Abstract
Free full text

Recombinant Dengue Type 2 Viruses with Altered E Protein Domain III Epitopes Are Efficiently Neutralized by Human Immune Sera
Abstract
Humans develop polyclonal, serotype-specific neutralizing antibody responses after dengue virus (DENV) infection. Many mouse antibodies that neutralize DENV bind to the lateral ridge or A strand epitopes on domain III of the viral envelope (EDIII) protein. It has been assumed that these epitopes are also the main target of human neutralizing antibodies. Using recombinant dengue serotype 2 viruses with altered EDIII epitopes, we demonstrate that EDIII epitopes are not the main target of human neutralizing antibody.
TEXT
Dengue viruses (DENV) are emerging, mosquito-borne flaviviruses of humans (10). Some people infected with dengue have asymptomatic or mild disease, while others develop classical dengue fever (DF) or dengue hemorrhagic fever (DHF), which can be fatal (12, 13). The DENV complex consists of 4 distinct but related viruses designated as serotypes. Although infection with one serotype stimulates an adaptive immune response that is highly cross-reactive between serotypes, this response only prevents reinfection with the homologous serotype (13). People experiencing a second infection with a new serotype face a much greater risk of developing DHF because preexisting, cross-reactive immunity can exacerbate disease. A leading theory to explain the association between preexisting immunity and severe disease is antibody-dependent enhancement (ADE), which postulates that cross-reactive, weakly neutralizing antibodies enhance the ability of DENVs to infect Fc receptor-bearing cells (13) and the amount of virions released from each infected cell (32). Antibodies also play a key role in neutralizing DENVs and appear to provide long-term protection from reinfection. Currently, several live attenuated dengue vaccines are being tested in clinical trials. Despite the advanced stage of live DENV vaccine development, we do not know the properties of human antibodies responsible for potent and long-term neutralization following natural infection.
The DENV envelope (E) protein that covers the surface of the virion is the main target of neutralizing antibodies. Each folded E protein molecule contains three distinct domains, designated EDI, EDII, and EDIII (Fig. 1A) (21–23). Most mouse monoclonal antibodies (MAbs) that strongly neutralize DENVs bind to epitopes on the lateral ridge (LR) and A strand of EDIII (Fig. 1B) (26, 28, 30, 31). The LR epitope is poorly conserved between DENV serotypes, and antibodies that target this epitope only bind and neutralize a single serotype (dengue type specific) (8, 31). The A strand epitope is more conserved between serotypes, and antibodies that bind to this epitope usually bind and neutralize more than one serotype (dengue subcomplex) (31). Some antibodies are sensitive to mutations in both the LR and A strand, indicating that the footprints of these antibodies span both epitopes (8, 9, 19, 31).
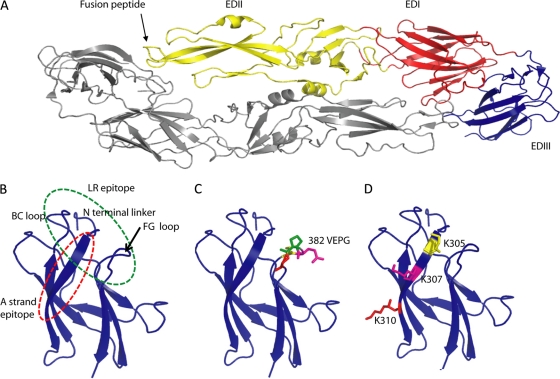
E protein structure of dengue virus type 2 and the location of EDIII mutations. (A) Individual subunits of E protein consist of three beta-barrel domains designated domain I (EDI; red), II (EDII; yellow), and III (EDIII; blue), with the native protein forming a head-to-tail homodimer that lies flat on the surface of the virus (21). (B) Enlarged view of EDIII. The lateral ridge (LR) epitope encompasses the BC loop, N linker region, and FG loop of the EDIII. The A strand epitope is centered on the A strand. (C) The lateral ridge epitope was disrupted by deleting 4 amino acids on the FG loop (382–385) or replacing amino acids 382 to 384 (VEP replaced with RGD) on the FG loop to generate viruses DV2IC20 and DV2IC21, respectively. (D) The A strand epitope was disrupted by replacing lysine at positions 305, 307, and 310 with glutamic acid (E).
As people exposed to DENV infections develop strong, type-specific or subcomplex-neutralizing polyclonal antibody responses, it was plausible that human neutralizing antibodies would also bind to epitopes on EDIII. However, our group and other groups recently demonstrated that people exposed to DENV infection develop low levels of EDIII binding, neutralizing antibody that accounted for 5 to 15% of the neutralizing activity in human immune sera (20, 33). For those studies, we used recombinant EDIII expressed as a fusion protein to deplete EDIII binding antibodies from immune sera and then assessed the neutralization titer of the depleted sera. One potential drawback to this approach is that conformation differences between the recombinant protein used for depletions and EDIII presented on the surface of the virion may lead to incomplete depletion of antibodies. To address this concern, we report here on the ability of human immune sera to neutralize recombinant dengue serotype 2 viruses (DENV2) that contain targeted mutations in the lateral ridge and A strand EDIII epitopes.
The specific amino acid changes introduced to create the recombinant DENV2s and the location of these mutations on the structure of EDIII are shown in Table 1 and Fig. 1C and D, respectively. The recombinant DENV2 (rDENV2) cDNAs were made by site-directed mutagenesis using the parental DENV2 clone, pD2/IC-30P-NBX, which was originally developed using DENV2 strain 16681, as previously described (7, 15). The rDENV2 viruses were derived by transfection of in vitro-transcribed RNA into the C6/36 Aedes albopictus cell line at 28°C, and viral RNAs extracted from resulting viruses were sequence analyzed to verify that the genomes contained the engineered substitutions without other unexpected mutations (15). Viruses were amplified in C6/36 cells with minimal essential medium (MEM) (Gibco) supplemented with 2% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) in the presence of 5% CO2. The FG loop mutant viruses (D2IC20 and D2IC21) replicated well in insect cells and mammalian cells, although replication in mammalian cells was slower than that of the parental virus (7) The A strand mutant virus (D2IC24) also replicated in insect and mammalian cells, although growth in both cell types was delayed compared to that of the parental virus (data not shown). Therefore, the incubation time for each mutant virus used in the neutralization assay was adjusted to obtain a similar number of infected cells as with the parental virus. These parental and recombinant viruses were used in the neutralization assays as described by Wahala et al. with minor modifications (33). Briefly, amounts of viruses predetermined to give a 10-to-15% rate of infection and the monoclonal antibodies or serum samples were incubated at 37°C for 1 h and then added to a human monocytic cell line (U937) engineered to express DC-SIGN, which is an attachment factor for DENVs. After being incubated for 2 h at 28°C, cells were washed with fresh medium and incubated for 48 to 60 h at 28°C. Cells were fixed, permeabilized, and stained with Alexa Fluor 488-conjugated 2H2 dengue antibody. The percentages of infected cells at different antibody concentrations were determined by flow cytometry, and the 50% neutralization titer was determined using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
Table 1
DENV2 EDIII mutant viruses used in the study
| Virus | Mutation | EDIII epitopea | Description |
|---|---|---|---|
| D2IC20 | Deletion of amino acids 382–385 | LR (FG loop) | ΔFG loop mutant |
| D2IC21 | VEP382-384RGD | LR (FG loop) | FG loop substituted |
| D2IC24 | K305E, K307E, K310E | A strand | A strand mutant |
| D2IC30 | None | None | Parental virus |
We used mouse MAbs that have previously been mapped to the LR and A strand epitopes on EDIII to establish that these epitopes were disrupted in the recombinant viruses used for this study. The MAbs used and their contact residues in the EDIII protein are summarized in Table 2. MAbs 1A1D2 and DV2-106 bind to the A strand epitope centered on positions 303 to 305 (303-305), 307, and 310 (3, 18, 30). Replacing lysine at positions 305, 307, and 310 with glutamic acid ablated neutralization by these two MAbs (Fig. 2), demonstrating that the A strand epitope was disrupted in this virus. However, the FG loop mutant viruses were neutralized by MAbs 1A1D2 and DV2-106, in agreement with previous epitope-mapping data, indicating that the FG loop was not a part of the A strand epitope. MAb 9F16 has been mapped to residues 301, 304–305, 327, 329, 383, and 384 (8, 31), which form the lateral ridge epitope on EDIII. All the mutant viruses used in this study but not the wild-type parental virus escaped neutralization by MAb 9F16. Our observation that both the mutations at the center of the lateral ridge (383-385) and the mutations extending into the A strand region led to escape from 9F16 was predicted from previous studies indicating that the 9F16 epitope includes both the LR and the A strand. We conclude that the LR and the A strand epitopes were severely compromised in the mutant recombinant DENV2s selected for this study. The effect was specific for MAbs that have been mapped to these epitopes, because non-EDIII antibodies (MAb DV2-30 and 4G2) neutralized the mutant and parental viruses to similar extents (Fig. 2).
Table 2
Properties of mouse MAbs used in current study
Lateral ridge and A strand epitope mutant viruses escape neutralization by EDIII binding mouse MAbs. Neutralization assays were performed using parental and mutant viruses and a panel of mouse MAbs that bind to different regions of E protein. The graph displays the mean percentages of neutralization from two independent experiments for each MAb used at a concentration of 10 μg/ml. Table 2 displays the binding sites of the MAbs used here. MAbs DV2-30 and 4G2 bind to epitopes on EDII. MAbs 1A1D2 and DV2-106 bind to the A strand of EDIII. MAb 9F16 binds to the lateral ridge epitope on EDIII. An epitope was considered disrupted when mutations led to nearly complete neutralization escape from an antibody known to bind to the epitope.
Previous studies have demonstrated that most DENV-neutralizing antibodies in human sera were not directed to epitopes on EDIII (20, 33). These studies were based on the use of bacterially expressed recombinant EDIII antigen to deplete EDIII antibodies in human sera. It is conceivable that the bacterially produced protein may not deplete all EDIII A strand and LR antibodies because of structural differences between recombinant EDIII and native EDIII expressed on the surface of the virus. As an alternate approach to assess whether the human neutralizing antibody response was directed to EDIII LR and A strand epitopes, we compared the ability of human dengue immune sera to neutralize the recombinant viruses with mutations in the LR and A strand epitopes. For these studies, we selected 4 dengue immune sera from individuals exposed to primary DENV2 infections (Table 3). All 4 sera neutralized DENV2 to high titers and showed undetectable or lower titers to the other serotypes. Serially diluted serum samples were tested to determine the neutralization titers against each of the mutant viruses, as well as the parental virus. As displayed in Fig. 3A, the 50% neutralization titers for each of the serum samples against the mutant viruses were not significantly different (P > 0.05 at 95% confidence interval) from that of the parental virus. These results demonstrate that LR and A strand epitopes targeted by strongly neutralizing mouse antibodies are not the main target of human neutralizing antibodies.
Table 3
Neutralization properties of human sera used in current study
| Serum | 50% neutralization titera | Inferred infecting serotype | |||
|---|---|---|---|---|---|
| DENV1 | DENV2 | DENV3 | DENV4 | ||
| 01 | <20 | 271 | <20 | 41 | DENV2 |
| 02 | 28 | 320 | 88 | 167 | DENV2 |
| 03 | <20 | 650 | 22 | 24 | DENV2 |
| 04 | 34 | 526 | 27 | 47 | DENV2 |
EDIII lateral ridge and A strand epitope mutant viruses remain sensitive to neutralization by dengue immune human sera. (A) Neutralization assays were performed using parental and mutant viruses and 4 immune sera from people exposed to DENV2 infections. Serially diluted serum samples were used to calculate the 50% neutralization titer against each virus. The mean 50% neutralization titers are displayed in the graph. The parental and mutant viruses were equally sensitive to neutralization by the DENV 2 monotypic sera (P > 0.05; confidence interval [CI], 95%). The properties of the sera used in the study are summarized in Table 3. (B) Neutralization assays were performed using parental and mutant viruses and serum from a rhesus macaque immunized with an alphavirus vector expressing dengue 2 E ectodomain (E residues 1 to 424). Macaque serum was diluted to 1:40, and the percentage of neutralization was calculated for each virus. At a 1:40 dilution, lateral ridge mutant viruses (D2IC20 and D2IC21) were significantly more resistant to neutralization than the parental and A strand mutant viruses (D2IC30 and D2IC24) (P < 0.001; CI, 95%).
We performed a control experiment to determine whether a polyclonal neutralizing antibody response directed against EDIII was influenced by mutations in the LR and A strand epitopes. For this experiment, we used serum from a rhesus macaque immunized with an alphavirus vector expressing the ectodomain of DENV2 E protein (residues 1 to 424), which induces EDIII neutralizing antibodies (L. J. White, V. Yingsiwaphat, W. M. P. B. Wahala, and A. M. de Silva, unpublished data). The serum was tested for its ability to neutralize parental and EDIII LR- and A strand mutant viruses. As depicted in Fig. 3B, the parental virus and the A strand mutant viruses were neutralized by the EDIII-targeted vaccine serum. In contrast, the LR mutant viruses were no longer neutralized by the EDIII vaccine serum. Further studies are needed to understand why immunization with the ectodomain of E protein stimulated EDIII lateral ridge but not A strand binding neutralizing antibodies, especially because some groups are pursuing recombinant E protein antigens as dengue vaccine candidates (2, 11, 14). We conclude that while EDIII-targeted immunizations can stimulate an LR-focused neutralizing antibody response, most neutralizing antibodies in people exposed to DENV2 infections are not directed against these epitopes.
Investigators have used the recombinant-antigen-based antibody depletion method to demonstrate that neutralizing antibodies that develop after natural infection with dengue and West Nile virus are not mainly directed to well-studied neutralizing antibody epitopes on EDIII (20, 27, 33). A significant concern with the antibody depletion approach is that conformational differences between the recombinant protein antigens and the native protein on the virion may lead to incomplete depletion of EDIII antibodies. Here, we used the alternate method of genetic alteration of key epitopes on EDIII to test whether these epitopes were the main target of human neutralizing antibodies. We used mutant DENVs with multiple amino acid substitutions or deletions to drastically alter the structure of these epitopes. Our results are consistent with the conclusions of antibody depletion studies and indicate that LR and A strand epitopes on EDIII are not the main target of human neutralizing antibodies. Studies with West Nile virus reporter virus particles (RVP) containing mutations in EDIII also indicated that West Nile virus-infected people develop very low levels of EDIII-focused neutralizing antibodies (24, 25, 27).
Several groups have recently reported on the properties of human MAbs generated from dengue virus- and West Nile virus-immune subjects (1, 5, 6, 16). Although EDIII LR and A strand binding human MAbs have been identified, most human antibodies that strongly neutralize DENVs bind to other epitopes (1, 5, 6, 16). It is unclear whether the preponderance of EDIII-directed neutralizing antibodies isolated from mice compared to the ratio in humans is a result of inherent species differences or a result of comparing mice immunized with dengue antigens or virions to naturally infected humans. Studies are currently in progress to compare antibody responses in mice and humans infected with DENV (Katherine Williams, W. M. B. P. Wahala, Aravinda de Silva, and Eva Harris, unpublished data). In summary, a growing body of work now demonstrates that serotype-specific, strongly neutralizing antibody responses that people develop after natural DENV infections recognize epitopes that are distinct from the well-studied LR and A strand epitopes engaged by strongly neutralizing mouse antibodies.
ACKNOWLEDGMENTS
We thank Michael Diamond from Washington University, St. Louis, MO, for MAbs DV2-106 and DV2-30 used in the current study.
These studies were supported by NIH grant no. U54 AI057157 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.06871-11
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/86/7/4019.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.06871-11
Article citations
The dengue-specific immune response and antibody identification with machine learning.
NPJ Vaccines, 9(1):16, 20 Jan 2024
Cited by: 4 articles | PMID: 38245547 | PMCID: PMC10799860
Immune-Mediated Pathogenesis in Dengue Virus Infection.
Viruses, 14(11):2575, 21 Nov 2022
Cited by: 15 articles | PMID: 36423184 | PMCID: PMC9699586
Review Free full text in Europe PMC
Innate Immune Response to Dengue Virus: Toll-like Receptors and Antiviral Response.
Viruses, 14(5):992, 07 May 2022
Cited by: 11 articles | PMID: 35632732 | PMCID: PMC9147118
Review Free full text in Europe PMC
Discovery of B-cell epitopes for development of dengue vaccines and antibody therapeutics.
Med Microbiol Immunol, 211(1):1-18, 21 Jan 2022
Cited by: 2 articles | PMID: 35059822
Review
Subdominance in Antibody Responses: Implications for Vaccine Development.
Microbiol Mol Biol Rev, 85(1):e00078-20, 25 Nov 2020
Cited by: 9 articles | PMID: 33239435 | PMCID: PMC7709521
Review Free full text in Europe PMC
Go to all (40) article citations
Protocols & materials
Related Immune Epitope Information - Immune Epitope Database and Analysis Resource
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antibodies targeting dengue virus envelope domain III are not required for serotype-specific protection or prevention of enhancement in vivo.
Virology, 429(1):12-20, 25 Apr 2012
Cited by: 66 articles | PMID: 22537810 | PMCID: PMC3683589
Engineered Dengue Virus Domain III Proteins Elicit Cross-Neutralizing Antibody Responses in Mice.
J Virol, 92(18):e01023-18, 29 Aug 2018
Cited by: 30 articles | PMID: 29976679 | PMCID: PMC6146717
Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions.
Proc Natl Acad Sci U S A, 109(19):7439-7444, 12 Apr 2012
Cited by: 269 articles | PMID: 22499787 | PMCID: PMC3358852
The human antibody response to dengue virus infection.
Viruses, 3(12):2374-2395, 25 Nov 2011
Cited by: 196 articles | PMID: 22355444 | PMCID: PMC3280510
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R56 AI097560
Grant ID: U54 AI057157
 a
a




