Abstract
Free full text

Electrical Activation of Wound-Healing Pathways
Abstract
Background
Effective wound healing has been a lasting and challenging topic in health care. Various strategies have been used to accelerate and perfect the healing process. One such strategy has involved the application of an exogenous electrical stimulus to chronic wounds with the aim of stimulating healing responses.
The Problem
The biology of electric stimulation to instigate healing, however, is very poorly understood. How does electric stimulation induce healing responses?
Basic/Clinical Science Advances
Recent research shows that the electric fields (EFs) activate multiple signaling pathways that are critical for wound healing. Importantly, the EFs provide a powerful, sometimes an overriding, directional signal for cell migration in wound healing. Unlike other stimuli, EFs have the intrinsic property of being directional. The EF-directed cell migration (electrotaxis/galvanotaxis) appears to be a consequence of EF-induced polarized signaling of epidermal growth factor receptors, integrins, and phosphoinositide 3 kinase/Pten, and may be mediated by protein kinase C, intracellular Ca2+, and cyclic adenosine monophosphate (cAMP). Because directional cell migration is a key component in wound healing, galvanotaxis may represent an important mechanism of wound healing.
Clinical Care Relevance
With the constantly enlarging diabetic and aging population, chronic or nonhealing wounds pose increasing health and economic problems, and currently there is no effective therapy available. Electric stimulation activates important intracellular signaling pathways that are polarized in the EF direction, resulting in enhanced and stimulated directional cell migration. Electric stimulation offers a novel approach to achieve better and accelerated wound healing.
Conclusion
Experimental evidence suggests a significant role of endogenous EFs in cell migration in wound healing. Most importantly, EFs are a very powerful signal to direct cell migration. Electric stimulation therefore may represent a promising and unique strategy to induce cell and tissue growth in a directional manner, to enhance wound healing, and to achieve better wound healing.
BACKGROUND
Injury elicits multiple signaling pathways.1–3 To restore tissue integrity and homeostasis effectively, those signaling pathways have to be well coordinated. Impairment of signaling orchestration may lead to defective healing, and chronic and nonhealing wounds.4 Many strategies have been used in an attempt to accelerate the healing response. One strategy is application of an exogenous electrical stimulus to activate healing.5–8 Despite encouraging results being reported and approval of Medicare and other insurance policies for use of electric stimulation in some types of chronic and nonhealing wounds, the advancement in using electric stimulation to achieve effective wound healing is limited.9 Yet, recent research has provided significant insights into electrical stimulation of wound healing.
Endogenous wound electric fields (EFs) are present at human skin wounds, generated immediately upon wounding. Application of EFs, in dose ranges of those generated naturally, activates signaling molecules critical for wound healing in many types of exposed cells, including epidermal growth factor receptors (EGFRs), integrins, and phosphoinositide 3 (PI3) kinases and Pten (phosphatase and tensin homolog).8,10–13 Significantly, these signaling molecules are activated in a directional manner, often on the side of the cell facing the cathodal pole of the EF. This provides a unique prohealing advantage to application of EF as a therapeutic modality, since the directional cueing of the repair response is maintained as long as the EF is present—unlike the topical application of growth factors or other stimulatory compounds, which normally are difficult to maintain as a stable gradient to sustain directional migratory or proliferative responses. Importantly, the electric signaling appears to be a predominant signaling mechanism in guiding cell migration in epithelial wound healing.11
Clinically, both direct current (DC) stimulation and alternating current (AC) stimulation have been tested for wound healing. The stimulation schemes include constant DCs, DC pulses, and ACs. The electric stimulation comes with a huge number of choices of different amplitudes, frequencies (AC and pulsed DC), duty cycles, durations, current strengths, and so on. Current is usually very small for DC stimulation (hundreds of μA–μA). Low-voltage pulsed currents are pulses with durations up to 1 s and voltages up to 150 V. Monophasic and biphasic pulsed currents can be low voltage and high voltage up to several hundred volts with short duration (μs).7,14–17 For example, a recent clinical study in Germany used Dermapulse® (Gerromed, Hamburg, Germany) with varied polarity, a pulse frequency of 128 Hz, and an average current strength of 300 μA. This double-blinded, placebo-controlled clinical study demonstrated pain relief, improved perfusion, and a tendency of improved healing in chronic venous leg ulcers.18 Because there are many modalities of using pulsed DC and AC, understanding the effects of those modalities on wound-healing signaling pathways will offer better choice of those many parameters.
In addition to wound healing, electric stimulation has been used in orthopedics, sports medicine, and neurology.19–22 For bone and cartilage cells, a specific, defined capacitively coupled electrical signal can result in significant upregulation of cartilage matrix protein production, and nitric oxide–dependent chondrocyte proliferation.14,19
This short perspective focuses on activation of signaling pathways by DC EFs. Because cells are responsive to DC EFs, exogenously applied electric stimulation is likely to affect cells through same or similar mechanisms. Those responses are likely to be cell-type specific, and stimulation parameter dependent.
CLINICAL PROBLEM ADDRESSED
The clinical problems presented by chronic, nonhealing wounds are familiar to the readers of this review. While acute wounds normally heal without many complications, the comorbidities of diabetes, malnutrition, pressure, infection, chronic inflammation, and aging can create a scenario that leads to nonhealing wounds. The biologic impediments that prevent healing are poorly understood.23 Chronic wounds generate significant economic burden to our health care system.4,24 Even with the best standard of care, up to 30% of venous leg ulcers and 65% of diabetic foot ulcers fail to heal25 and few therapeutic advances have significantly altered these figures. Thus, new treatment paradigms are needed to address this burgeoning problem.
RELEVANT BASIC SCIENCE CONTEXT
Du-Bois Reymond, a founder of electrophysiology, detected wound EFs on a cut in his fingertip 160 years ago.26 The existence of endogenous wound EFs has been confirmed in all wounds that have been measured using various modern techniques. When guinea pig skin is wounded, a large and steady EF arises, measuring ~ 140 mV/mm.12,27,28 The vibrating probe technique has allowed nonin-vasive measurements with great temporal and spatial resolution of endogenous electric currents.11,29,30 The electric currents (flow of positive charge) are oriented toward the wound and, therefore, are well positioned to guide and stimulate the migration and other healing responses of cells at wounds (Fig. 1A).
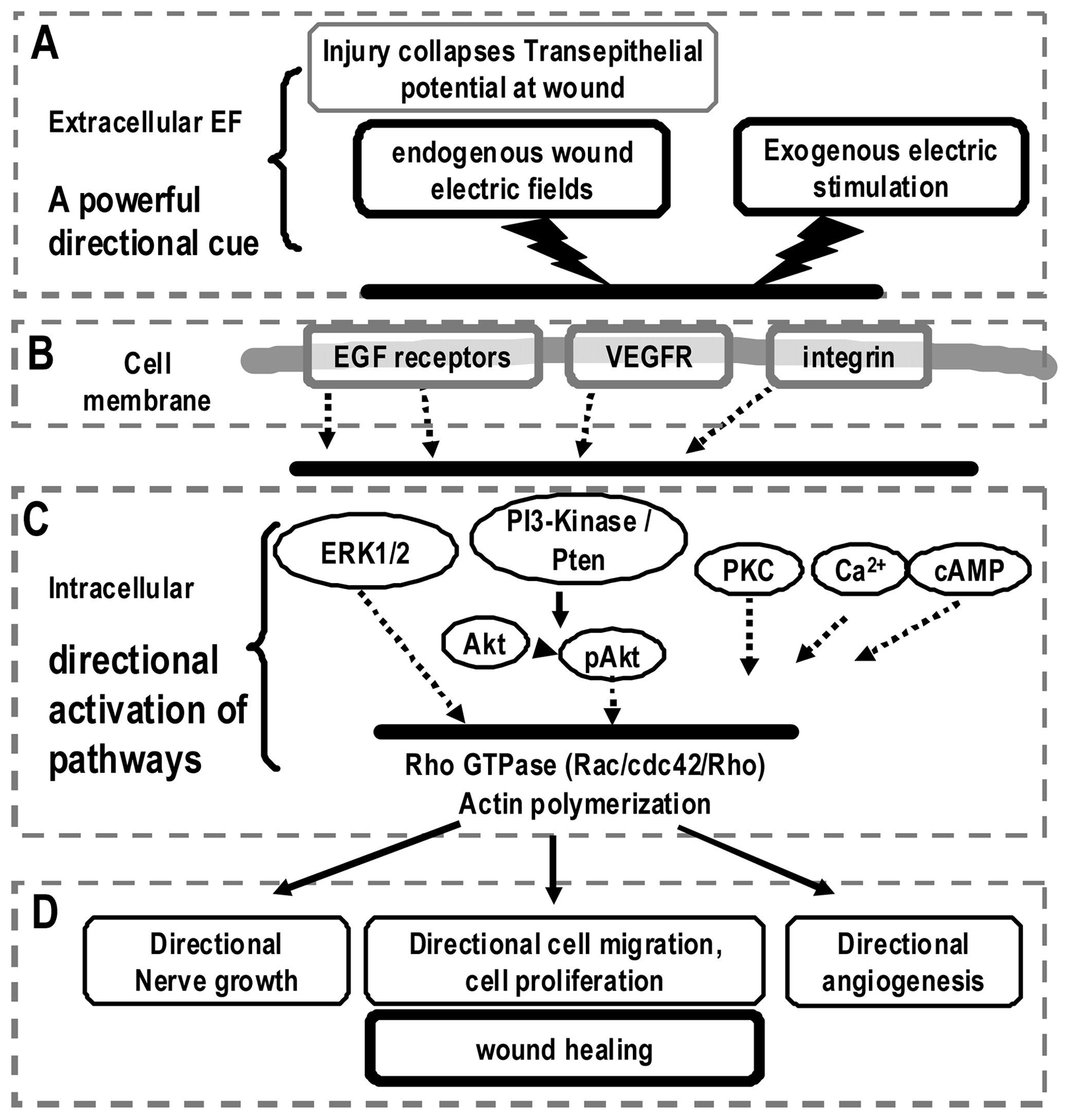
Electrical activation of wound-healing pathways. Schematic diagram shows the generation of the endogenous wound electric signals and the electrical activation of some important signaling pathways for cell migration and wound healing. (A) Injury breaks the epithelial barrier and induces endogenous wound electric fields (EFs). Electric stimulation can also be exogenously applied; (B) extracellular EFs may activate epidermal growth factor receptors (EGFRs), vascular endothelial growth factor receptors (VEGFRs), and integrins; (C) polarized activation of membrane receptors induces asymmetric intracellular signaling, notably extracellular-signaling regulated kinase 1/2 (ERK1/2), phosphoinositide 3 (PI3) kinase/Akt, Rac, and protein kinase C (PKC) to induce downstream cascades; (D) the orchestration of the polarized intracellular signaling results in directional cell migration and proliferation, and perhaps directional nerve growth and new blood vessel formation. These effects may lead to improved wound-healing response.
Impaired cell migration into wounds is characteristic of chronic wounds in the elderly, pressure ulcers, and venous stasis ulcers of the skin.2,24,31 To heal a wound effectively, cells must not only become motile, but also migrate in the correct direction. Many types of cells migrate directionally in an EF, a phenomenon called electrotaxis or galvano-taxis.32 Because the cells have to migrate directionally to heal a wound, and because endogenous EFs naturally occur at wounds and cells migrate directionally in EFs, it has long been postulated that the wound EFs might have a role in wound healing in directing cell and tissue growth.33–35
EXPERIMENTAL MODEL OR MATERIAL—ADVANTAGES AND LIMITATIONS
We have used a vibrating probe system to measure the endogenous wound electric currents at epithelial wounds of skin and cornea. While not a chronic wound, the acute wound offers the advantage of being easier to manipulate. Using the vibrating probe system requires specialized instrument and special expertise, which limits the use of this technique in the clinical situation. A noninvasive device currently being developed may offer opportunities to measure EFs in patients and determine to what extent the changes in wound EFs correlate to impaired wound healing.28 This may lead to diagnostic and prognostic application of measuring EFs and indicate manipulation of wound EFs to achieve better wound healing.
We use digital video microscopy to observe EF-induced cellular responses, for example, cell migration, division, and signaling mechanisms. This allows us to mimic the effects of endogenous EFs on the cells. Our results show that keratinocytes, corneal epithelial cells, fibroblasts, endothelial cells, and neutrophils—when cultured in an EF—all migrate directionally. The advantages of this approach include precise control of the experimental conditions, the stimulating parameters, and direct evaluation of the cellular response and signaling mechanisms. We have identified some important signaling mechanisms that are activated by EFs. Most importantly, the electric signaling gives cells a sense of direction by activating EGFR, extracellular-signaling regulated kinase 1/2 (ERK1/2), and PI3 kinase in a polarized manner. The directionality of EFs when combined with promising therapies of using biochemical stimulators such as growth factors may achieve better wound healing, especially in chronic, nonhealing wounds. However, the extrapolation and translation of the results to clinical application are still a major challenge.
With further understanding of the molecular and cellular mechanisms, and development in techniques of application of EFs, electric stimulation may lead to effective therapies.
DISCUSSION OF FINDINGS AND RELEVANT LITERATURE
Newly developed tools such as micro-needle arrays and a bioelectric imager have confirmed and greatly extended our understanding of the existence of wound EFs. A shallow slit wound (~1 mm in depth and ~1–2 mm in length) on human finger tip produces an electric current of 8–10 μA/cm2.29 The microneedle arrays and bioelectric imager have measured very similar wound EFs (100–177 mV/mm) on human skin immediately upon wounding.27,28 EFs of similar strength activate multiple signaling pathways important to wound healing (Fig. 1).
EFs relocalize and activate EGFRs and downstream signaling pathways
EFs induce polarization and activation of EGFRs in keratinocytes and corneal epithelial cells. We have observed cathodal redistribution of EGFRs (Fig. 1B) on keratinocytes and corneal epithelial cells as early as 10 min in an EF. The downstream signaling–dua-phosphorylated ERK1/2 and actin filament are also polarized to the cathodal side.8,12,41 Long-time exposure in combination with extracellular matrix (ECM) upregulates expression of EGFRs in corneal epithelial cells in culture.36 EGFR signaling is a ubiquitous signaling mechanism following wounding, and its activation results in many important prohealing responses, such as enhanced directional cell motility and proliferation. Activation of EGFRs also has antiapoptotic effects.
EFs have the intrinsic property of being directional, and do not easily dissipate like soluble chemicals. EFs thus provide a persistent directional signal through activation of intracellular pathways in a polarized manner, different from activation of intracellular signaling by diffusible molecules. This gives the therapeutic approach of electrical stimulation an advantage over conventional approaches with diffusing agents.
Signaling through integrins—Rac
EFs redistribute integrins in fibroblasts.37 Integrins are cell surface receptors that cells use to bind and respond to the ECM. Integrins have diverse functions. They regulate cell adhesion to ECM and to neighbor cells. They also contribute to the control of cell proliferation, shape, and motility. Integrins also mediate many important signal pathways. Knockout of β4 integrin abrogated the electrotaxis of keratinocytes in the absence of EGF, which can be recovered by transfection and expression of β4 integrin.13 Two distinct but synergistic signaling pathways—EGFR and integrin β4—coordinate galvanotaxis and are required for the activation of Rac that was essential for directional migration of keratinocytes in response to an EF (Fig. 1B,C).
PI3 kinase and Pten are key molecules that mediate electrotaxis
PI3 kinase/Akt and Pten are compass molecules in directional sensing and migration of cells in response to chemical gradients. EFs activate PI3 kinase/Akt signaling pathway in neutrophils and keratinocytes that are cultured in serum-free medium. The activation of PI3 kinase/Akt and several other kinases in keratinocytes is rapid and specific.11 Most strikingly, the activation of those signaling pathways is polarized in the direction of cell migration. When the polarity of EF is reversed, PI3 kinase signaling is activated at the new cathode-facing side, membrane protrusion, and directional migration in the new direction ensue.10,11 Genetic disruption or pharmacological inhibition of PI3 kinase abrogated electrotaxis of corneal epithelial cells and keratinocytes in wound healing in monolayer and organ cultures. Those results suggest that the EF-induced directional activation of PI3 kinase underlies the directional cell migration (Fig. 1C).
EFs are an overriding guidance cue directing cell migration in wound healing
It is generally accepted that the following cues give cells a sense of direction and the cells migrate and grow directionally: (i) injury stimulation per se; (ii) gradients of chemoattractants; (iii) contact inhibition release; (iv) wound void; (v) population pressure—growth of adjacent cells; and (vi) changes in mechanical force after injury.1–3,38 However, when an EF of physiological strength is applied in the opposite direction of those cues, cell migration simply follows the direction of EF,11 suggesting that EFs are able to override other cues in directing cell migration.
EFs induce and direct proangiogenic responses and nerve growth
In addition to guiding epithelial cells, EFs may direct angiogenesis and nerve growth.39 An EF upregulates the expression of vascular endothelial growth factor (VEGF). EFs as low as 75–100 mV/mm direct the reorientation, elongation, and migration of endothelial cells in culture. These proangiogenic responses require VEGF receptor (VEGFR) activation and are mediated through PI3 kinase-Akt and the small GTPase Rho kinase (ROCK) signaling pathways, resulting in reorganization of the actin cytoskeleton. Thus, the endogenous EFs might play a role in angiogenesis in vivo by stimulating the VEGFR signaling pathway, to induce angiogenic responses. Nerve sprouting at corneal wounds increases in number and grows more directionally when the endogenous would EFs are increased.12
CAUTION, CRITICAL REMARKS, AND RECOMMENDATIONS
Currently, electric stimulation is being used in certain refractory and nonhealing wounds. While there are reports with encouraging outcomes, there is no FDA-approved device specifically for the indication of skin wound healing. Great discrepancy exists in the reported clinical protocols that claim to have achieved improved wound healing using electric stimulation. Well-controlled, randomized clinical trials and standardization of the device and protocols for electric stimulation are needed.
Practical application of electrical stimulation itself may need to be carefully considered and designed. The endogenous EFs are generated at the leading edge, very focally because of tightly regulated ion transport at the breach to skin electrical resistant barrier. Experimentally applied EFs in culture dish can be precisely controlled. Exogenously applied electric stimulation at wounds in patients, however, can generate highly uneven current density, path, and voltage distribution. Those are significantly confounding factors in electric stimulation for wound healing in patients. These confounding factors may explain the discrepant results reported in some of the clinical trials.
At the same time, electric stimulation for wound healing requires thorough understanding of the underlying mechanisms before therapeutic applications may be more successful, reliable, and consistent. Because electric stimulation may have numerous signaling and cellular targets, appropriate tests are needed to ensure that any beneficial outcome of electric stimulation targeting one type of cell or some particular signaling pathway is not counteracted by other effects on different types of cells and signaling pathways. Understanding how EFs may affect cellular behaviors and signaling pathways in tissues and their functional consequences should bring a new tool to manage chronic wounds.
FUTURE DEVELOPMENT
Despite the remarkable guidance effects of EF on many types of cells, it is still not known how the cells sense the EFs. This type of basic scientific understanding is fundamental to develop effective therapies. It would be important to delineate the signaling pathways and mechanisms of EF-induced cellular responses and the effects on the biology of wound healing.
It is highly important that devices and practice be standardized for wound care clinical trials. From a therapeutic standpoint, the development of safe, effective, and reliable stimulators to specifically manipulate cellular and tissue response in vitro as well as in vivo could have tremendous impact in this field. We are developing pharmacological approaches to affect the endogenous wound EFs through manipulation of ion transport in epithelial cells. This may represent a practical and easier way to standardize for manipulation of local EFs rather than by application of physical electrodes.
Acknowledgments
Wound healing research in the authors’ laboratory is supported by the Wellcome Trust, UC Davis Dermatology Development Fund (to M.Z.) and NIH R01-AR44518 (to R.R.I.). The project described was also supported by Award Number R01EY019101 from the National Eye Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
Abbreviations and Acronyms
- AC
- alternating current
- Akt
- a downstream effecter of PI3 kinase. Akt is activated as a result of PI3 kinase activity
- cAMP
- cyclic adenosine monophosphate (cyclic AMP or 3′-5′-cyclic adenosine monophosphate) is a second messenger that is important in many biological processes
- chemoattractant
- inorganic or organic substances possessing chemotaxis inducer effect in motile cells
- chemotaxis
- the phenomenon in which cells direct their movements according to certain chemicals in their environment
- contact inhibition
- a natural process of arresting cell growth and movement when two or more cells come into contact with each other
- DC EFs
- direct current electric fields
- ECM
- extracellular matrix
- EF(s)
- electric field(s)
- EGFR
- epidermal growth factor receptor
- ERK1/2
- extracellular-signaling regulated kinase 1/2
- FAD
- Food and Drug Administration
- galvanotaxis/electrotaxis
- directional cell migration in an electric field
- integrins
- cell surface receptors that interact with the ECM and mediate various intracellular signals. They define cellular shape, mobility, and regulate the cell cycle
- pAkt
- activated form of Akt
- PI3 kinase
- phosphoinositide 3-kinases (PI 3-kinases or PI3Ks) are a family of related enzymes that are capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol (PtdIns). They are also known as phosphatidylinositol-3-kinases
- PKC
- protein kinase C
- population pressure
- growth of adjacent cells form a pressure to push cells to move into a wound
- Pten
- short of phosphatase and tensin homolog is a protein that acts as a tumor suppressor gene through the action of its phosphatase protein product. This phosphatase is involved in the regulation of the cell cycle, preventing cells from growing and dividing too rapidly
- Rac
- subfamily of the Rho family of GTPases, small (~21 kDa) signaling G proteins (more specifically a GTPases)
- transepithelial potential difference
- electric potential difference between the basal side and apical side of epithelial layers
- VEGF
- vascular endothelial growth factor
- VEGFR
- vascular endothelial growth factor receptor
References
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/122780799
Article citations
Electrical stimulation: a novel therapeutic strategy to heal biological wounds.
RSC Adv, 14(44):32142-32173, 11 Oct 2024
Cited by: 0 articles | PMID: 39399261 | PMCID: PMC11467653
Review Free full text in Europe PMC
Review of Piezoelectrical Materials Potentially Useful for Peripheral Nerve Repair.
Biomedicines, 11(12):3195, 01 Dec 2023
Cited by: 3 articles | PMID: 38137416 | PMCID: PMC10740581
Review Free full text in Europe PMC
Synergistic effects of electroactive antibacterial material and electrical stimulation in enhancing skin tissue regeneration: A next-generation dermal wound dressing.
Skin Res Technol, 29(11):e13465, 01 Nov 2023
Cited by: 2 articles | PMID: 38009021 | PMCID: PMC10603310
Quantum molecular resonance electrotherapy (Rexon-Eye) for recalcitrant dry eye in an Asian population.
Front Med (Lausanne), 10:1209886, 12 Sep 2023
Cited by: 1 article | PMID: 37771976 | PMCID: PMC10523309
Self-powered enzyme-linked microneedle patch for scar-prevention healing of diabetic wounds.
Sci Adv, 9(28):eadh1415, 14 Jul 2023
Cited by: 9 articles | PMID: 37450590 | PMCID: PMC10348682
Go to all (34) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Electrical fields in wound healing-An overriding signal that directs cell migration.
Semin Cell Dev Biol, 20(6):674-682, 25 Dec 2008
Cited by: 273 articles | PMID: 19146969
Review
Electric field-induced suppression of PTEN drives epithelial-to-mesenchymal transition via mTORC1 activation.
J Dermatol Sci, 85(2):96-105, 18 Nov 2016
Cited by: 7 articles | PMID: 27919618
Electrically stimulated cell migration and its contribution to wound healing.
Burns Trauma, 6:20, 09 Jul 2018
Cited by: 44 articles | PMID: 30003115 | PMCID: PMC6036678
Review Free full text in Europe PMC
New Opportunities for Electric Fields in Promoting Wound Healing: Collective Electrotaxis.
Adv Wound Care (New Rochelle), 15 Jul 2024
Cited by: 0 articles | PMID: 38780799
Review
Funding
Funders who supported this work.
NEI NIH HHS (2)
Grant ID: R01 EY019101-01A2
Grant ID: R01 EY019101
NIAMS NIH HHS (1)
Grant ID: R01 AR044518



