Abstract
Free full text

Correlation of NRAS Mutations with Clinical Response to High Dose IL-2 in Patients with Advanced Melanoma
Abstract
The purpose of this study is to identify clinical and molecular characteristics of melanoma patients that predict response to high-dose interleukin-2 (HD IL-2) in order to improve patient selection for this approved but toxic therapy.
We reviewed the records of 208 patients with unresectable stage III/IV melanoma treated with HD IL-2 at The University of Texas M.D Anderson Cancer Center (MDACC) (n=100) and Beth Israel Deaconess Medical Center (BIDMC) (n=108) between 2003 and 2009. The BRAF and NRAS mutation status of the tumors was determined for patients with available tissue samples and the mutation status and clinical characteristics were compared to clinical outcomes. Pre-treatment serum lactate dehydrogenase (LDH) levels were available for most patients (n=194). Tissue was available for mutational analysis on a subset of patients (n=103) and the prevalence of mutations was as follows: BRAF 60%, NRAS 15%, WT/WT 25%. In the subset of patients for which mutational analysis was available, there was a significant difference in the response rate based on the mutation status: NRAS 47%, BRAF 23%, and WT/WT 12% (p=0.05). Patients with NRAS mutations had non-statistically longer overall survival (5.3 versus 2.4 years, p=0.30) and progression free survival (214 versus 70 days, p=0.13). Patients with an elevated LDH had a decreased PFS (46 versus 76 days, p<0.0001), decreased OS (0.56 versus 1.97 years, p<0.0001), and trended toward a decreased response rate (7% versus 21%, p=0.08). NRAS mutational status is a new candidate biomarkers for selecting patients with melanoma for HD IL-2 treatment.
Introduction
Melanoma is the most aggressive form of skin cancer causing an estimated 8790 deaths per year in the United States.1 Patients with metastatic melanoma have very poor outcomes, with a median overall survival (OS) of 6 to 8 months.2, 3 Currently there are three Food and Drug Administration (FDA) approved treatments for patients with metastatic melanoma, dacarbazine, high dose bolus interleukin-2 (HD IL-2), and ipilimumab.4,5 HD IL-2 was approved primarily due to it achievement of durable (i.e. > 5 years) disease remissions, albeit in a relatively small proportion of patients (~6%).6, 7 However, the use of HD IL-2 is limited by the toxicity of the regimen, which affects virtually every organ system, and caused treatment-related deaths in 1% to 2% in initial studies.8, 9 Thus, there is a clear need for biomarkers that identify the patients most likely to benefit from HD IL-2 therapy, as well as those whose chance of response is too low to justify the associated risks.
Activating mutations in the RAS-RAF-MEK-MAPK signaling pathway are the most common somatic genetic events in cutaneous melanomas. An estimated 40–50% of patients with cutaneous melanoma harbor a mutation in BRAF and an additional 15–20% of patients harbor a mutation in NRAS.10–12 Mutations in BRAF and NRAS cause constitutive activation of the MAP kinase pathway leading to increased cellular proliferation.11, 13 In addition to increasing cell growth, there is emerging evidence that activation of the MAP kinase pathway influences the immunophenotype of the tumor.14 Expression of mutant BRAF in melanoma cell lines decreased the expression of the several melanocytic antigens, including Melan-A/MART-1.13 More recently, investigators demonstrated that treatment with the mutant-specific BRAF inhibitor PLX4032/RG7204 and or MEK inhibitors increased the expression of melanoma differentiation antigens in BRAF-mutant melanoma cell lines.15
To test the hypothesis that mutations in BRAF or NRAS affect the clinical efficacy of HD IL-2 in patients with advanced melanoma, we retrospectively reviewed the clinical characteristics, mutation status, and clinical outcomes of a contemporary cohort of patients treated with HD IL-2 at The University of Texas M.D. Anderson Cancer Center (MDACC) and the Beth Israel Deaconess Medical Center (BIDMC). Our primary endpoint was the relationship between the response rate (CR or PR) and tumor mutation status. Response rate was selected as the primary endpoint because this is most direct early measure of immunotherapy success. Secondary endpoints included determining the relationship between mutation status and overall survival (OS) and progression free survival (PFS), and to determine if other clinical characteristics (ie, gender, age, LDH) were associated with HD IL-2 outcomes in this contemporary cohort.
Methods
Patients
Under an Institutional Review Board-approved protocol, clinical data was collected on 208 patients with advanced melanoma treated with HD IL-2 at MDACC (n=100) or BIDMC (n=108). Patients at MDACC were treated from December 16, 2003 to June 1, 2009 and patients at BIDMC were treated from August 15, 2005 to September 15, 2009. All patients who received at least one dose of IL-2 were included in the analysis. Patients who received concurrent vaccine therapy (e.g. gp100 peptide16) were excluded from the analysis. Patient demographics at the start of HD IL-2 therapy were collected including: age, gender, AJCC clinical stage, and serum LDH. LDH status (Normal, Elevated) was defined by the upper limit of normal for the assay used at each institution. At MDACC the upper limit of normal for LDH was 618 IU/L and at BIDMC the upper limit of normal was 250 IU/L. Systemic therapies received prior to HD IL-2 were also collected.
IL-2 Administration and Treatment Response Evaluation
IL-2 600,000–720,000 U/kg IV was administered in 15-minute infusions every 8 hours for up to 14 doses in 5 days. At MDACC patients were given a two-week recovery time before they were re-evaluated for an additional course of IL-2. At BIDMC, patients were treated on days 1–5 and 15–19 of a 12-week cycle and offered a second cycle of therapy if there was any evidence of tumor regression. At MDACC the range of cycles was from 1–8, and at BIDMC the range of cycles was from 1–2. Tumor response for each patient was assessed after two 5-day cycles of therapy at each institution. For 206 of the 208 patients, clinical response was evaluated retrospectively by review of radiographic studies and documented skin exams using RECIST17 criteria. At MDACC, an independent radiologist reviewed and confirmed the radiographic findings for all patients who were categorized as either a complete (CR) or partial responder (PR). At BIDMC an independent clinician reviewed and confirmed all radiographic complete (CR) and partial responses (PR). In addition, one patient classified as a CR had complete resolution of a nasopharyngeal melanoma documented by a formal ENT exam and direct visualization, while another patient classified as a CR patient had radiographic resolution of assessable lesions and complete resolution of malignant ascites. Progression free survival (PFS) was calculated from the date the first dose of IL-2 was given to the date of progression per the evaluating physician, or the last documented clinical evaluation for patients who had not progressed. No patients were lost to follow up before the best response could be quantified. OS was calculated from the date the first dose of IL-2 was given to the last documented contact with the patient. While some patients were lost to follow up after progression on HD IL-2, progression was able to be determined on all patients. Results were updated through June 10, 2011.
Tissue Collection and Mutation Analysis
Tissue was available for analysis on a subset of patients. At MDACC, 34 patients had available results of Clinical Laboratory Improvement Amendment (CLIA) certified pyrosequencing of BRAF (exon 15) and NRAS (codons 12, 13, 61). For the remaining patients at MDACC who did not have CLIA mutational analysis (n=30) and all of the patients at BIDMC (n=37), archived available formalin-fixed paraffin embedded (FFPE) tissues were analyzed. Attempts were made to use tissue that had been harvested prior to administration to HD IL-2, however 30% of the tissue used in the mutational analysis came from tissue after HD IL-2 was administered. The MDACC Biospecimen Core Facility extracted DNA from samples with at least 50% tumor content. Mass-spectroscopy genotyping for BRAF (exon 15) and NRAS (codons 12, 13, 61) mutations was performed as previously described.4 Patients without a BRAF or NRAS mutation were classified as wild-type (WT/WT).
Statistical Analysis
The association between patient characteristics (gender, disease stage, mutation status, and initial LDH level) and clinical response (CR, PR) was evaluated using Fisher’s exact test. The association between disease stage and response was assessed using the Kruskal-Wallis test to take into account the ordering of the disease stages. The association of age with clinical response was assessed using a Wilcoxon rank-sum test. Distributions of PFS and OS were estimated using the Kaplan-Meier method,18 and distributions were compared between groups with log-rank tests. No adjustments were made for the multiplicity of testing.
Results
Patient Demographics
The patient demographics for the 208 patients treated with HD IL-2 are shown in Table 1. The median patient age was 51 years (range, 15 to 74 years) and 60% were male. The vast majority (88%) of the patients had stage IV disease, normal LDH level (79%), and measurable disease (98%). The best clinical response rates were as follows: 6% CR, 13% PR, 14% stable disease (SD), and 67% progressive disease (PD). The median OS duration was 1.9 years (range, 0.1 to 6.4 years), and the median PFS duration was 104 days (range, 11 to 2188 days). The 5 year survival rate is 39% and the median follow up for surviving patients is 2.5 years.
Table 1
Baseline Demographics
| ALL (n=208) | Tested (n=103) | Untested (n=105) | P value | |
|---|---|---|---|---|
| Age median (range) | 51.3 (14.9–74.0) | 50.2 (14.9–70.3) | 53.0 (25.0–74.0) | 0.02† |
| Gender (%) | 0.48§ | |||
 Female Female | 84 (40) | 39 (46) | 45 (54) | |
 Male Male | 124 (60) | 64 (52) | 60 (48) | |
| Adjuvant IFN | 92 (44) | 54 (58) | 38 (41) | 0.03§ |
| Prior Systemic Therapy for Metastatic Disease | ||||
 Chemo Chemo | 41 (20) | 25 (61) | 16 (39) | 0.12§ |
 None None | 152 (73) | 74 (49) | 78 (51) | 0.48§ |
| LDH(%) | 0.17
 | |||
 Normal Normal | 164 (79) | 89 (54) | 75 (46) | |
 Elevated Elevated | 30 (14) | 12 (40) | 18 (60) | |
 Unavailable Unavailable | 14 (7) | 2 (14) | 12 (86) | |
| Stage (%) | 0.07§ | |||
 IIIc IIIc | 25 (12) | 17 (68) | 8 (32) | |
 IV M1a IV M1a | 25 (12) | 15 (60) | 10 (40) | |
 IV M1b IV M1b | 61 (29) | 31 (51) | 30 (49) | |
 IV M1c IV M1c | 97 (47) | 40 (41) | 57 (59) | |
| Measurable Disease (%) | 1.0 | |||
 Yes Yes | 203 (98) | 100 (49) | 103 (51) | |
| Survival | ||||
 OS years (range) OS years (range) | 1.9 (0–6.4) | 2.6 (0–6.4) | 1.3 (0.1–6.1) | 0.0002‡ |
 PFS days (range) PFS days (range) | 104 (11–2188) | 169 (11–2038) | 71 (14–2188 | 0.15‡ |
| Best Response | 0.11§** | |||
 Complete Response Complete Response | 13 (6) | 9 (69) | 4 (31) | |
 Partial Response Partial Response | 26 (13) | 15 (58) | 11 (42) | |
 Stable Disease Stable Disease | 35 (17) | 20 (57) | 15 (43) | |
 Progressive Disease Progressive Disease | 134 (64) | 59 (44) | 75 (56) |
Note-
 Fisher’s exact excludes LDH unavailable
Fisher’s exact excludes LDH unavailableOf the 208 patients, tumor tissue was available for mutational analysis for 103 (50%). Patients for whom tissue was available (Tested) had several clinical differences from patients for whom tissue wasn’t available (Untested). The patients in the tested cohort were younger (50 years versus 53 years; p=0.02), survived longer (2.6 years versus 1.3 years; p=0.0002), and were more likely to have received adjuvant IFN prior to HD IL2 (p=0.03). The Tested patients demonstrated a trend for a higher rate of CR/PR (24% versus 15%, p=0.11) and longer PFS (169 days versus 71, p=0.15), and survived longer from the start of HD IL-2 treatment (2.6 years versus 1.3, p=0.0002).
Clinical and Molecular Characteristics of Patients Who Responded to HD-IL2
To identify factors that correlated with clinical response to HD IL-2, we analyzed the patients’ clinical characteristics for significant association with the chance of achieving as CR or PR as the best response to HD IL-2, as compared to SD or PD (Table 2). There was no significant association identified for patient age, gender, or disease stage. Only two (6%) of 31 patients with elevated LDH before starting HD IL-2 achieved a CR/PR, compared with 34 (21%) of 164 with a normal LDH level (p=0.08). In addition we also analyzed the association of an LDH cut off of 0.75 or 1.25 of normal, and we did not find a significant association (data not shown). We did not find any significant difference in age or gender between the patients with elevated versus normal LDH (data not shown).
Table 2
Clinical Characteristics or Responders and Non-Responders to HD-IL2
| CR N=13 (6%) | PR N=26 (13%) | SD N=35 (17%) | PD N=134 (64%) | p-value* | |
|---|---|---|---|---|---|
| Age median (range) | 56 (24–69) | 50 (30–67) | 51 (22–68) | 51 (15–74) | 0.58† |
| Gender (%) | |||||
 Female Female | 6 (7) | 11 (13) | 16 (19) | 51 (61) | 0.71§ |
 Male Male | 7 (6) | 15 (12) | 19 (15) | 83 (67) | |
| Adjuvant IFN | 8 (9) | 11 (12) | 17 (18) | 56 (61) | 0.59§ |
| Prior Systemic Therapy for Metastatic Disease | |||||
 Chemo Chemo | 2 (5) | 4 (10) | 11 (27) | 24 (59) | 0.51§ |
 None None | 10 (7) | 19 (13) | 25 (16) | 98 (64) | 1.0§ |
| LDH | 0.08
 | ||||
 Normal Normal | 12 (7) | 22 (14) | 32 (21) | 98 (64) | |
 Elevated Elevated | 0 | 2 (7) | 1 (3) | 27 (90) | |
 Unknown Unknown | 1 (8) | 2 (8) | 2 (6) | 9 (7) | |
| Stage (%) | 0.46§ | ||||
 IIIc IIIc | 5 (20) | 1 (4) | 6 (24) | 13 (54) | |
 IV M1a IV M1a | 4 (16) | 3 (12) | 6 (24) | 12 (48) | |
 IV M1b IV M1b | 1 (2) | 9 (15) | 12 (20) | 39 (64) | |
 IV M1c IV M1c | 3 (23) | 13 (50) | 11 (31) | 70 (52) | |
| Outcomes | |||||
 OS years (range) OS years (range) | NR (1.4–5.2) | NR (0.6–6.4) | 3.1 (0.1–5.5) | 1.1 (0.1–6.1) | <0.0001‡ |
 PFS days (range) PFS days (range) | NR (96–1887) | NR (75–2188) | 308 (42–1035) | 48 (11–327) | <0.0001‡ |
Note -
NR indicates “Not Reached”
 Fisher’s exact excludes LDH unavailable
Fisher’s exact excludes LDH unavailableWe next analyzed the clinical characteristics and outcomes for the patients tested for BRAF and NRAS mutations (n=103) (Table 3). Sixty-two patients (60% of the cohort) had an activating BRAF mutation, 15 (15%) had an NRAS mutation, and 26 (25%) were WT/WT. Mutation status significantly correlated with patient age (p=0.006) and gender (p=0.02). Patients with a BRAF mutation were younger (median age, 48 years) than those with a NRAS mutation (53 years) or WT/WT (56 years) patients. Women were more likely to have a BRAF mutation than men (77% versus 50%). Disease stage (p=0.24) and serum LDH (p=0.13) did not correlate with mutation status. There was a trend towards a difference in prior treatment with 79% of BRAF, 73% of NRAS, and 54% of WT patients undergoing HD IL-2 as first systemic therapy for metastatic disease (p=0.06). There was a significant association between tumor mutation status and the chance of achieving a CR/PR with HD IL-2 therapy. Among the patients who achieved a CR or PR, 58% had a BRAF mutation, 29% an NRAS mutation, and 13% were WT/WT. While a similar 60% of the SD/PD patients had a BRAF mutation, only 10% had an NRAS mutation, and 30% were WT/WT (p=0.05 by Fisher’s exact test). Overall, 47% of the NRAS-mutant patients achieved a CR/PR, as compared to 23% with a BRAF mutation, and 12% of WT/WT patients. Patients with NRAS mutations had a significantly higher response rate when compared to patients with BRAF mutations or were WT (Table 4) (47% versus 19%, p=0.04)
Table 3
Clinical Characteristics of Patients with BRAF/NRAS Mutations
| All Tested n=103 | BRAF n=62 (60%) | NRAS n=15 (15%) | WT/WT n=26 (25%) | p-value | |
|---|---|---|---|---|---|
| Age median (range) | 50 (15–70) | 48 (15–69) | 53 (33–68) | 56 (24–70) | 0.006† |
| Gender (%) | 0.02§ | ||||
 Female Female | 39 (38) | 30 (77) | 4 (10) | 5 (13) | |
 Male Male | 64 (62) | 32 (50) | 11 (17) | 21 (33) | |
| Adjuvant IFN | 54 (52) | 34 (63) | 9 (17) | 11 (20) | 0.46§ |
| Prior Systemic Therapy for Metastatic Disease | |||||
 Chemo Chemo | 25 (24) | 13 (52) | 6 (24) | 6 (24) | 0.33§ |
 None None | 74 (72) | 49 (66) | 11 (15) | 14 (19) | 0.06§ |
| Stage | 0.24§ | ||||
 IIIc IIIc | 17 (17) | 14 (82) | 2 (12) | 1 (6) | |
 M1a M1a | 15 (15) | 9 (60) | 3 (20) | 3 (20) | |
 M1b M1b | 31 (30) | 19 (61) | 5 (16) | 7 (23) | |
 M1c M1c | 40 (39) | 20 (50) | 5 (13) | 15 (38) | |
| Initial LDH | 0.13
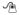 | ||||
 Normal Normal | 89 (86) | 55 (62) | 14 (16) | 20 (22) | |
 Elevated Elevated | 12 (12) | 5 (42) | 1 (8) | 6 (50) | |
 Unknown Unknown | 2 (2) | 2 (100) | 0 | 0 | |
| Survival | |||||
 OS-Years (range) OS-Years (range) | 2.6 (0.1–6.4) | 2.2 (0.2–5.6) | 5.3 (0.6–6.4) | 2.4 (0.1–5.5) | 0.57‡ |
 PFS-Days (range) PFS-Days (range) | 169 (11–2038) | 178 (16–2038) | 350 (35–1887) | 76 (11–1035) | 0.12‡ |
| Response | 0.05* | ||||
 CR/PR (n=38) CR/PR (n=38) | 24 (23) | 14 (58) | 7 (29) | 3 (13) | |
 SD/PD (n=127) SD/PD (n=127) | 79 (77) | 48 (61) | 8 (10) | 23 (33) |
Note -
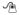 Fisher’s exact excludes LDH unavailable
Fisher’s exact excludes LDH unavailableTable 4
Analysis of NRAS vs BRAF/WT in Predicting Response
| Variable | NRAS n=15 | BRAF and WT n=88 | p-value |
|---|---|---|---|
| CR/PR | 7 (47%) | 27 (19%) | 0.04§ |
| OS (years) | 5.3 | 2.4 | 0.30‡ |
| PFS (days) | 214 | 70 | 0.13‡ |
Progression-Free and Overall Survival of Patients Treated with HD IL-2
OS and PFS were significantly longer for patients who achieved a CR/PR than for those who had SD/PD after HD IL-2 therapy (Table 2). Patients with elevated LDH (n=30) had decreased PFS (46 days versus 76 days, p<0.0001) and OS (0.56 years versus 1.97 years, p<0.0001) compared to patients with a normal LDH (Figure 1). There was no statistically significant difference in PFS or OS in patients based on the BRAF/NRAS mutational status (Figure 2). However, patients with NRAS mutations had the longest median PFS and OS, which trended toward but did not reach statistical significance when compared to BRAF and WT patients (p=0.13 for PFS, p=0.30 for OS).
Patients with an elevated serum LDH (n=30) at the start of treatment with HD IL-2 had shorter (A) overall survival (median 0.56 years versus 1.97 years, p<0.0001) and (B) progression free survival (median 46 days versus 76 days, p<0.0001) compared to patients with normal serum LDH (n=164).
In a three way comparison of BRAF, NRAS, and WT tumor mutation status did not correlate with (A) overall survival (p=0.57) or (B) progression-free survival (p=0.12) among patients treated with HD IL-2 in a three way comparison. Comparing NRAS versus BRAF and WT patients, (C) overall survival (p=0.30) and (D) PFS remain non-statistically different (p=0.13)
Discussion
The main conclusion from this multi-center retrospective study patients with metastatic melanoma treated with HD IL-2 is that there was a statistically significant difference in the response rate (CR or PR) based on the tumor mutation status. Patients with NRAS mutations were more than twice as likely to respond to HD IL-2 than patients who were WT for NRAS (47% versus 19%, p=0.04). This is the first time that mutation status has been associated with response to HD IL-2 therapy for melanoma. We also observed a strong trend for a lower chance of response to HD IL2 among patients with elevated serum LDH, which also has not been reported previously.
While tumor mutation status is critical to outcomes of melanoma patients treated with selective BRAF inhibitors PLX403219, 20 and GSK2118436,21 its predictive significance for other therapies is largely unknown. As preclinical studies suggest that activation of the RAS-RAF-MEK-MAPK pathway promotes an immunosuppressive phenotype,14, 15, 22 we hypothesized that patients with a BRAF or NRAS mutation would have worse outcomes when treated with HD IL-2. Surprisingly, patients with NRAS-mutant melanoma were the most likely to achieve a CR/PR to HD IL-2 in this cohort. This finding is particularly interesting in light of recent reports demonstrating that the presence of an NRAS mutation predicts shorter overall survival from initial diagnosis of melanoma, and from the diagnosis of stage IV disease.23, 24 The mechanism underlying this improved responsiveness to HD IL-2 among NRAS-mutant melanoma patients is not clear. Perhaps activation of the MAPK pathway, or other signaling pathways, by the NRAS mutation leads to an immunosuppressive phenotype that is most readily reversed by HD IL-2. Alternatively, the mutant NRAS protein may be immunogenic, and thus promotes the anti-tumor immune response. In contrast to BRAF-mutant melanoma, currently there are no specific, effective treatment strategies for patients with NRAS mutations. Given that we found increased responsiveness to HD IL-2 among patients with NRAS mutations, we believe that similar mutational analyses should be performed for other melanoma immunotherapies (e.g. ipilimumab). In addition, we believe future studies should correlate tumor mutations status with immunological endpoints. Importantly, if subsequent studies of HD IL-2 and/or other immunotherapies confirm the increased responsiveness of patients with NRAS mutations, this could serve as a preferred treatment strategy for these patients.
Elevated LDH is a validated predictor of poor prognosis for patients with melanoma.25, 26 In patients with metastatic colon cancer, elevated LDH correlates with increased vascular endothelial growth factor (VEGF).27 While the relationship between LDH and VEGF has not been demonstrated in patients with melanoma, VEGF is known to cause immunosuppression by inhibiting dendritic cell maturation,28, 29 and elevated serum VEGF has been associated with reduced survival to HD-IL2 in patients with metastatic melanoma and renal cell carcinoma.30 Regardless of the biologic mechanism linking elevated LDH with a decreased response to HD IL2, the widespread availability of serum LDH testing support the clinical potential of this candidate biomarker.
Multiple studies have identified potential biomarkers for predicting response to HD IL-2, however most of these studies are small and from single institutions. In renal cell carcinoma (RCC), high tumor expression of carbonic anhydrase IX was initially shown to correlate with improved response to immunotherapy as well as improved overall prognosis,31 however this was later shown not to hold up in a prospective fashion.32 In a single institution study involving patients with metastatic melanoma and RCC, high pretreatment levels of serum fibronectin, as well as VEGF, correlated with a decreased response to HD IL-2.30 Finally, investigators have identified an expression signature of immune related genes correlated with response to HD IL-2 in patientswith metastatic melanoma.33 While other biomarkers that predict response to HD IL-2 exist, we feel that using the mutation status of the tumor and pre-treatment LDH levels has several advantages. First, LDH is an established prognostic marker in stage IV melanoma with a simple laboratory test that is universally available. Second, in the era of targeted therapy for melanoma, patients will increasingly have their tumors tested for mutational status before beginning systemic therapy for metastatic disease. While these two biomarkers are readily available, we conclude that our results will require validation in a prospective fashion.
While the results of our study are intriguing, we recognize that they have several limitations. The primary limitation of our study is that only a subset of patients had tissue available for mutational analysis, and these patients had a statistically significant longer OS, and a trend towards an increased PFS and response rate, when compared to patients for whom no tissue was available (Table 1). We speculate that the Tested population was enriched for patients with good outcomes for three reasons. First, 20 (20%) of the Tested population received BRAF inhibitors after IL-2 and their median survival is not yet reached (data not shown). Second, we retrospectively obtained consent for tissue and therefore the Tested population was biased towards patients that were alive and available to consent. Third, 30% of samples used for mutational analysis were obtained after the administration of IL-2 further enriching this population for patients who survived long enough to undergo procedures after IL-2 and therefore either had slower growing tumors or some response to treatment. However, the prevalence of mutations was similar in the biopsies taken before and after HD IL-2 therapy (p = 0.68; Supplemental Table 1), perhaps mitigating the impact of this factor on the analysis on the predictive value of the tumor mutations. Another limitation is that longer follow-up is necessary to determine if responses are durable and translate into long-term survival. Finally, while the NRAS cohort had the best clinical outcomes, it was also the smallest molecularly defined cohort (n=15) in this study. We also recognize the limitation of determining response and PFS is limited by a retrospective study. Given these limitations, our finding of the association between an increased response and NRAS mutation should be viewed as hypothesis generating and not practice changing.
In conclusion, this paper is the first to link RAS-RAF-MEK-MAPK pathway mutation status as a predictor of response to HD IL-2 in patients with advanced melanoma. If validated in independent, prospective studies, the presence of an NRAS mutation may help identify the patients who are most likely to benefit from treatment with HD IL-2, and support this therapeutic approach for patients with that mutation. This data also provides important new information for the appropriate planning of future clinical trials combining HD IL-2 with BRAF or other RAS-RAF-MEK-MAPK pathway inhibitors in genotyped melanoma patients. In addition, we believe these results support a likely link between the molecular characteristics of melanomas and the immunophenotype of the tumor. This data therefore provides further support for the need for integrated analyses of both molecular and immunologic parameters in the future development and evaluation of therapies for this aggressive disease.
Supplementary Material
1
Supplementary Table 1:Mutation distribution in tissue obtained either before or after receiving HD-IL2. The majority of patients who had tissue available before being treated with HD IL-2 (70%). There was no difference in the mutation status in patients who had tissue that was biopsied before or after treatment of IL-2.
Acknowledgments
We thank all participating patients, nurses, laboratory scientist, and data coordinators for help on this project. In addition, we thank the American Society of Clinical Oncology who in part funded this work through the ASCO Young Investigator Award and Career Development Award.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/cji.0b013e3182372636
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3241890?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Anorectal mucosal melanoma.
Semin Colon Rectal Surg, 34(4):100990, 17 Oct 2023
Cited by: 0 articles | PMID: 38746826
A real-world study of adjuvant anti-PD -1 immunotherapy on stage III melanoma with BRAF, NRAS, and KIT mutations.
Cancer Med, 12(15):15945-15954, 05 Jul 2023
Cited by: 2 articles | PMID: 37403699 | PMCID: PMC10469738
MicroRNA-708 emerges as a potential candidate to target undruggable NRAS.
PLoS One, 18(4):e0284744, 21 Apr 2023
Cited by: 1 article | PMID: 37083947 | PMCID: PMC10120925
Case report: Later onset of NRAS-mutant metastatic melanoma in a patient with a partially-excised giant congenital melanocytic nevus.
Front Med (Lausanne), 9:1086473, 08 Dec 2022
Cited by: 0 articles | PMID: 36569151 | PMCID: PMC9773131
Association of NRAS Mutation With Clinical Outcomes of Anti-PD-1 Monotherapy in Advanced Melanoma: A Pooled Analysis of Four Asian Clinical Trials.
Front Immunol, 12:691032, 05 Jul 2021
Cited by: 13 articles | PMID: 34290710 | PMCID: PMC8289467
Go to all (74) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association Between NRAS and BRAF Mutational Status and Melanoma-Specific Survival Among Patients With Higher-Risk Primary Melanoma.
JAMA Oncol, 1(3):359-368, 01 Jun 2015
Cited by: 98 articles | PMID: 26146664 | PMCID: PMC4486299
NRAS mutation status is an independent prognostic factor in metastatic melanoma.
Cancer, 118(16):4014-4023, 16 Dec 2011
Cited by: 415 articles | PMID: 22180178 | PMCID: PMC3310961
Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma.
Cancer, 119(21):3821-3829, 06 Aug 2013
Cited by: 64 articles | PMID: 23922205
Targeting NRAS in melanoma.
Cancer J, 18(2):132-136, 01 Mar 2012
Cited by: 37 articles | PMID: 22453013
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: P50 CA093459
Grant ID: P50 CA093459-01A1
Grant ID: P30 CA016672





