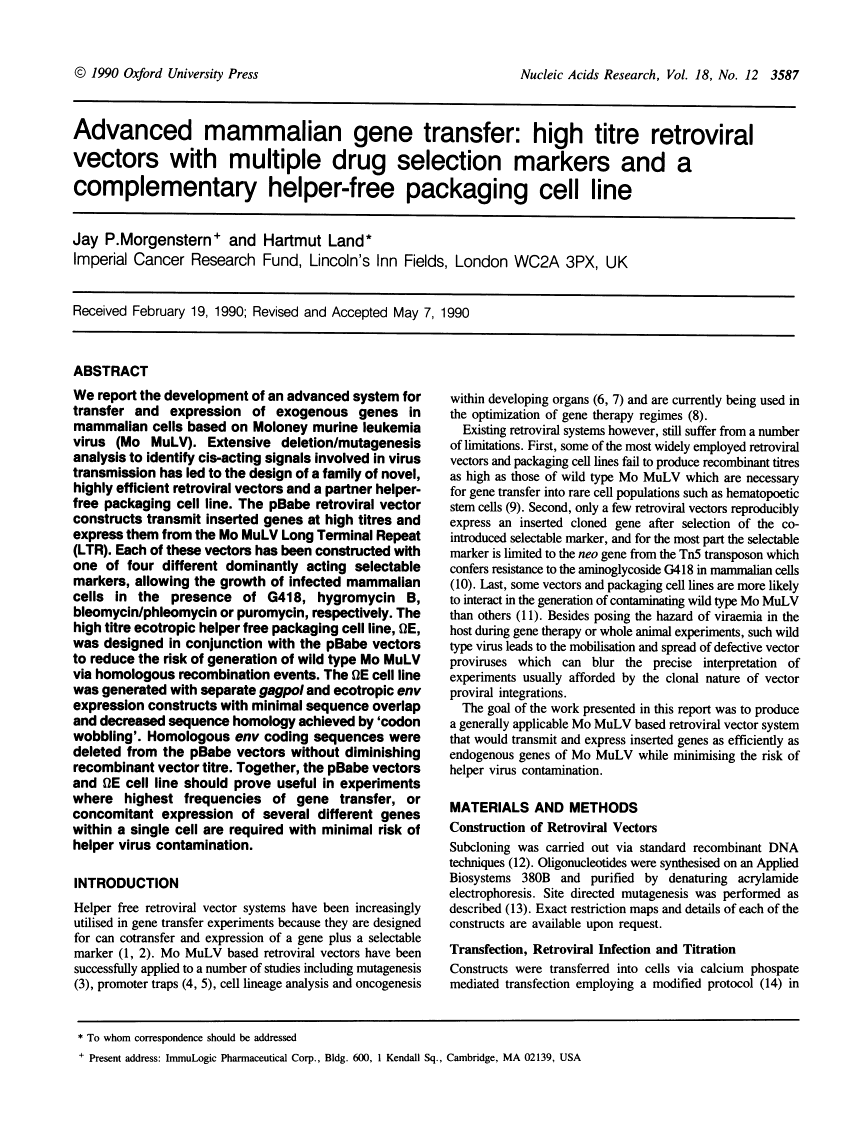
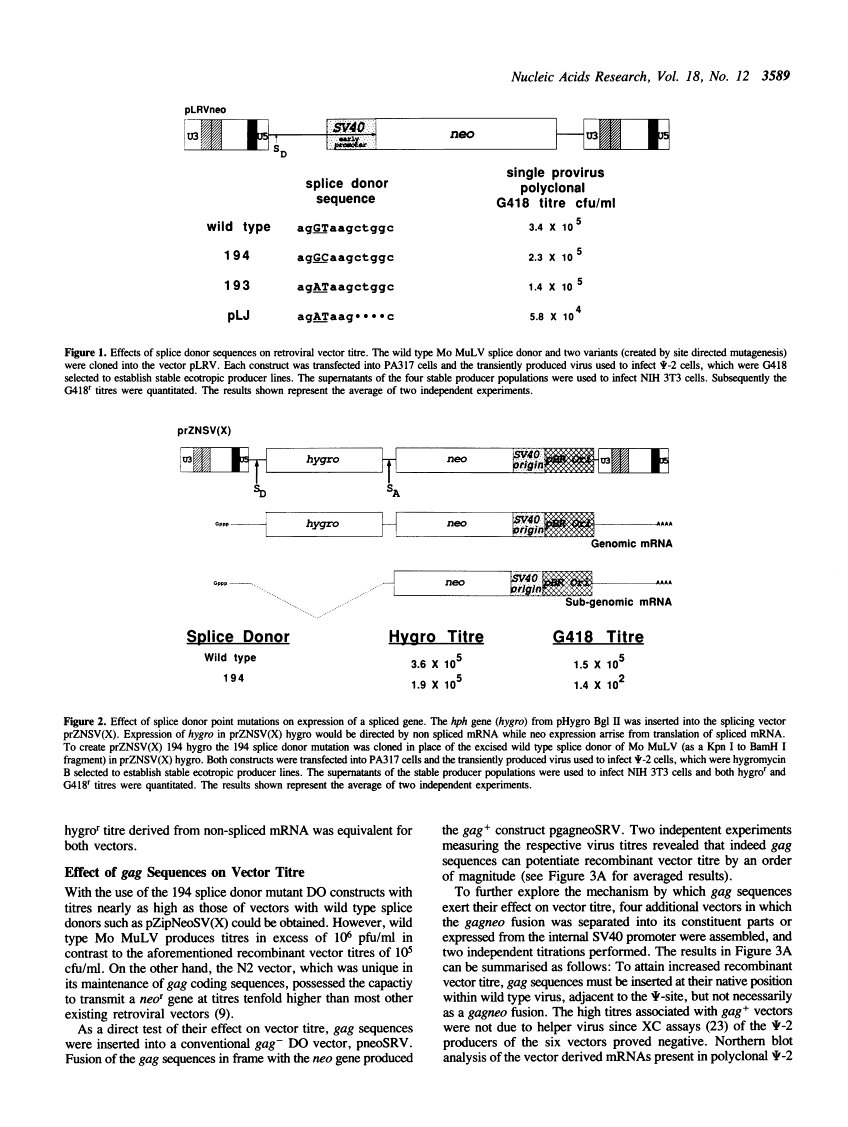
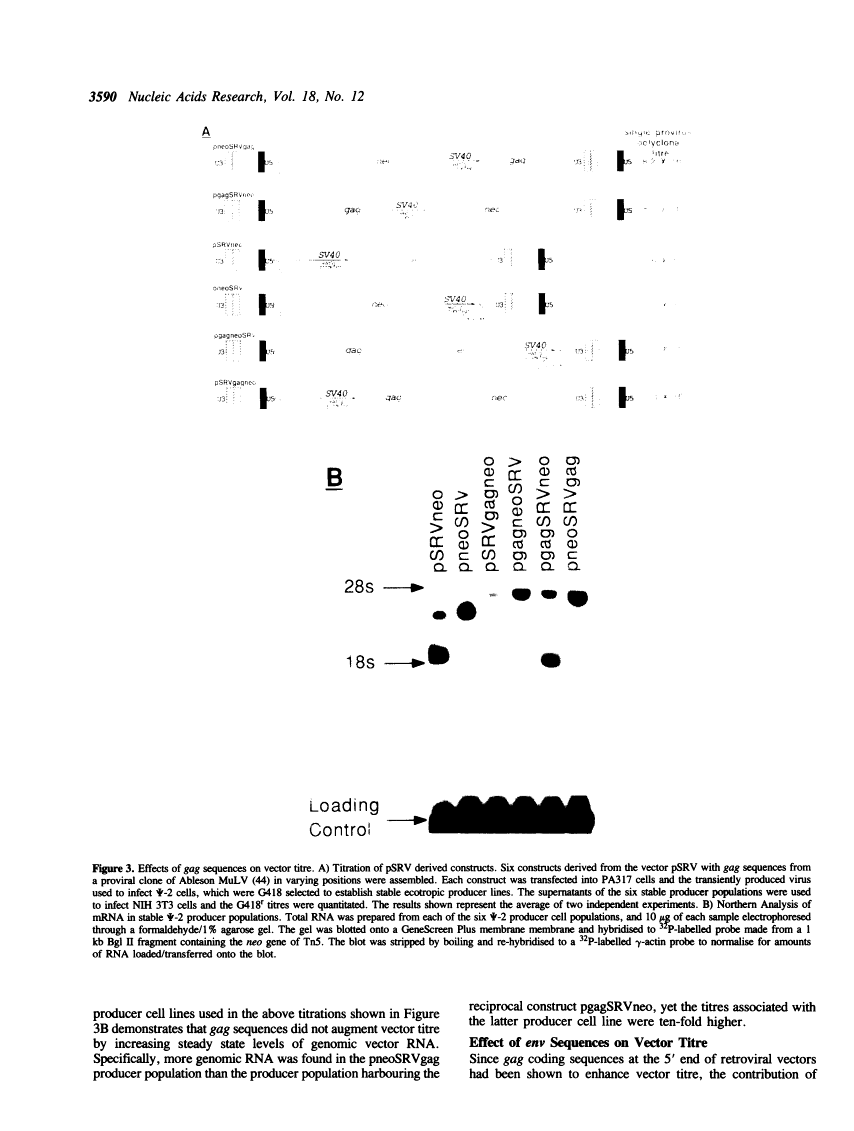
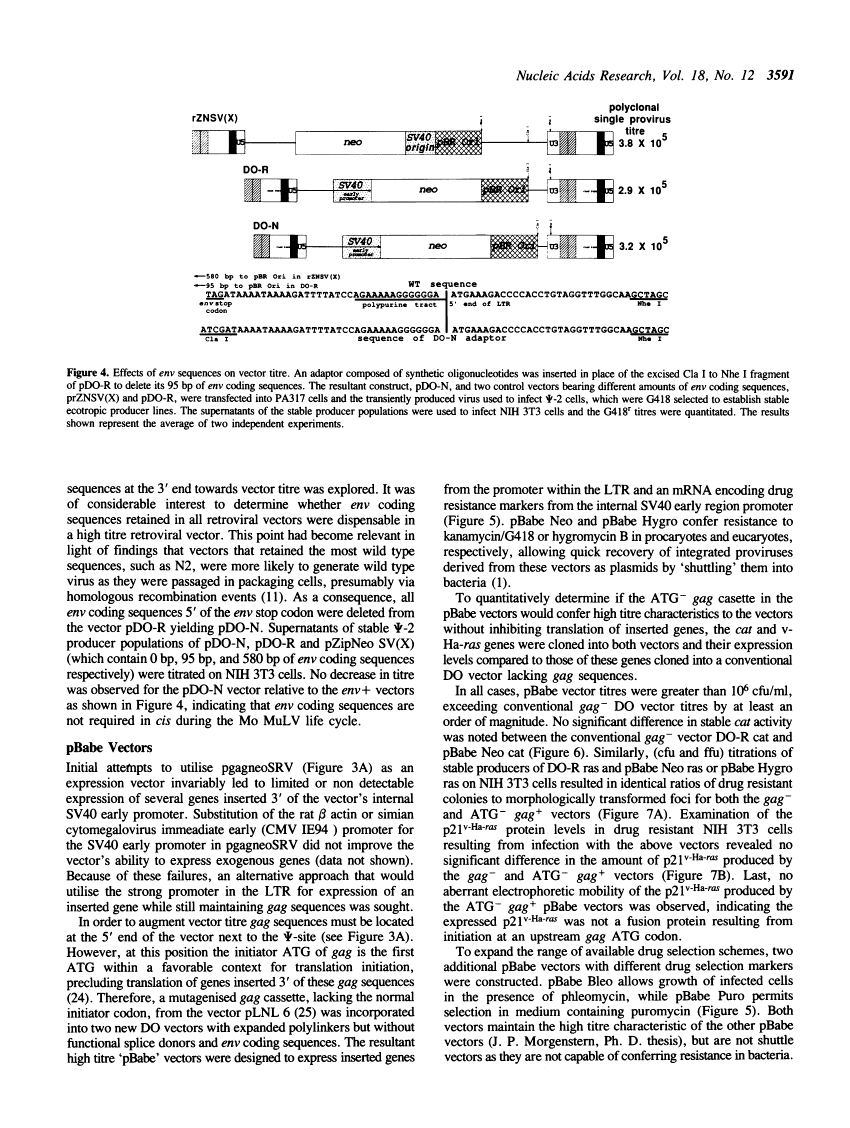
Abstract
Free full text

Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line.
Abstract
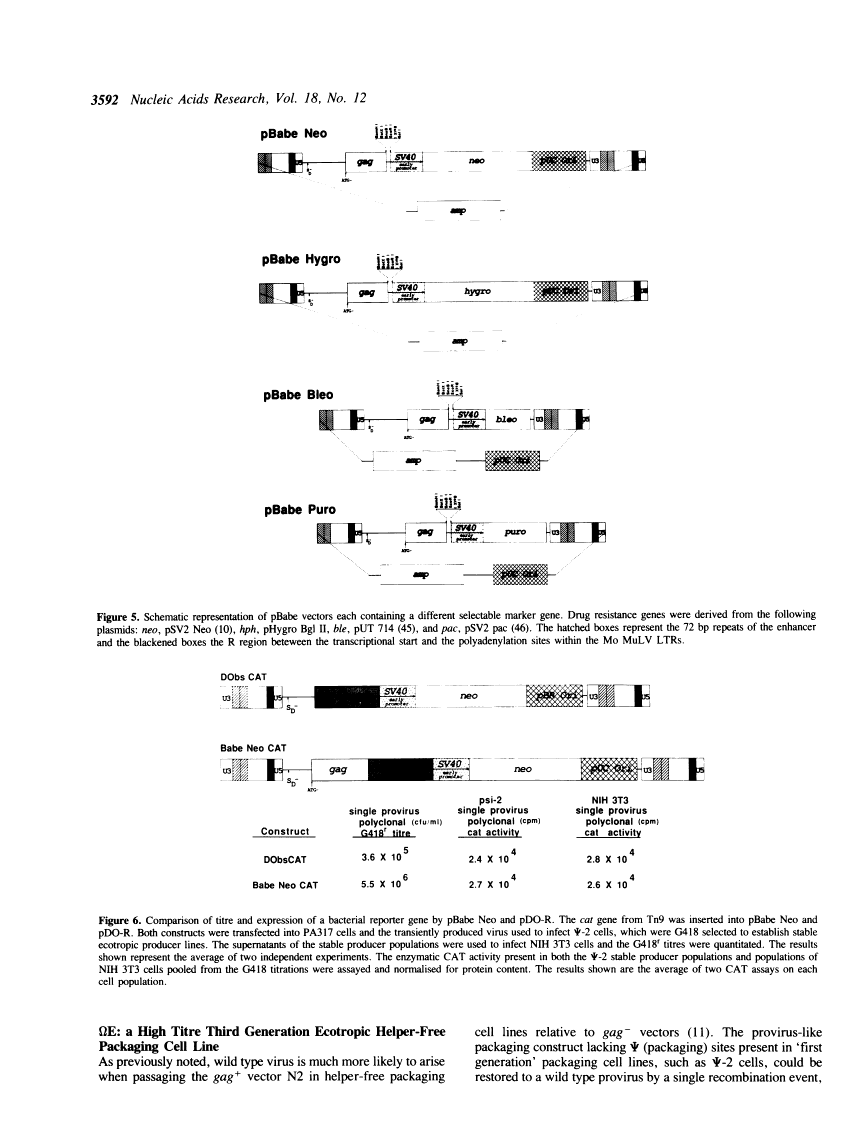
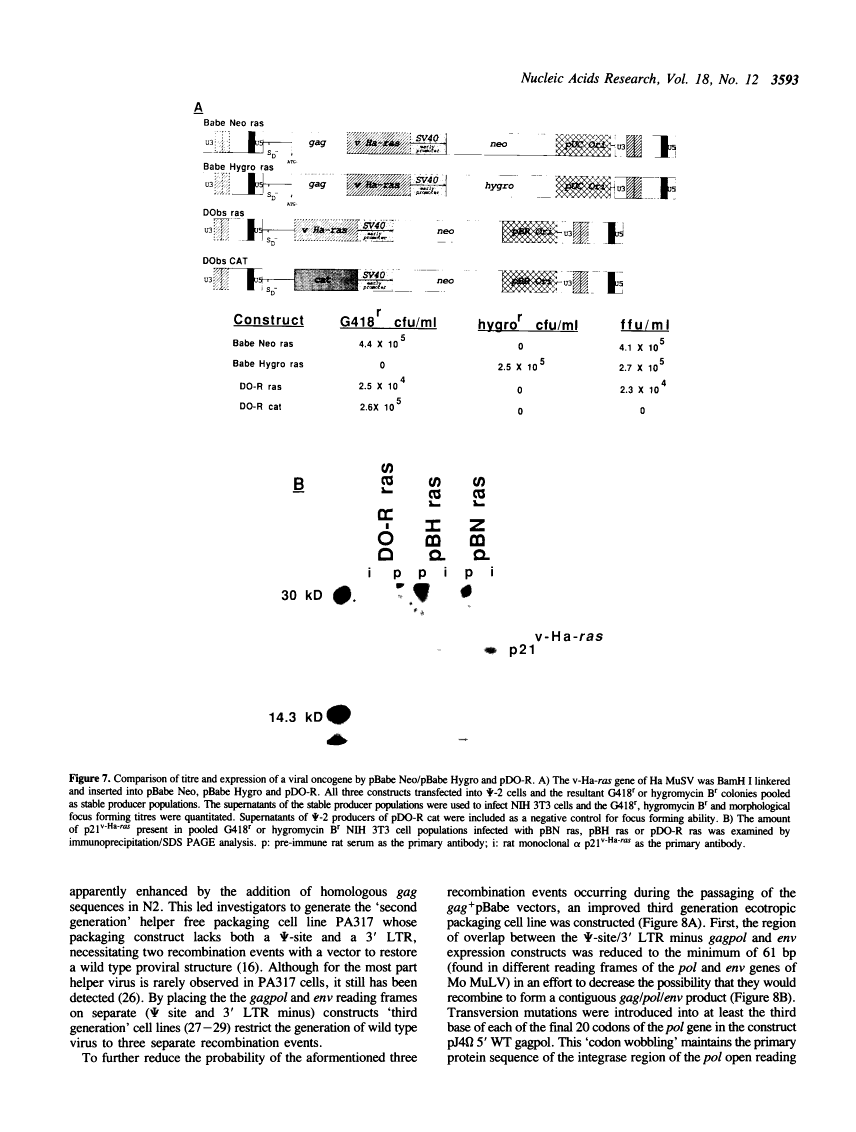
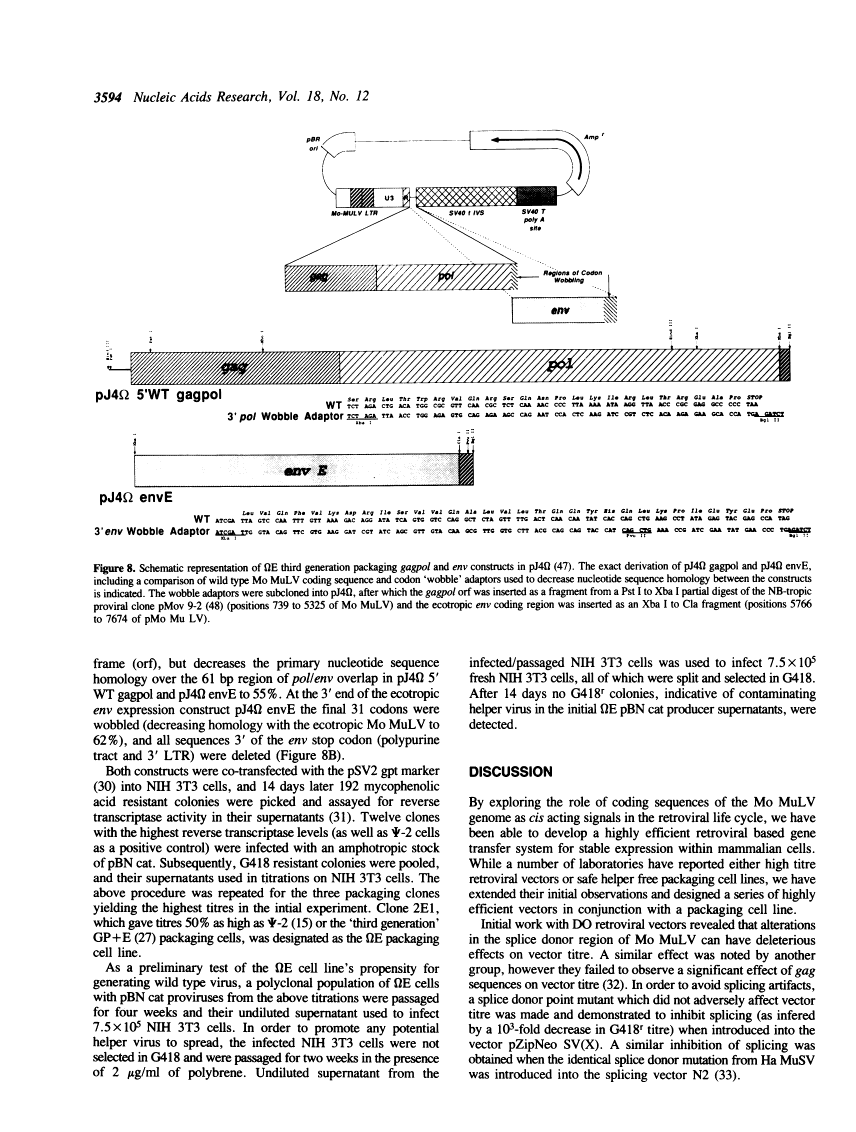
We report the development of an advanced system for transfer and expression of exogenous genes in mammalian cells based on Moloney murine leukemia virus (Mo MuLV). Extensive deletion/mutagenesis analysis to identify cis-acting signals involved in virus transmission has led to the design of a family of novel, highly efficient retroviral vectors and a partner helper-free packaging cell line. The pBabe retroviral vector constructs transmit inserted genes at high titres and express them from the Mo MuLV Long Terminal Repeat (LTR). Each of these vectors has been constructed with one of four different dominantly acting selectable markers, allowing the growth of infected mammalian cells in the presence of G418, hygromycin B, bleomycin/phleomycin or puromycin, respectively. The high titre ecotropic helper free packaging cell line, omega E, was designed in conjunction with the pBabe vectors to reduce the risk of generation of wild type Mo MuLV via homologous recombination events. The omega E cell line was generated with separate gagpol and ecotropic env expression constructs with minimal sequence overlap and decreased sequence homology achieved by 'codon wobbling'. Homologous env coding sequences were deleted from the pBabe vectors without diminishing recombinant vector titre. Together, the pBabe vectors and omega E cell line should prove useful in experiments where highest frequencies of gene transfer, or concomitant expression of several different genes within a single cell are required with minimal risk of helper virus contamination.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cepko CL, Roberts BE, Mulligan RC. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. [Abstract] [Google Scholar]
- Miller AD, Law MF, Verma IM. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. [Europe PMC free article] [Abstract] [Google Scholar]
- Davidson RL, Broeker P, Ashman CR. DNA base sequence changes and sequence specificity of bromodeoxyuridine-induced mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4406–4410. [Europe PMC free article] [Abstract] [Google Scholar]
- Brenner DG, Lin-Chao S, Cohen SN. Analysis of mammalian cell genetic regulation in situ by using retrovirus-derived "portable exons" carrying the Escherichia coli lacZ gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5517–5521. [Europe PMC free article] [Abstract] [Google Scholar]
- von Melchner H, Ruley HE. Identification of cellular promoters by using a retrovirus promoter trap. J Virol. 1989 Aug;63(8):3227–3233. [Europe PMC free article] [Abstract] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. [Abstract] [Google Scholar]
- Thompson TC, Southgate J, Kitchener G, Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell. 1989 Mar 24;56(6):917–930. [Abstract] [Google Scholar]
- Miller AD, Bender MA, Harris EA, Kaleko M, Gelinas RE. Design of retrovirus vectors for transfer and expression of the human beta-globin gene. J Virol. 1988 Nov;62(11):4337–4345. [Europe PMC free article] [Abstract] [Google Scholar]
- Eglitis MA, Kantoff P, Gilboa E, Anderson WF. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. [Abstract] [Google Scholar]
- Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [Abstract] [Google Scholar]
- Miller AD, Trauber DR, Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. [Abstract] [Google Scholar]
- Carter P, Bedouelle H, Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. [Europe PMC free article] [Abstract] [Google Scholar]
- Parker BA, Stark GR. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. [Europe PMC free article] [Abstract] [Google Scholar]
- Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. [Abstract] [Google Scholar]
- Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. [Europe PMC free article] [Abstract] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. [Abstract] [Google Scholar]
- Sleigh MJ. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. [Abstract] [Google Scholar]
- Korman AJ, Frantz JD, Strominger JL, Mulligan RC. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. [Europe PMC free article] [Abstract] [Google Scholar]
- Maniatis T, Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. [Abstract] [Google Scholar]
- Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH, Jr, Stephenson JR. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. [Europe PMC free article] [Abstract] [Google Scholar]
- Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. [Abstract] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. [Abstract] [Google Scholar]
- Bender MA, Palmer TD, Gelinas RE, Miller AD. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. [Europe PMC free article] [Abstract] [Google Scholar]
- Bosselman RA, Hsu RY, Bruszewski J, Hu S, Martin F, Nicolson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Moloney murine leukemia virus structural genes via the metallothionein promoter. Mol Cell Biol. 1987 May;7(5):1797–1806. [Europe PMC free article] [Abstract] [Google Scholar]
- Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. [Europe PMC free article] [Abstract] [Google Scholar]
- Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [Abstract] [Google Scholar]
- Danos O, Mulligan RC. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulligan RC, Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. [Europe PMC free article] [Abstract] [Google Scholar]
- Goff S, Traktman P, Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Guild BC, Finer MH, Housman DE, Mulligan RC. Development of retrovirus vectors useful for expressing genes in cultured murine embryonal cells and hematopoietic cells in vivo. J Virol. 1988 Oct;62(10):3795–3801. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980–990. [Europe PMC free article] [Abstract] [Google Scholar]
- Armentano D, Yu SF, Kantoff PW, von Ruden T, Anderson WF, Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. [Europe PMC free article] [Abstract] [Google Scholar]
- Mann R, Baltimore D. Varying the position of a retrovirus packaging sequence results in the encapsidation of both unspliced and spliced RNAs. J Virol. 1985 May;54(2):401–407. [Europe PMC free article] [Abstract] [Google Scholar]
- Adam MA, Miller AD. Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretroviral RNA into virions. J Virol. 1988 Oct;62(10):3802–3806. [Europe PMC free article] [Abstract] [Google Scholar]
- Murphy JE, Goff SP. Construction and analysis of deletion mutations in the U5 region of Moloney murine leukemia virus: effects on RNA packaging and reverse transcription. J Virol. 1989 Jan;63(1):319–327. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson JM, Johnston DE, Jefferson DM, Mulligan RC. Correction of the genetic defect in hepatocytes from the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4421–4425. [Europe PMC free article] [Abstract] [Google Scholar]
- Osborne WR, Miller AD. Design of vectors for efficient expression of human purine nucleoside phosphorylase in skin fibroblasts from enzyme-deficient humans. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6851–6855. [Europe PMC free article] [Abstract] [Google Scholar]
- Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. [Europe PMC free article] [Abstract] [Google Scholar]
- Boulter CA, Wagner EF. A universal retroviral vector for efficient constitutive expression of exogenous genes. Nucleic Acids Res. 1987 Sep 11;15(17):7194–7194. [Europe PMC free article] [Abstract] [Google Scholar]
- Del Pozzo G, Guardiola J. Construction of hygromycin-resistant retroviral cloning vectors. Nucleic Acids Res. 1988 Dec 9;16(23):11372–11372. [Europe PMC free article] [Abstract] [Google Scholar]
- Small J, Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983 Jan 14;219(4581):174–176. [Abstract] [Google Scholar]
- Reddy EP, Smith MJ, Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulsant P, Gatignol A, Dalens M, Tiraby G. Phleomycin resistance as a dominant selectable marker in CHO cells. Somat Cell Mol Genet. 1988 May;14(3):243–252. [Abstract] [Google Scholar]
- Vara JA, Portela A, Ortín J, Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986 Jun 11;14(11):4617–4624. [Europe PMC free article] [Abstract] [Google Scholar]
- Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990 Feb 25;18(4):1068–1068. [Europe PMC free article] [Abstract] [Google Scholar]
- Jaenisch R, Jähner D, Nobis P, Simon I, Löhler J, Harbers K, Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. [Abstract] [Google Scholar]
Associated Data
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/18.12.3587
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc331014?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/nar/18.12.3587
Article citations
Chromatin protein complexes involved in gene repression in lamina-associated domains.
EMBO J, 43(21):5260-5287, 25 Sep 2024
Cited by: 0 articles | PMID: 39322756 | PMCID: PMC11535540
ERK5 suppression overcomes FAK inhibitor resistance in mutant KRAS-driven non-small cell lung cancer.
EMBO Mol Med, 16(10):2402-2426, 13 Sep 2024
Cited by: 0 articles | PMID: 39271958 | PMCID: PMC11473843
Histone chaperone HIRA, promyelocytic leukemia protein, and p62/SQSTM1 coordinate to regulate inflammation during cell senescence.
Mol Cell, 84(17):3271-3287.e8, 22 Aug 2024
Cited by: 2 articles | PMID: 39178863
ProDOL: a general method to determine the degree of labeling for staining optimization and molecular counting.
Nat Methods, 21(9):1708-1715, 08 Aug 2024
Cited by: 0 articles | PMID: 39117875 | PMCID: PMC11399104
The SEMA3F-NRP1/NRP2 axis is a key factor in the acquisition of invasive traits in in situ breast ductal carcinoma.
Breast Cancer Res, 26(1):122, 13 Aug 2024
Cited by: 0 articles | PMID: 39138514 | PMCID: PMC11320849
Go to all (1,406) article citations
Protocols & materials
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Retroviral gene transfer using safe and efficient packaging cell lines.
Ann N Y Acad Sci, 612:407-414, 01 Jan 1990
Cited by: 25 articles | PMID: 2291567
Characterization of recombinant helper retroviruses from Moloney-based vectors in ecotropic and amphotropic packaging cell lines.
Virology, 180(2):849-852, 01 Feb 1991
Cited by: 25 articles | PMID: 1989392
Infection of human cells by murine ecotropic viruses: retroviral vectors carrying the hygromycin resistance-encoding gene.
Gene, 170(2):255-259, 01 May 1996
Cited by: 0 articles | PMID: 8666255
Amplified and tissue-directed expression of retroviral vectors using ping-pong techniques.
J Mol Med (Berl), 73(3):113-120, 01 Mar 1995
Cited by: 8 articles | PMID: 7633947
Review