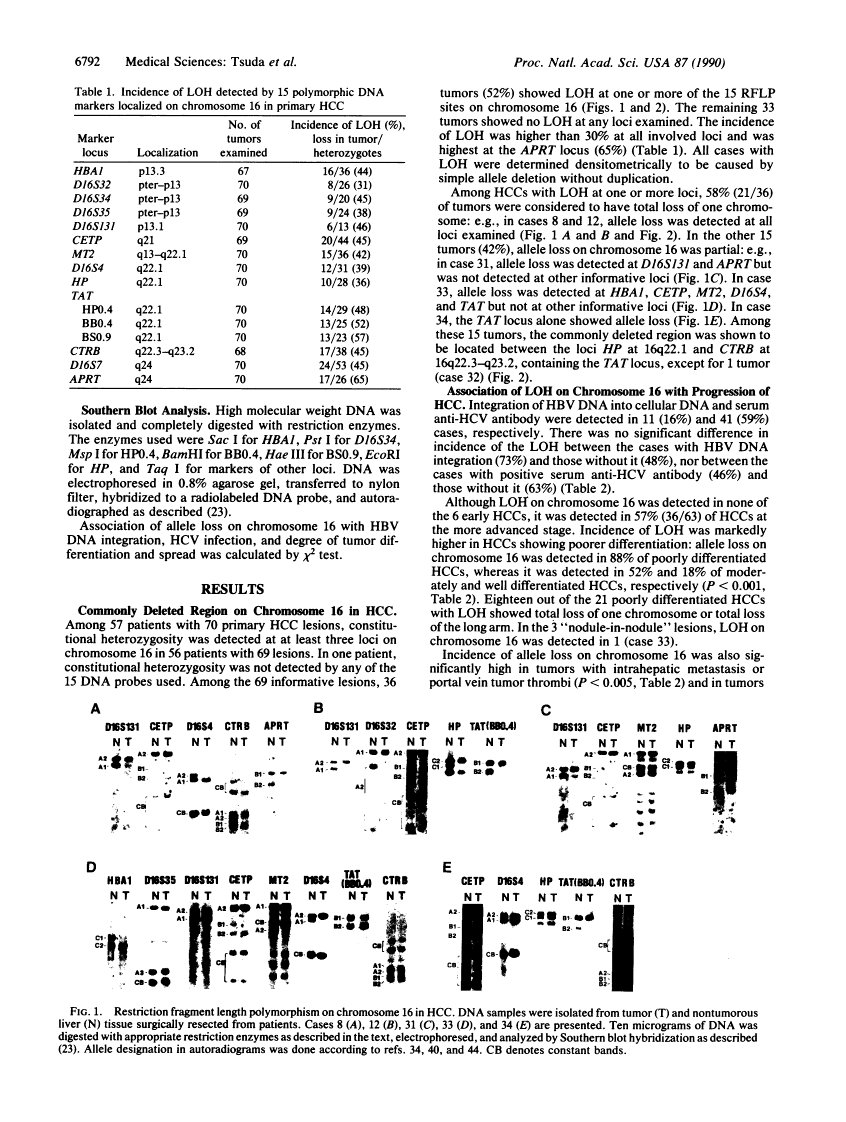
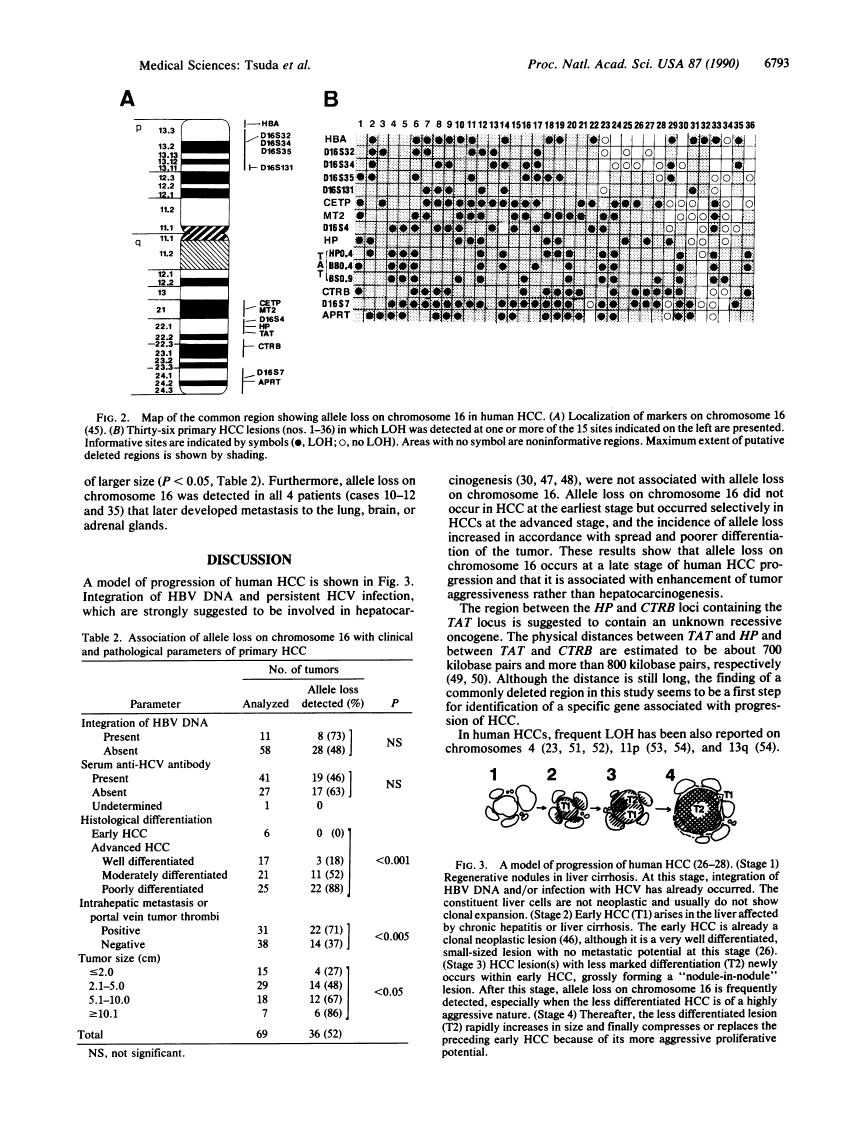
Loss of heterozygosity on chromosome 16 is a common genetic alteration in human hepatocellular carcinoma (HCC). To clarify the pathogenetic significance of allele loss on chromosome 16, we performed restriction fragment length polymorphism analysis of 70 surgically resected tumors by using 15 polymorphic DNA markers for chromosome 16. Loss of heterozygosity on chromosome 16 was detected in 36 (52%) of 69 informative cases, and the common region of allele loss in these 36 tumors was located between the HP locus (16q22.1) and the CTRB locus (16q22.3-q23.2). These losses occurred more frequently in HCCs of poor differentiation, of larger size, and with metastasis, whereas they were not detected in HCC at the earliest stage. In addition, these losses were not associated with presence or absence of hepatitis B virus DNA integration or hepatitis C virus infection. These results show that loss of heterozygosity on chromosome 16 is a late event occurring after hepatocarcinogenesis and strongly suggest that this phenomenon is involved in enhancement of tumor aggressiveness during progression of HCC.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavenee WK, Dryja TP, Phillips RA, Benedict WF, Godbout R, Gallie BL, Murphree AL, Strong LC, White RL. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature. 305(5937):779–784. [Abstract] [Google Scholar]
- Lee EY, To H, Shew JY, Bookstein R, Scully P, Lee WH. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. [Abstract] [Google Scholar]
- Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. [Abstract] [Google Scholar]
- Yokota J, Wada M, Shimosato Y, Terada M, Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. [Europe PMC free article] [Abstract] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. [Abstract] [Google Scholar]
- Bodmer WF, Bailey CJ, Bodmer J, Bussey HJ, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. [Abstract] [Google Scholar]
- Solomon E, Voss R, Hall V, Bodmer WF, Jass JR, Jeffreys AJ, Lucibello FC, Patel I, Rider SH. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. [Abstract] [Google Scholar]
- Leppert M, Dobbs M, Scambler P, O'Connell P, Nakamura Y, Stauffer D, Woodward S, Burt R, Hughes J, Gardner E, et al. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science. 1987 Dec 4;238(4832):1411–1413. [Abstract] [Google Scholar]
- Fong CT, Dracopoli NC, White PS, Merrill PT, Griffith RC, Housman DE, Brodeur GM. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. [Europe PMC free article] [Abstract] [Google Scholar]
- Dracopoli NC, Harnett P, Bale SJ, Stanger BZ, Tucker MA, Housman DE, Kefford RF. Loss of alleles from the distal short arm of chromosome 1 occurs late in melanoma tumor progression. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4614–4618. [Europe PMC free article] [Abstract] [Google Scholar]
- Naylor SL, Johnson BE, Minna JD, Sakaguchi AY. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. [Abstract] [Google Scholar]
- Yokota J, Tsukada Y, Nakajima T, Gotoh M, Shimosato Y, Mori N, Tsunokawa Y, Sugimura T, Terada M. Loss of heterozygosity on the short arm of chromosome 3 in carcinoma of the uterine cervix. Cancer Res. 1989 Jul 1;49(13):3598–3601. [Abstract] [Google Scholar]
- Ali IU, Lidereau R, Theillet C, Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. [Abstract] [Google Scholar]
- Scrable HJ, Witte DP, Lampkin BC, Cavenee WK. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. [Abstract] [Google Scholar]
- Knudson AG., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [Abstract] [Google Scholar]
- Huang HJ, Yee JK, Shew JY, Chen PL, Bookstein R, Friedmann T, Lee EY, Lee WH. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. [Abstract] [Google Scholar]
- Harbour JW, Lai SL, Whang-Peng J, Gazdar AF, Minna JD, Kaye FJ. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. [Europe PMC free article] [Abstract] [Google Scholar]
- Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF, Nakamura Y, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. [Abstract] [Google Scholar]
- Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, Levitt M, Pass H, Gazdar AF, Minna JD. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. [Abstract] [Google Scholar]
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. [Abstract] [Google Scholar]
- Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. [Abstract] [Google Scholar]
- Zhang WD, Hirohashi S, Tsuda H, Shimosato Y, Yokota J, Terada M, Sugimura T. Frequent loss of heterozygosity on chromosomes 16 and 4 in human hepatocellular carcinoma. Jpn J Cancer Res. 1990 Feb;81(2):108–111. [Europe PMC free article] [Abstract] [Google Scholar]
- Arthur DC, Bloomfield CD. Partial deletion of the long arm of chromosome 16 and bone marrow eosinophilia in acute nonlymphocytic leukemia: a new association. Blood. 1983 May;61(5):994–998. [Abstract] [Google Scholar]
- Le Beau MM, Larson RA, Bitter MA, Vardiman JW, Golomb HM, Rowley JD. Association of an inversion of chromosome 16 with abnormal marrow eosinophils in acute myelomonocytic leukemia. A unique cytogenetic-clinicopathological association. N Engl J Med. 1983 Sep 15;309(11):630–636. [Abstract] [Google Scholar]
- Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987 Aug 15;60(4):810–819. [Abstract] [Google Scholar]
- Kenmochi K, Sugihara S, Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver. 1987 Feb;7(1):18–26. [Abstract] [Google Scholar]
- Kobayashi M, Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984 Oct;30(1-3):227–232. [Abstract] [Google Scholar]
- Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. [Abstract] [Google Scholar]
- Miyamura T, Saito I, Katayama T, Kikuchi S, Tateda A, Houghton M, Choo QL, Kuo G. Detection of antibody against antigen expressed by molecularly cloned hepatitis C virus cDNA: application to diagnosis and blood screening for posttransfusion hepatitis. Proc Natl Acad Sci U S A. 1990 Feb;87(3):983–987. [Europe PMC free article] [Abstract] [Google Scholar]
- Assum G, Griese EU, Horst J. Detection of a restriction site polymorphism within the human alpha-globin gene complex. Hum Genet. 1985;69(2):144–146. [Abstract] [Google Scholar]
- Harris P, Lalande M, Stroh H, Bruns G, Flint A, Latt SA. Construction of a chromosome 16-enriched phage library and characterization of several DNA segments from 16p. Hum Genet. 1987 Oct;77(2):95–103. [Abstract] [Google Scholar]
- Hyland VJ, Friend K, Sutherland GR. A TaqI RFLP detected by the probe VK45C6 [D16S131] at 16p13.11. Nucleic Acids Res. 1989 Aug 11;17(15):6430–6430. [Europe PMC free article] [Abstract] [Google Scholar]
- Drayna D, Lawn R. Multiple RFLPs at the human cholesteryl ester transfer protein (CETP) locus. Nucleic Acids Res. 1987 Jun 11;15(11):4698–4698. [Europe PMC free article] [Abstract] [Google Scholar]
- Hyland VJ, Grist S, West A, Richards RI, Sutherland GR. A 5' flanking region of the metallothionein, MT2A, gene identifies two moderately frequent RFLPs. Nucleic Acids Res. 1987 Feb 11;15(3):1350–1350. [Europe PMC free article] [Abstract] [Google Scholar]
- Hyland VJ, Grist S, Sutherland GR. Restriction fragment length polymorphisms detected by anonymous DNA probes mapped to defined intervals of human chromosome 16. Hum Genet. 1988 Jul;79(3):277–279. [Abstract] [Google Scholar]
- Maeda N, Yang F, Barnett DR, Bowman BH, Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984 May 10;309(5964):131–135. [Abstract] [Google Scholar]
- Westphal EM, Natt E, Grimm T, Odievre M, Scherer G. The human tyrosine aminotransferase gene: characterization of restriction fragment length polymorphisms and haplotype analysis in a family with tyrosinemia type II. Hum Genet. 1988 Jul;79(3):260–264. [Abstract] [Google Scholar]
- Wullich B, Natt E, Wienker TF, Scherer G. A BAM HI RFLP at the human tyrosine aminotransferase (TAT) gene locus at 16q. Nucleic Acids Res. 1989 Apr 25;17(8):3331–3331. [Europe PMC free article] [Abstract] [Google Scholar]
- Bell GI, Quinto C, Quiroga M, Valenzuela P, Craik CS, Rutter WJ. Isolation and sequence of a rat chymotrypsin B gene. J Biol Chem. 1984 Nov 25;259(22):14265–14270. [Abstract] [Google Scholar]
- Bufton L, Mohandas TK, Magenis RE, Sheehy R, Bestwick RK, Litt M. A highly polymorphic locus on chromosome 16q revealed by a probe from a chromosome-specific cosmid library. Hum Genet. 1986 Dec;74(4):425–431. [Abstract] [Google Scholar]
- Murray AM, Drobetsky E, Arrand JE. Cloning the complete human adenine phosphoribosyl transferase gene. Gene. 1984 Nov;31(1-3):233–240. [Abstract] [Google Scholar]
- Pearson PL, Kidd KK, Willard HF. Report of the committee on human gene mapping by recombinant DNA techniques. Cytogenet Cell Genet. 1987;46(1-4):390–566. [Abstract] [Google Scholar]
- Reeders ST, Hildebrand CE. Report of the committee on the genetic constitution of chromosome 16. Cytogenet Cell Genet. 1989;51(1-4):299–318. [Abstract] [Google Scholar]
- Tsuda H, Hirohashi S, Shimosato Y, Terada M, Hasegawa H. Clonal origin of atypical adenomatous hyperplasia of the liver and clonal identity with hepatocellular carcinoma. Gastroenterology. 1988 Dec;95(6):1664–1666. [Abstract] [Google Scholar]
- Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. [Abstract] [Google Scholar]
- Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6547–6549. [Europe PMC free article] [Abstract] [Google Scholar]
- Westphal EM, Burmeister M, Wienker TF, Lehrach H, Bender K, Scherer G. Tyrosine aminotransferase and chymotrypsinogen B are linked to haptoglobin on human chromosome 16q: comparison of genetic and physical distances. Genomics. 1987 Dec;1(4):313–319. [Abstract] [Google Scholar]
- Natt E, Magenis RE, Zimmer J, Mansouri A, Scherer G. Regional assignment of the human loci for uvomorulin (UVO) and chymotrypsinogen B (CTRB) with the help of two overlapping deletions on the long arm of chromosome 16. Cytogenet Cell Genet. 1989;50(2-3):145–148. [Abstract] [Google Scholar]
- Buetow KH, Murray JC, Israel JL, London WT, Smith M, Kew M, Blanquet V, Brechot C, Redeker A, Govindarajah S. Loss of heterozygosity suggests tumor suppressor gene responsible for primary hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8852–8856. [Europe PMC free article] [Abstract] [Google Scholar]
- Pasquinelli C, Garreau F, Bougueleret L, Cariani E, Grzeschik KH, Thiers V, Croissant O, Hadchouel M, Tiollais P, Bréchot C. Rearrangement of a common cellular DNA domain on chromosome 4 in human primary liver tumors. J Virol. 1988 Feb;62(2):629–632. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogler CE, Sherman M, Su CY, Shafritz DA, Summers J, Shows TB, Henderson A, Kew M. Deletion in chromosome 11p associated with a hepatitis B integration site in hepatocellular carcinoma. Science. 1985 Oct 18;230(4723):319–322. [Abstract] [Google Scholar]
- Wang HP, Rogler CE. Deletions in human chromosome arms 11p and 13q in primary hepatocellular carcinomas. Cytogenet Cell Genet. 1988;48(2):72–78. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.17.6791
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc54623?pdf=render
Citations & impact
Impact metrics
Article citations
Machine learning and multi-omics data reveal driver gene-based molecular subtypes in hepatocellular carcinoma for precision treatment.
PLoS Comput Biol, 20(5):e1012113, 10 May 2024
Cited by: 0 articles | PMID: 38728362 | PMCID: PMC11230636
Integrated Analysis of Ulcerative Colitis Revealed an Association between PHLPP2 and Immune Infiltration.
Dis Markers, 2022:4983471, 16 Mar 2022
Cited by: 3 articles | PMID: 35308140 | PMCID: PMC8931176
PHLPPing the Script: Emerging Roles of PHLPP Phosphatases in Cell Signaling.
Annu Rev Pharmacol Toxicol, 61:723-743, 30 Sep 2020
Cited by: 16 articles | PMID: 32997603
Review
Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma.
Sci Rep, 5:10466, 23 Jun 2015
Cited by: 43 articles | PMID: 26099564 | PMCID: PMC4479133
WD repeat protein WDR48 in complex with deubiquitinase USP12 suppresses Akt-dependent cell survival signaling by stabilizing PH domain leucine-rich repeat protein phosphatase 1 (PHLPP1).
J Biol Chem, 288(48):34545-34554, 21 Oct 2013
Cited by: 46 articles | PMID: 24145035 | PMCID: PMC3843068
Go to all (169) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Frequent loss of heterozygosity on chromosomes 16 and 4 in human hepatocellular carcinoma.
Jpn J Cancer Res, 81(2):108-111, 01 Feb 1990
Cited by: 72 articles | PMID: 1970554 | PMCID: PMC5963891
Hepatitis B virus integration event in human chromosome 17p near the p53 gene identifies the region of the chromosome commonly deleted in virus-positive hepatocellular carcinomas.
Cancer Res, 51(1):49-54, 01 Jan 1991
Cited by: 77 articles | PMID: 1670994
Allelic loss at chromosome band 8p21.3-p22 is associated with progression of hepatocellular carcinoma.
Genes Chromosomes Cancer, 7(3):152-157, 01 Jul 1993
Cited by: 62 articles | PMID: 7687868
[Cytogenetic and molecular genetic alterations on chromosome 4q in human hepatocellular carcinoma].
Ai Zheng, 27(9):998-1005, 01 Sep 2008
Cited by: 0 articles | PMID: 18799044
Review