Abstract
Free full text

Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats.
Abstract
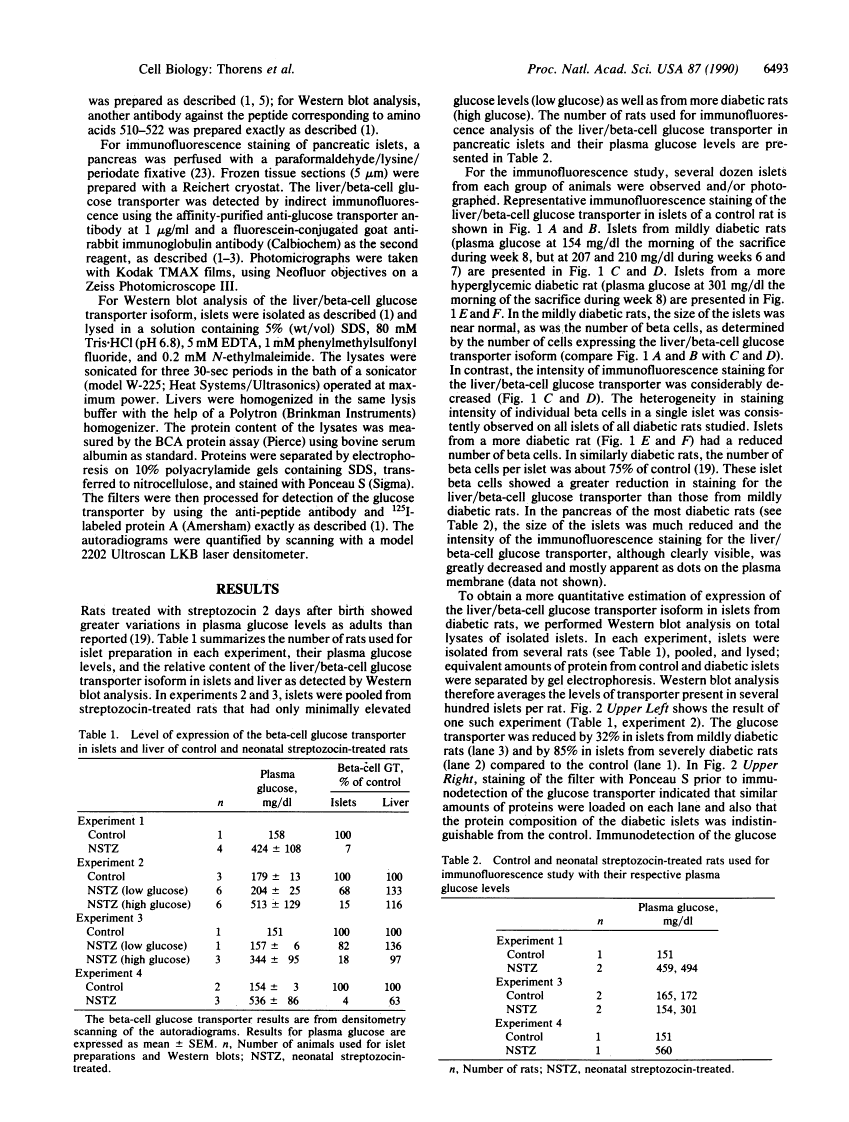
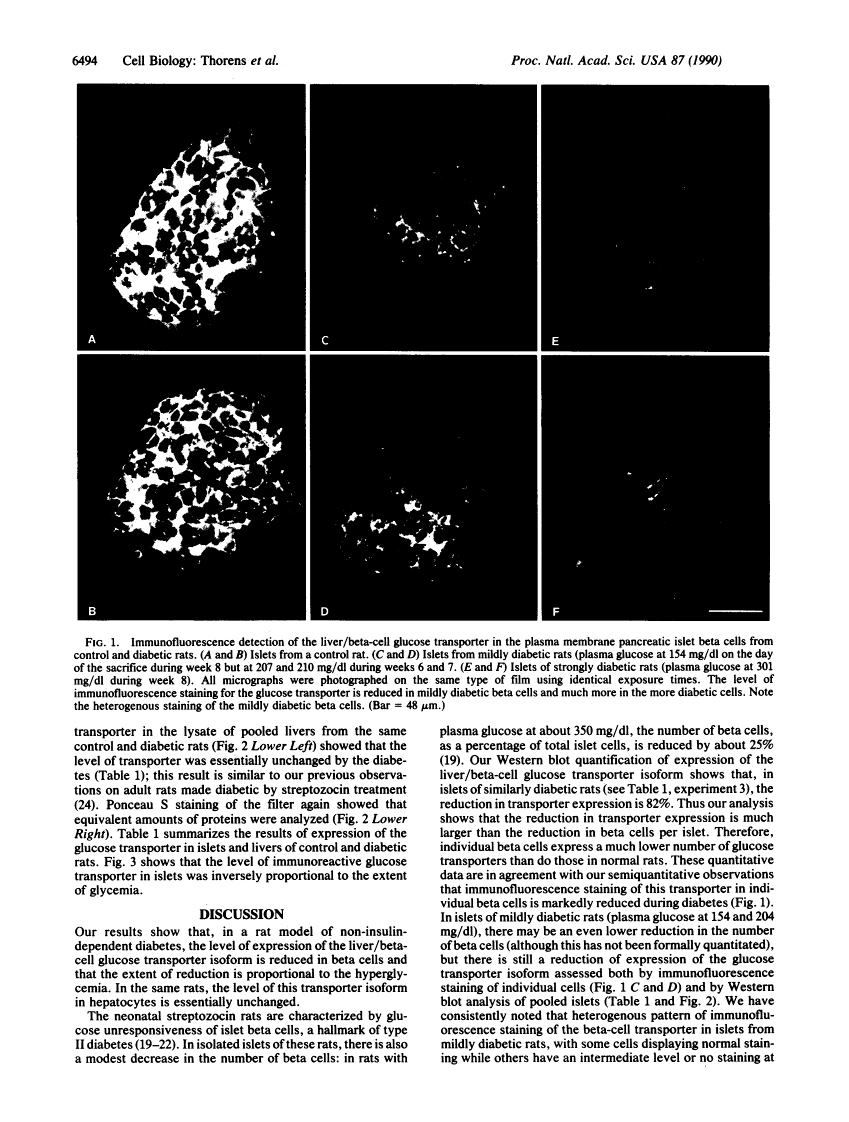
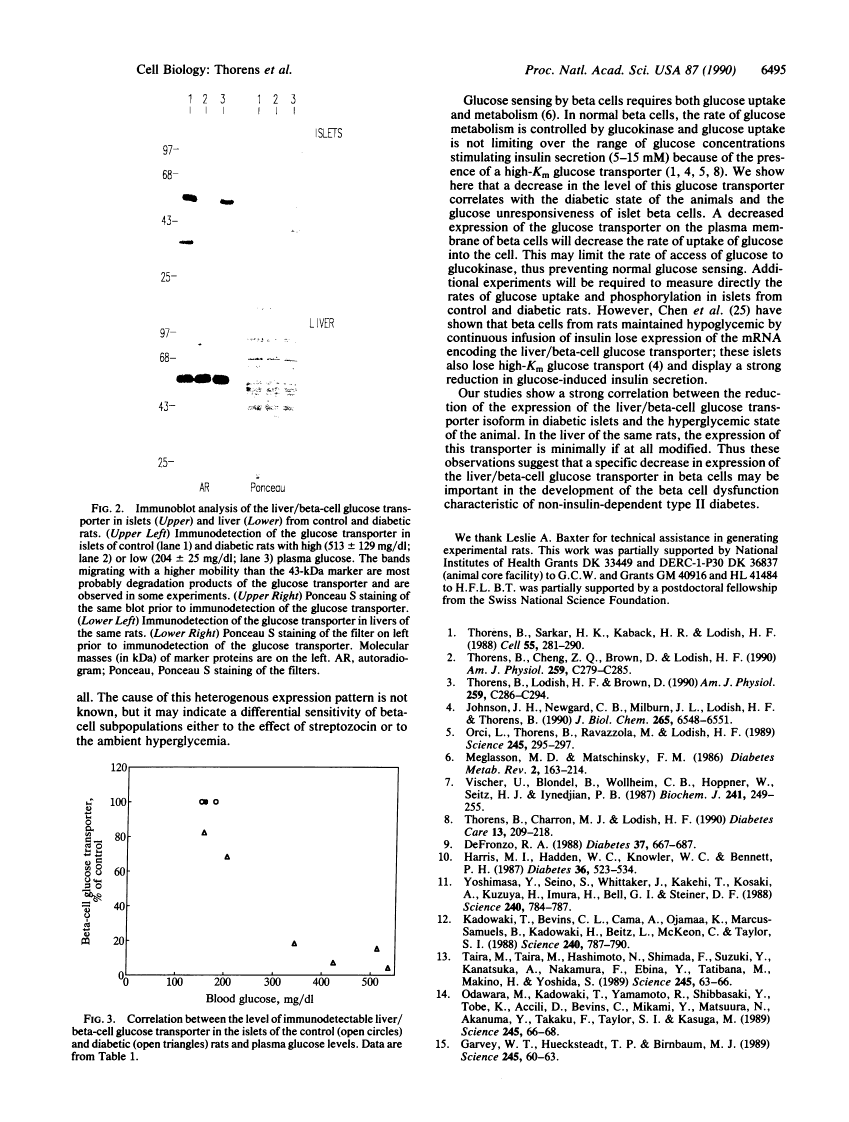
Rats injected with a single dose of streptozocin at 2 days of age develop non-insulin-dependent diabetes 6 weeks later. The pancreatic beta islet cells of these diabetic rats display a loss of glucose-induced insulin secretion while maintaining sensitivity to other secretagogues such as arginine. We analyzed the level of expression of the liver/beta-cell glucose transporter isoform in diabetic islets by immunofluorescence staining of pancreas sections and by Western blotting of islet lysates. Islets from diabetic animals have a reduced expression of this beta-cell-specific glucose transporter isoform and the extent of reduction is correlated with the severity of hyperglycemia. In contrast, expression of this transporter isoform in liver is minimally modified by the diabetes. Thus a decreased expression of the liver/beta-cell glucose transporter isoform in beta cells is associated with the impaired glucose sensing characteristic of diabetic islets; our data suggest that this glucose transporter may be part of the beta-cell glucose sensor.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. [Abstract] [Google Scholar]
- Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. [Abstract] [Google Scholar]
- Thorens B, Lodish HF, Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990 Dec;259(6 Pt 1):C286–C294. [Abstract] [Google Scholar]
- Johnson JH, Newgard CB, Milburn JL, Lodish HF, Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [Abstract] [Google Scholar]
- Orci L, Thorens B, Ravazzola M, Lodish HF. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. [Abstract] [Google Scholar]
- Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. [Abstract] [Google Scholar]
- Vischer U, Blondel B, Wollheim CB, Höppner W, Seitz HJ, Iynedjian PB. Hexokinase isoenzymes of RIN-m5F insulinoma cells. Expression of glucokinase gene in insulin-producing cells. Biochem J. 1987 Jan 1;241(1):249–255. [Europe PMC free article] [Abstract] [Google Scholar]
- Thorens B, Charron MJ, Lodish HF. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. [Abstract] [Google Scholar]
- DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. [Abstract] [Google Scholar]
- Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes. 1987 Apr;36(4):523–534. [Abstract] [Google Scholar]
- Yoshimasa Y, Seino S, Whittaker J, Kakehi T, Kosaki A, Kuzuya H, Imura H, Bell GI, Steiner DF. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988 May 6;240(4853):784–787. [Abstract] [Google Scholar]
- Kadowaki T, Bevins CL, Cama A, Ojamaa K, Marcus-Samuels B, Kadowaki H, Beitz L, McKeon C, Taylor SI. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science. 1988 May 6;240(4853):787–790. [Abstract] [Google Scholar]
- Taira M, Taira M, Hashimoto N, Shimada F, Suzuki Y, Kanatsuka A, Nakamura F, Ebina Y, Tatibana M, Makino H, et al. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. [Abstract] [Google Scholar]
- Odawara M, Kadowaki T, Yamamoto R, Shibasaki Y, Tobe K, Accili D, Bevins C, Mikami Y, Matsuura N, Akanuma Y, et al. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):66–68. [Abstract] [Google Scholar]
- Garvey WT, Huecksteadt TP, Birnbaum MJ. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. [Abstract] [Google Scholar]
- Sivitz WI, DeSautel SL, Kayano T, Bell GI, Pessin JE. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. [Abstract] [Google Scholar]
- Berger J, Biswas C, Vicario PP, Strout HV, Saperstein R, Pilch PF. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. [Abstract] [Google Scholar]
- Kahn BB, Charron MJ, Lodish HF, Cushman SW, Flier JS. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. [Europe PMC free article] [Abstract] [Google Scholar]
- Bonner-Weir S, Trent DF, Honey RN, Weir GC. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes. 1981 Jan;30(1):64–69. [Abstract] [Google Scholar]
- Weir GC, Clore ET, Zmachinski CJ, Bonner-Weir S. Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes. 1981 Jul;30(7):590–595. [Abstract] [Google Scholar]
- Portha B, Levacher C, Picon L, Rosselin G. Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes. 1974 Nov;23(11):889–895. [Abstract] [Google Scholar]
- Giroix MH, Portha B, Kergoat M, Bailbe D, Picon L. Glucose insensitivity and amino-acid hypersensitivity of insulin release in rats with non-insulin-dependent diabetes. A study with the perfused pancreas. Diabetes. 1983 May;32(5):445–451. [Abstract] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. [Abstract] [Google Scholar]
- Thorens B, Flier JS, Lodish HF, Kahn BB. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. [Abstract] [Google Scholar]
- Chen L, Alam T, Johnson JH, Hughes S, Newgard CB, Unger RH. Regulation of beta-cell glucose transporter gene expression. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4088–4092. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.17.6492
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc54562?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.87.17.6492
Article citations
NGF effects promote the maturation of rat pancreatic beta cells by regulating GLUT2 levels and distribution, and glucokinase activity.
PLoS One, 19(6):e0303934, 14 Jun 2024
Cited by: 0 articles | PMID: 38875221 | PMCID: PMC11178159
Missense mutation of ISL1 (E283D) is associated with the development of type 2 diabetes.
Diabetologia, 67(8):1698-1713, 31 May 2024
Cited by: 1 article | PMID: 38819467
Metformin increases the uptake of glucose into the gut from the circulation in high-fat diet-fed male mice, which is enhanced by a reduction in whole-body Slc2a2 expression.
Mol Metab, 77:101807, 16 Sep 2023
Cited by: 2 articles | PMID: 37717665 | PMCID: PMC10550722
Exploring of the ameliorative effects of Nerium (Nerium oleander L.) ethanolic flower extract in streptozotocin induced diabetic rats via biochemical, histological and molecular aspects.
Mol Biol Rep, 50(5):4193-4205, 10 Mar 2023
Cited by: 2 articles | PMID: 36897524
Single-cell RNA-seq transcriptomic landscape of human and mouse islets and pathological alterations of diabetes.
iScience, 25(11):105366, 14 Oct 2022
Cited by: 10 articles | PMID: 36339258 | PMCID: PMC9626680
Go to all (79) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recovery of pancreatic beta cells in response to long-term normoglycemia after pancreas or islet transplantation in severely streptozotocin diabetic adult rats.
Pancreas, 23(2):186-196, 01 Aug 2001
Cited by: 13 articles | PMID: 11484921
Evidence that down-regulation of beta-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia.
Proc Natl Acad Sci U S A, 87(24):9953-9957, 01 Dec 1990
Cited by: 76 articles | PMID: 2263645 | PMCID: PMC55292
Recovery of glucose-induced insulin secretion in a rat model of NIDDM is not accompanied by return of the B-cell GLUT2 glucose transporter.
Diabetes, 41(10):1320-1327, 01 Oct 1992
Cited by: 12 articles | PMID: 1397706
The pancreatic beta-cell glucose sensor.
Trends Biochem Sci, 19(12):535-538, 01 Dec 1994
Cited by: 55 articles | PMID: 7846765
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (2)
Grant ID: 1-P30 DK 36837
Grant ID: DK 33449
NIGMS NIH HHS (1)
Grant ID: GM 40916