Abstract
Purpose of review
This review highlights recent progress toward understanding complex interactions between diarrhea, pneumonia, and undernutrition among children in low-income and middle-income countries.Recent findings
New studies parallel earlier reports that diarrhea and pneumonia impair children's growth and that underlying malnutrition is a major risk factor for these conditions. Episodes of diarrhea may predispose to pneumonia in undernourished children. Additional studies support breastfeeding and micronutrient supplementation for the prevention and control of diarrhea and pneumonia. Malnutrition may partially account for the reduced efficacy of oral rotavirus vaccines in low-income countries. Immunization of pregnant women against influenza also appears to reduce intrauterine growth retardation. Immunization of infants against Streptococcus pneumoniae may improve their growth. New genetic studies indicate that polymorphisms in apolipoprotein E or the leptin receptor modulate children's risk for diarrhea and Entamoeba histolytica infection, respectively, thereby linking two genes important for lipid metabolism to enteric infections.Summary
Significant advances have been made in understanding the vicious cycle of malnutrition, diarrhea, and pneumonia in developing countries. Future challenges will be to translate this progress into effective and widely accessible public health measures.Free full text

Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries
Abstract
Purpose of review
This review highlights recent progress toward understanding complex interactions between diarrhea, pneumonia, and undernutrition among children in low-income and middle-income countries.
Recent findings
New studies parallel earlier reports that diarrhea and pneumonia impair children’s growth and that underlying malnutrition is a major risk factor for these conditions. Episodes of diarrhea may predispose to pneumonia in undernourished children. Additional studies support breastfeeding and micronutrient supplementation for the prevention and control of diarrhea and pneumonia. Malnutrition may partially account for the reduced efficacy of oral rotavirus vaccines in low-income countries. Immunization of pregnant women against influenza also appears to reduce intrauterine growth retardation. Immunization of infants against Streptococcus pneumoniae may improve their growth. New genetic studies indicate that polymorphisms in apolipoprotein E or the leptin receptor modulate children’s risk for diarrhea and Entamoeba histolytica infection, respectively, thereby linking two genes important for lipid metabolism to enteric infections.
Summary
Significant advances have been made in understanding the vicious cycle of malnutrition, diarrhea, and pneumonia in developing countries. Future challenges will be to translate this progress into effective and widely accessible public health measures.
Introduction
Pneumonia and diarrhea together account for more than 3 million child deaths each year—or nearly one in three child deaths worldwide [1]. Both conditions have long been linked to a reciprocal cycle of malnutrition and infection among vulnerable children in low-income and middle-income countries; hence, the global burden of these diseases may extend beyond their staggering toll on child survival to include long-term adverse outcomes for child growth and neurodevelopment. This review addresses recent clinical research findings that elucidate the nutrition–infection cycle in developing countries and highlights issues involved in translating these advances into interventions.
Diarrhea and pneumonia as risk factors for and outcomes of undernutrition
Prior to the 1960s, child malnutrition in developing countries was attributed almost exclusively to poor diet; the view that common childhood infections might directly influence growth was still controversial. Seminal studies by Mata and colleagues [2–4] in Guatemala provided the first rigorous evidence in support of this hypothesis by carefully tracking the growth and illnesses of newborn children in the village of Santa Maria Cauque. An illustrative example of their findings is seen in Fig. 1, showing the growth curve of a child with a normal birth weight who continued to gain weight normally during the first 6 months of life while breastfed [3]. Upon weaning, a series of gut, respiratory, and skin infections began. These illnesses occurred both discretely and concomitantly, were accompanied by no net gains in weight for 1 year, and were associated with inadequate weight gain for 18 months thereafter. Five decades later, this child’s story is shared by millions of children worldwide who, in part because of preventable infectious diseases, will not reach their full growth potential.
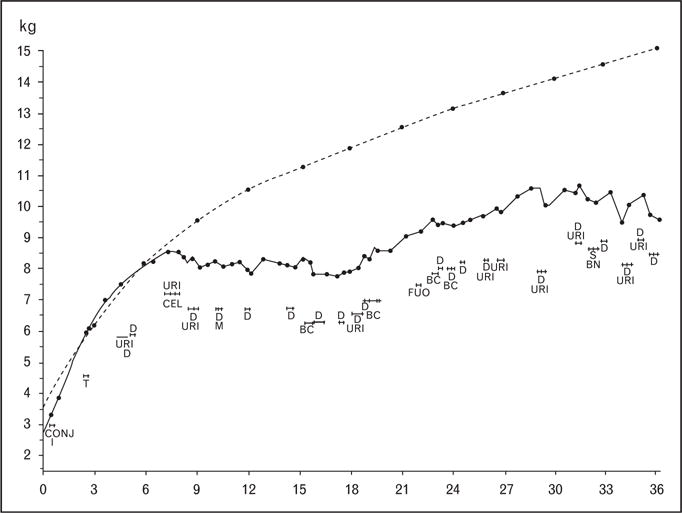
Solid line represents child’s weight-for-age; dashed line represents expected weight-for-age; D, diarrhea; URI, upper respiratory illness; BC, bronchitis; BN, bronchopneumonia; FUO, fever of unknown origin; CONJ, conjunctivitis; T, thrush; CEL, cellulitis. Reproduced with permission [3].
Studies from West Africa in the 1980s by Rowland et al. [5] reported similar findings. The authors followed a cohort of 126 newborns in a Gambian township, where the mean weight-for-age in the first 6 months of life exceeded National Center for Health Statistics standards. However, by 1 year of age, a mean weight deficit of 1.2 kg emerged. Diarrheal diseases accounted for one-half of the deficit, or 14.4 g of weight loss per day of infection. Acute lower respiratory tract infections (ALRI) accounted for one-quarter, or 14.7 g of weight loss per day of infection.
Recent findings from Brazil build on and extend earlier findings by demonstrating a bidirectional relationship between nutritional status and the duration of diarrheal illnesses. In a decade-long birth cohort study of 414 children followed for prolonged episodes of acute diarrhea (ProD, duration ≥7 and <14 days) and persistent diarrhea (PD, duration ≥14 days), Moore et al. [6•] found that ProD and PD comprised only 16% of diarrheal episodes, yet accounted for 50% of all days with diarrhea. They further found that children’s height-for-age prior to a first ProD or PD episode was significantly below WHO/Centers for Disease Control (CDC) international standards (Fig. 2). In the months following ProD, children experienced further declines in height-for-age (indicating stunting), as well as weight-for-age (indicating underweight). PD illnesses were followed by significant decreases in weight-for-age and weight-for-height (an indicator of wasting). Substandard height and weight were more likely prior to either a first ProD or PD illness compared with a first acute (AD, duration <7 days) illness. Furthermore, a ProD illness in the first year of life robustly predicted which children were at increased risk of later developing PD. Importantly, these short-term nutritional effects of diarrhea may have long-term consequences for children’s growth. Despite the phenomenon of catch-up growth, recent multicountry data reveal that diarrhea in early childhood remains highly predictive of stunting at age 2 years and beyond [7].
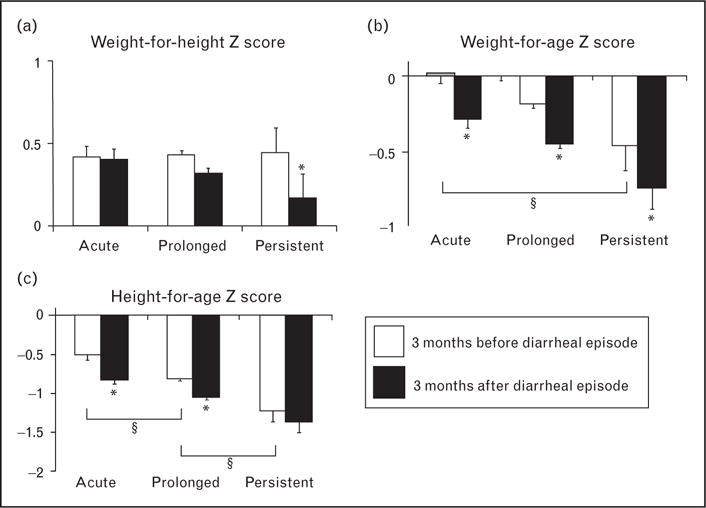
Nutritional Z-scores (age-adjusted and sex-adjusted standard deviations above or below WHO/CDC medians) were compared 3 months before and after children’s first acute (n = 308), prolonged (n = 145), and persistent (n = 62) (a) Weight-for-height (WHZ) Z-scores declined significantly following persistent episodes, but not after episodes. acute or prolonged episodes. (b) Weight-for-age (WAZ) Z-scores decreased with all episode types and mean WAZ of children prior to acute diarrhea was significantly greater than mean WAZ prior to persistent diarrhea. (c) Height-forage (HAZ) Z-scores declined following acute and prolonged episodes, but not after persistent episodes. Mean HAZ prior to acute diarrhea was greater than mean HAZ prior to prolonged or persistent diarrhea. (Error bars indicate SEM; *P < 0.005, paired t-test of Z-scores before and after diarrhea; §P < 0.05, unpaired t-test of Z-scores prior to acute vs. prolonged, acute vs. persistent, or prolonged vs. persistent episodes). Reproduced with permission [6•].
New studies from Bangladesh, Colombia, Ghana, and Israel further support the paradigm that malnutrition is a key risk factor for diarrhea and pneumonia and, in many cases, specific infectious causes. Drawing on nearly three decades of hospital-based surveillance in Dhaka, Khatun et al. [8] report findings from a study of 3648 children less than 5 years of age with positive cultures for Shigella. Of these 3648 children, 8–10% were severely underweight, stunted, or wasted and this subgroup had a 1.8–2.2-fold greater risk of developing Shigella compared with well nourished controls. Mondal et al. [9] report findings from a community-based prospective cohort study in Dhaka showing that enterotoxigenic E. coli, Cryptosporidium, and E. histolytica were identified more frequently in stools from undernourished vs. well-nourished children with diarrhea. In a nationally representative sample of 5333 Bangladeshi children, Rahman et al. [10] report that respiratory illnesses with fever or cough were more frequent in children with moderate or severe wasting. In Colombia, Villamor and colleagues [11,12] show that stunting was significantly associated with an increase in the incidence of cough with fever or infection with Giardia duodenalis among school children from low-income and middle-income households. In Ghana, a case–control study by Opintan et al. [13] of 274 children, with or without diarrhea, identified moderate or severe wasting as a risk factor. Lastly, Coles et al. [14] report that low weight-for-age increased the risk of symptomatic giardiasis among young Arab-Bedouin children in Israel. Taken together, these geographically and methodologically diverse studies bolster earlier findings that diarrhea and pneumonia are significantly associated with malnutrition both before and following the illness [15,16].
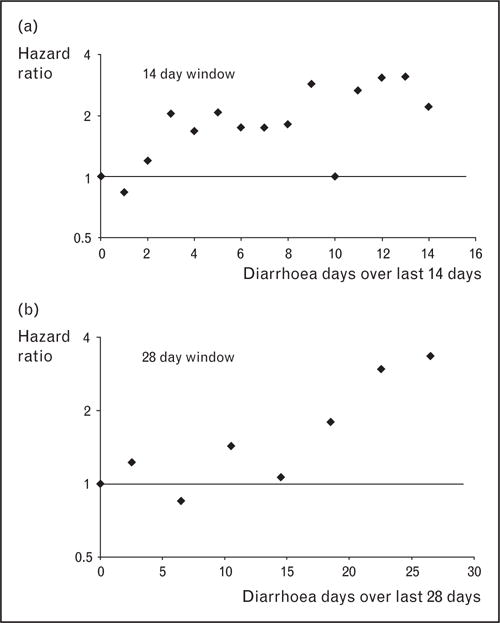
Of special interest, a novel analysis by Schmidt et al. [17] demonstrates that diarrhea frequently precedes pneumonia in undernourished children. Using a time-to-event analysis in a cohort of children from Ghana at high-risk of diarrhea and malnutrition, they found that each additional day of diarrhea in the preceding 2 weeks augmented the risk of developing pneumonia by a factor of 1.08, demonstrating a linear relationship between the number of days of diarrhea in the preceding 28 days and the subsequent risk of pneumonia (Fig. 3). They did not find similar associations in a cohort of better-nourished Brazilian children; hence, diarrhea may exacerbate underlying moderate or severe malnutrition to further impair children’s immunity to pneumonia. In their commentary on the study, Walker and Black [18] suggest that increased loss of zinc in stools or electrolyte imbalances following diarrhea may be potential mechanisms of increased risk of subsequent infections and that undernourished children should be closely monitored for other infections in the weeks following resolution of their diarrheal illnesses. Studies by Stephensen [19] have shown that children with diarrhea have increased renal losses of vitamin A and suggest another mechanism by which diarrhea might predispose to pneumonia. Importantly, the findings of Schmidt et al. indicate that interventions for diarrhea may also have a substantial impact on reducing the burden of pneumonia in undernourished children.
Nutritional interventions for diarrhea and pneumonia
In 2009, the United Nations Children’s Fund and WHO published recommendations for prevention and treatment of diarrhea and included breastfeeding, zinc, and vitamin A as key nutritional strategies, with well-documented benefits for the reduction of child mortality from diarrhea [20]. Adding strength to these guidelines are several recent studies from sub-Saharan Africa, where it is estimated that 50% of all childhood diarrheal deaths occur. The first is a case–control analysis by Arvelo et al. [21] of a diarrhea outbreak in Botswana, which showed that infants less than 12 months of age who were not currently breastfeeding had a 30-fold increase in risk of diarrhea compared with breastfed controls. A second study, by Mwiru et al. [22] in Tanzania, found that exclusive breastfeeding was associated with a significant decrease in the risk of respiratory and diarrheal diseases in the first 6 months of life among 666 children born to HIV-infected mothers, thereby adding support to the recommendation to promote exclusive breastfeeding to 6 months of age among HIV-infected women who choose to breastfeed.
Additional reports focus on supplementation with multivitamins, zinc, and vitamins A and D. A trial of multivitamin supplementation conducted by Mda et al. [23], in 118 children less than 2 years of age hospitalized for diarrhea or pneumonia in South Africa, reports that daily supplementation with a multivitamin containing vitamins A, B complex, C, D, E, and folic acid, and copper, iron, and zinc led to a statistically-significant 1.7-day decrease in duration of hospitalization with diarrhea or pneumonia. A Cochrane Collaboration meta-analysis of the effects of vitamin A supplementation on prevention of morbidity and mortality in preschool children showed substantial reductions in diarrhea morbidity and mortality, but no significant benefit for cause-specific pneumonia [24]. To evaluate the effects of prenatal zinc supplementation on diarrhea morbidity in infants, Iannotti et al. [25] conducted a trial in Peru. Pregnant participants received either daily zinc or placebo and their infants were followed from birth until 12 months of age for diarrhea. Diarrhea was less prevalent in the zinc group vs. the control group, and prenatal zinc supplementation also reduced the likelihood of ProD, PD, or stools with mucus.
Vitamin D deficiency and susceptibility to infectious diseases was first recognized by Davies et al. [26] in patients with tuberculosis. In Yemen, Karatekin et al. [27] report that vitamin D levels in newborns with ALRI were lower than healthy controls. More recently, Banajeh [28] demonstrates a significant association between rickets, circulating neutrophils, oxygen saturation, and treatment outcomes in severe pneumonia. Taken together, these findings suggest that vitamin D supplementation of deficient children may help prevent and treatment pneumonia and other respiratory infections [29].
In addition to breastfeeding and micronutrients, recent attention has also been given to supplementation with essential amino acids. In a trial of lysine supplementation in children and adults in Ghana, Ghosh et al. [30•] found that lysine reduced diarrheal morbidity among children by 48%, but had no significant protective effect on diarrhea in adults. Although lysine supplementation had no apparent effect on children’s growth, the authors did note a significant inverse correlation between increased amounts of diarrhea and weight gain during the study.
Nutritional aspects of vaccine and antibiotic interventions for diarrhea and pneumonia
Beyond evaluations of interactions of growth, nutrients, and infection, other approaches to assess these complex relationships include the use of antimicrobials or vaccines as interventions to eliminate or reduce select microorganisms. In well-designed trials, the only difference between two populations would be the drug or vaccine effect; therefore, differences between groups can be attributed to reduction or elimination of specific pathogens. Likely mechanisms for these effects are: reduction of the inflammatory responses and immune activation generated by the pathogen, reduction of illness-related anorexia, and a reduction in the malabsorption associated with gut infections [31]. For respiratory pathogens, the effect of inflammatory/immune activation and anorexia are likely to be the major mechanisms. Because immune activation requires metabolic energy, this energy is not available for growth, which in turn will be reduced. A recent study of adults showed that the resting metabolic rate was 8–14% higher during minor respiratory illnesses compared with recovery, showing the magnitude of the metabolic cost of immune activation [32].
Studies of substantial effects of anthelmintics on both growth and school performance show the effectiveness of using treatments to learn about pathogenesis [33]. Similarly, recent trials of vaccines against respiratory pathogens have generated unexpected and welcome results regarding infant nutrition (Table 1). Steinhoff and colleagues in Bangladesh randomized 340 pregnant women to pneumococcal or influenza vaccine [34]. Their 320 infants were then randomized to receive three doses of pneumococcal conjugate vaccine (PCV7) or Haemophilus influenzae type B (Hib) vaccine [35]. Infants in the PCV7 group were somewhat taller and heavier. Further analyses showed that mean heights and weights were not statistically different in boys, but were substantially higher in PCV7 girls at 6 months of age (a 9%, or 507 g, increase in mean weight). Maternal influenza and infant PCV7 immunizations were independently associated with increased length for age. Furthermore, maternal flu vaccination during a period of high flu circulation led to a significant reduction in small-for-gestational-age births (29% vs. 44% in controls) and improvements in mean birth weights (3178 g vs. 2978 g in controls) [36]. These data suggest that influenza infections in pregnancy adversely affect intrauterine growth, and further studies are needed to assess this novel observation; to our knowledge, these are the first examples of vaccines improving nutrition.
Table 1
Effect of respiratory infections on growth
| Illness or vaccine | Deficit | Age | Source |
|---|---|---|---|
| Acute lower respiratory tract infections | 14.7 g/day of illness | 0–1 year | Rowland et al. [5], Gambia |
| Coughing illness | 11 mm/6 mo. 10–18 g/6 mo. | Kossmann et al. [15], Sudan | |
| Respiratory infection | 0.6 kg at 12 mo. | 0–12 mo. prospective | Moffat [16], Nepal |
| Influenza vaccine in pregnancy | +200 ga | Newborn | Steinhoff et al. [36], Bangladesh |
| PCV7 vaccine in infants | +507 ga | 0–6 month | Steinhoff et al. [35], Bangladesh |
This table summarizes key studies identifying an adverse effect of malnutrition secondary to respiratory infections. The second column identifies the growth deficit from respiratory infections or growth benefit from a vaccine against respiratory infections observed in each study. ALRI, acute lower respiratory infection; PCV7, conjugate pneumoccal conjugate vaccine.
Trials of rotavirus vaccines in developing countries reveal the substantial challenges in developing effective oral vaccines for underprivileged populations. When comparing the immune response to Rotarix across countries representing different income strata, Patel et al. [37] find that high-income, middle-income, and low-income countries have mean seroconversion rates of 86, 75, and 63%, respectively. Maternal antibodies in breast milk, coinfections, malnutrition, and overwhelming exposure to rotavirus are possible explanations that require further study. It has not yet been reported whether rotavirus vaccination offers secondary protection against under-nutrition.
Genetic determinants of the infection–nutrition cycle
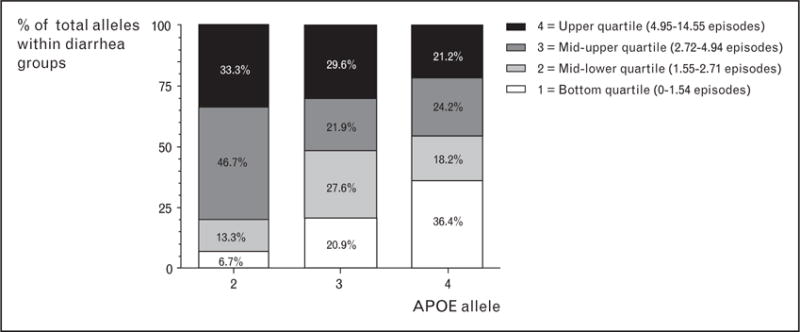
Two recent studies contribute to a novel genetic understanding of how undernutrition contributes to diarrhea morbidity. The first, by Oria et al. [38], follows up on an earlier study in which they demonstrated that apolipoprotein (APO)E4, an allele well known to predispose to Alzheimer’s disease and cardiovascular disease, protected the cognitive function of a cohort of Brazilian children with heavy diarrhea burdens. They have now determined that APOE4 also protects against early childhood diarrhea [39]. Figure 4 shows that of the three APOE alleles, children with APOE2 and APOE3 have significantly greater burdens of diarrhea in early childhood compared with children with APOE4. This suggests that the distinctive mechanisms of APOE4-mediated cholesterol transport and metabolism that predispose to Alzheimer’s disease may also protect undernourished children (e.g. recent weanlings with insufficient dietary fat) against enteric infections and their long-term sequelae, hence its continued presence in the genomic pool.
A role for lipid homeostasis in protection against specific enteric pathogens is supported by recent findings by Duggal et al. [40]. Building on earlier laboratory observations that mice lacking leptin (an adipose-derived hormone that mediates satiety) or its receptor (LEPR) are more susceptible to infection, they prospectively followed a cohort of Bangladeshi children for E. histolytica infection and tested them for genetic variants in leptin and LEPR. They report that a single amino acid substitution in the cytokine receptor homology domain 1 of LEPR was associated with a nearly four-fold increase in risk for infection compared with children homozygous for the typical allele. Furthermore, they find that this LEPR mutation was associated with amebic liver abscesses in adults and demonstrate that mice engineered to express mutant LEPR were more highly susceptible to severe infection with E. histolytica.
Conclusion
Pneumonia and diarrhea account for nearly one in three child deaths worldwide, and both conditions are linked to a reciprocal cycle of malnutrition and infection. Beginning with classic descriptions in Guatemala, subsequent research has continued to elucidate the detrimental nutritional consequences of recurrent or severe episodes of diarrhea and pneumonia. Additionally, malnourished children are more likely to acquire these illnesses and experience greater morbidity and mortality as a result. New and novel intervention trials, addressing immunization and supplementation, along with a better understanding of the mechanisms and genetic determinants of undernutrition, offer hope of solutions. Although significant advances have been made in understanding this vicious cycle, further research is needed in order to translate this progress into effective and large-scale public health measures.
Acknowledgments
Authors thank Elizabeth Maier for assistance in preparing the review. E.P.S. is supported by NIH grant 5 R24 TW007988-03, M.C.S. is supported by the Bill and Melinda Gates Foundation, and S.R.M. is supported by NIH grant K12HD028827.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 512–513).
Full text links
Read article at publisher's site: https://doi.org/10.1097/qco.0b013e328349287d
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5454480?pdf=render
Citations & impact
Impact metrics
Article citations
Wasting and its associated factors among under-two years children in Ethiopia: a systematic review and meta-analysis.
BMC Public Health, 24(1):2547, 19 Sep 2024
Cited by: 0 articles | PMID: 39300428 | PMCID: PMC11411762
Review Free full text in Europe PMC
Predictive modeling and socioeconomic determinants of diarrhea in children under five in the Amhara Region, Ethiopia.
Front Public Health, 12:1366496, 01 Aug 2024
Cited by: 0 articles | PMID: 39157521 | PMCID: PMC11327862
Factors associated with severe pneumonia among children <5 years, Kasese District, Uganda: a case-control study, January-April 2023.
Pneumonia (Nathan), 16(1):13, 25 Jul 2024
Cited by: 0 articles | PMID: 39049136 | PMCID: PMC11270805
The Impact of Rapid Handpump Repairs on Diarrhea Morbidity in Children: Cross-Sectional Study in Kwale County, Kenya.
JMIR Public Health Surveill, 10:e42462, 16 Jan 2024
Cited by: 1 article | PMID: 38227359 | PMCID: PMC10828938
Multimodal machine learning for modeling infant head circumference, mothers' milk composition, and their shared environment.
Sci Rep, 14(1):2977, 05 Feb 2024
Cited by: 1 article | PMID: 38316895 | PMCID: PMC10844250
Go to all (74) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments.
Clin Infect Dis, 59 Suppl 4:S193-206, 01 Nov 2014
Cited by: 248 articles | PMID: 25305287
Global burden of childhood pneumonia and diarrhoea.
Lancet, 381(9875):1405-1416, 12 Apr 2013
Cited by: 1144 articles | PMID: 23582727 | PMCID: PMC7159282
Review Free full text in Europe PMC
Health practices and indices of a poor urban population in Indonesia. Part II: Immunization, nutrition, and incidence of diarrhea.
Asia Pac J Public Health, 7(4):224-227, 01 Jan 1994
Cited by: 2 articles | PMID: 7605697
Rotavirus A infection in children under five years old with a double health problem: undernutrition and diarrhoea - a cross-sectional study in four provinces of Mozambique.
BMC Infect Dis, 21(1):18, 06 Jan 2021
Cited by: 8 articles | PMID: 33407207 | PMCID: PMC7788695
Funding
Funders who supported this work.
FIC NIH HHS (2)
Grant ID: 5 R24 TW007988-03
Grant ID: R24 TW007988
NICHD NIH HHS (2)
Grant ID: K12HD028827
Grant ID: K12 HD028827