Abstract
Free full text

Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells.
Abstract
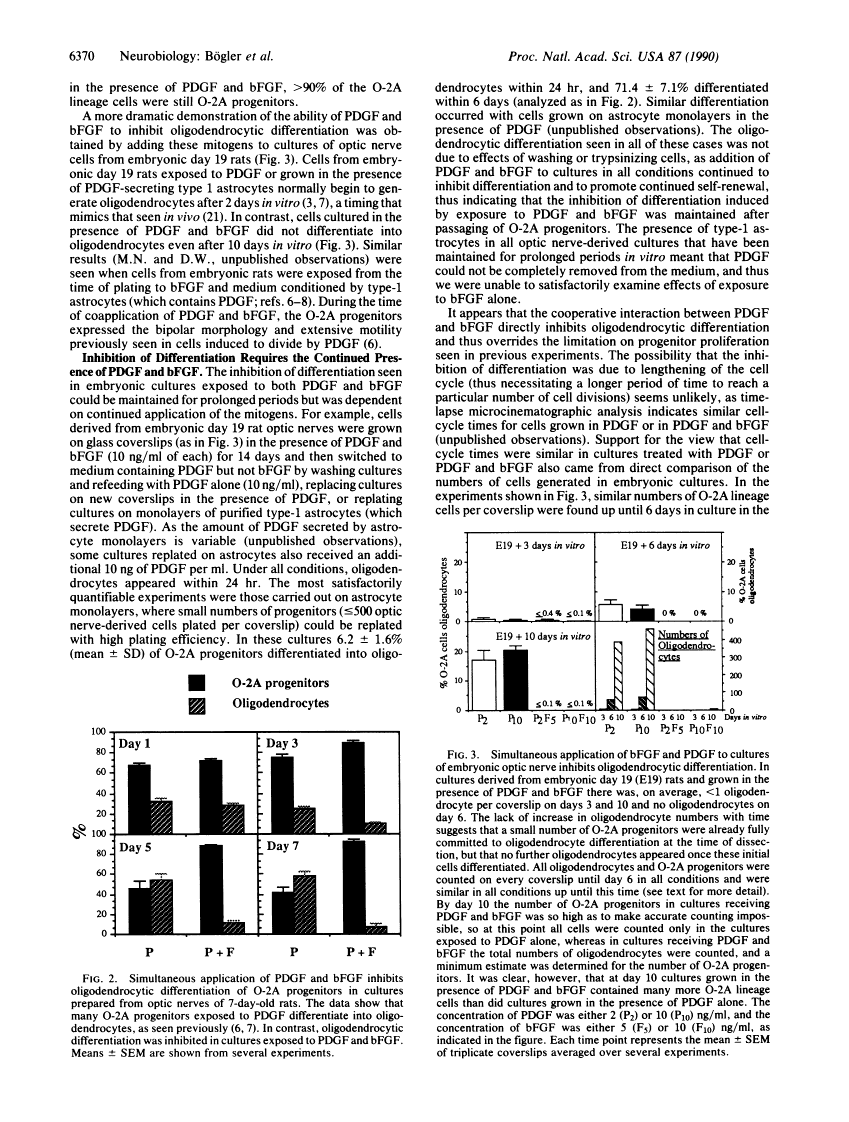
Bipotential oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells, which give rise to oligodendrocytes and type-2 astrocytes in cultures of rat optic nerve, are one of the few cell types in which most aspects of proliferation and differentiation can be manipulated in a defined in vitro environment. Previous studies have shown that O-2A progenitors exposed to platelet-derived growth factor (PDGF) divide as migratory bipolar cells a limited number of times, with a cell cycle time of 18 hr, before clonally related progenitors differentiate into nondividing oligodendrocytes with a timing similar to that seen in vivo. In contrast, O-2A progenitors grown in the absence of mitogen do not divide but instead differentiate prematurely into oligodendrocytes, and progenitors exposed to appropriate inducing factors differentiate into type-2 astrocytes. We now have found that O-2A progenitors can be induced to undergo continuous self-renewal in the absence of oligodendrocytic differentiation by exposure to a combination of PDGF and basic fibroblast growth factor (bFGF). With the exception of the inhibition of differentiation, the O-2A progenitors exposed to PDGF and bFGF behaved similarly to those exposed to PDGF alone. In contrast, progenitors exposed to basic bFGF alone were multipolar, had a cell-cycle length of 45 hr, showed little migratory behavior, underwent premature oligodendrocytic differentiation, and did not cease division upon expression of oligodendrocyte marker antigens. Thus, inhibition of differentiation required the presence of both mitogens. Our results demonstrate that PDGF and bFGF act on O-2A progenitors as both inducers of division and as regulators of differentiation that modulate multiple aspects of O-2A progenitor development and, additionally, reveal a previously unrecognized means of regulating self-renewal processes, wherein cooperation between growth factors promotes continuous division in the absence of differentiation.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. [Abstract] [Google Scholar]
- Noble M, Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J. 1984 Oct;3(10):2243–2247. [Europe PMC free article] [Abstract] [Google Scholar]
- Raff MC, Abney ER, Fok-Seang J. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell. 1985 Aug;42(1):61–69. [Abstract] [Google Scholar]
- Hughes SM, Lillien LE, Raff MC, Rohrer H, Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988 Sep 1;335(6185):70–73. [Abstract] [Google Scholar]
- Lillien LE, Sendtner M, Rohrer H, Hughes SM, Raff MC. Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron. 1988 Aug;1(6):485–494. [Abstract] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988 Jun 9;333(6173):560–562. [Abstract] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. [Abstract] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. [Abstract] [Google Scholar]
- Small RK, Riddle P, Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. [Abstract] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. [Europe PMC free article] [Abstract] [Google Scholar]
- Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. [Europe PMC free article] [Abstract] [Google Scholar]
- Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. [Europe PMC free article] [Abstract] [Google Scholar]
- Pruss RM. Thy-1 antigen on astrocytes in long-term cultures of rat central nervous system. Nature. 1979 Aug 23;280(5724):688–690. [Abstract] [Google Scholar]
- Raff MC, Mirsky R, Fields KL, Lisak RP, Dorfman SH, Silberberg DH, Gregson NA, Leibowitz S, Kennedy MC. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [Abstract] [Google Scholar]
- Johnson GD, Davidson RS, McNamee KC, Russell G, Goodwin D, Holborow EJ. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. [Abstract] [Google Scholar]
- Eccleston PA, Silberberg DH. Fibroblast growth factor is a mitogen for oligodendrocytes in vitro. Brain Res. 1985 Aug;353(2):315–318. [Abstract] [Google Scholar]
- Saneto RP, de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985 May;82(10):3509–3513. [Europe PMC free article] [Abstract] [Google Scholar]
- Logan A, Logan SD. Distribution of fibroblast growth factor in the central and peripheral nervous systems of various mammals. Neurosci Lett. 1986 Aug 29;69(2):162–165. [Abstract] [Google Scholar]
- Gonzalez AM, Buscaglia M, Ong M, Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membranes of diverse tissues. J Cell Biol. 1990 Mar;110(3):753–765. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller RH, David S, Patel R, Abney ER, Raff MC. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev Biol. 1985 Sep;111(1):35–41. [Abstract] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988 Dec 15;336(6200):688–690. [Abstract] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. [Abstract] [Google Scholar]
- Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, Nice EC, Kelso A, Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987 Dec 20;6(13):3995–4002. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann MJ, Patterson PH. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989 Dec 15;246(4936):1412–1416. [Abstract] [Google Scholar]
- Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986 Mar 14;44(5):773–779. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.16.6368
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc54535?pdf=render
Citations & impact
Impact metrics
Article citations
Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis.
Proc Natl Acad Sci U S A, 121(32):e2407974121, 31 Jul 2024
Cited by: 2 articles | PMID: 39083422 | PMCID: PMC11317586
ADAMTS4 Enhances Oligodendrocyte Differentiation and Remyelination by Cleaving NG2 Proteoglycan and Attenuating PDGFRα Signaling.
J Neurosci, 43(24):4405-4417, 15 May 2023
Cited by: 2 articles | PMID: 37188512 | PMCID: PMC10278682
Low sulfated heparan sulfate mimetic differentially affects repair in immune-mediated and toxin-induced experimental models of demyelination.
Glia, 71(7):1683-1698, 21 Mar 2023
Cited by: 2 articles | PMID: 36945189 | PMCID: PMC10952530
Exogenous FGF-1 Differently Regulates Oligodendrocyte Replenishment in an SCI Repair Model and Cultured Cells.
Biomedicines, 10(11):2724, 27 Oct 2022
Cited by: 0 articles | PMID: 36359244 | PMCID: PMC9687664
Fixing the GAP: The role of RhoGAPs in cancer.
Eur J Cell Biol, 101(2):151209, 10 Feb 2022
Cited by: 16 articles | PMID: 35180567 | PMCID: PMC9081277
Review Free full text in Europe PMC
Go to all (363) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Control of division and differentiation in oligodendrocyte-type-2 astrocyte progenitor cells.
Ciba Found Symp, 150:227-43; discussion 244-9, 01 Jan 1990
Cited by: 16 articles | PMID: 2373025
Measurement of time in oligodendrocyte-type-2 astrocyte (O-2A) progenitors is a cellular process distinct from differentiation or division.
Dev Biol, 162(2):525-538, 01 Apr 1994
Cited by: 35 articles | PMID: 8150211
The inhibition of oligodendrocytic differentiation of O-2A progenitors caused by basic fibroblast growth factor is overridden by astrocytes.
Glia, 8(1):12-19, 01 May 1993
Cited by: 52 articles | PMID: 8509161