Abstract
Free full text

Nitric oxide in adaptation to altitude
Abstract
This review summarizes published information on levels of nitric oxide gas (NO) in the lungs and NO-derived liquid phase molecules in the acclimatization of visitors newly arrived at altitudes of 2500m or more and adaptation of populations whose ancestors arrived thousands of years ago. Studies of acutely exposed visitors to high altitude focus on the first 24–48 hours with just a few extending to days or weeks. Among healthy visitors, NO levels in the lung, plasma and/or red blood cells fell within three hours, but then returned toward baseline or slightly higher by 48 hours, and increased above baseline by 5 days. Among visitors ill with high-altitude pulmonary edema at the time of the study or in the past, NO levels were lower than their healthy counterparts. As for highland populations, Tibetans had NO levels in the lung, plasma and red blood cells that were at least double and in some cases orders of magnitude greater than other populations regardless of altitude. Red blood cell associated nitrogen oxides were more than two hundred times higher. Other highland populations had generally higher levels although not to the degree showed by Tibetans. Overall, responses of those acclimatized and those presumed to be adapted are in the same direction although the Tibetans have much larger responses. Missing are long-term data on lowlanders at altitude showing how similar they become to the Tibetan phenotype. Also missing are data on Tibetans at low altitude to see the extent to which their phenotype is a response to the immediate environment or expressed constitutively. The mechanisms causing the visitors’ and the Tibetans’ high levels of NO and NO-derived molecules at altitude remain unknown. Limited data suggest processes including hypoxic upregulation of NO synthase gene expression, hemoglobin-NO reactions and genetic variation. Gains in understanding will require integrating appropriate methods and measurement techniques with indicators of adaptive function under hypoxic stress.
Introduction
This review summarizes and evaluates published information on levels of nitric oxide in the lungs and circulation of people at altitudes above 2500m and, when available, the causal mechanisms or functional consequences. It discusses methodological issues, describes the effects of acute exposure on measures of NO among visitors and among populations indigenous to high altitude. The aim is to determine if there is a scientific consensus on the effect of high altitude on levels of NO and to identify research needed to discover its roles in offsetting the severe stress of high altitude hypoxia.
In 1990, Gustafsson, Persson, Moncada and collaborators reported that hypoxia decreased pulmonary NO and caused vasoconstriction of isolated rabbit lungs. They suggested their findings could account for the puzzling, yet well-known hypoxic pulmonary vasoconstriction response to high altitude [1, 2]. In 1996, Scherrer and collaborators reported that inhaled NO reduced pulmonary artery pressure and improved oxygen saturation among patients ill with high altitude pulmonary edema (HAPE), a maladaptation characterized by exaggerated hypoxic pulmonary vasoconstriction [3]. Since then, an accumulating body of data has demonstrated that NO in various forms and locations in the body plays roles at all levels of the oxygen delivery cascade from the pulmonary to the cardiovascular, hematological and mitochondrial [4–11]. The role of NO in oxygen delivery under the stress of high altitude hypoxia is an area of active investigation because of the potential for improving understanding of human biology and health. A first step toward understanding is establishing how much is available, where it is located and in what form.
Background on Nitric Oxide
Nitric oxide (NO), originally described as endothelium-derived relaxation factor, is a product of the NO synthases (NOS), which convert L-arginine to NO and L-citrulline in a reaction that requires oxygen, NADPH, and cofactors FAD, FMN, tetrahydrobiopterin. NOS enzymes include neuronal, inducible and endothelial forms (nNOS, iNOS and eNOS, respectively) [12]. The nNOS and eNOS are generally expressed in the brain and the vascular endothelium, while iNOS is constitutively expressed in respiratory epithelium and is upregulated in other tissues in response to numerous factors including inflammation and hypoxia [13]. An increased expression of iNOS and eNOS under hypoxia through the activation of gene expression by hypoxia-inducible transcription factors (HIF1 and HIF2) leads to the expectation of more NO, but NO production in the lung decreases immediately under acute hypoxia, which may be due to limitation of oxygen substrate for the reaction [14]. A NOS independent pathway for generating NO operates through the reduction of nitrate and nitrite [11, 15, 16]. Thus, a priori, the effect of high hypoxia on nitric oxide synthesis, metabolism and clearance is uncertain.
The vascular effects of NO are generally attributed to eNOS-derived NO production and its reaction products. Once produced in the endothelium, NO gas is freely diffusible and enters smooth muscle cells to activate soluble guanylate cyclase to produce 3′, 5′-cyclic monophosphate [cyclic GMP (cGMP)], which leads to vasodilatation. Nitrogen oxides measurable in blood, saliva, urine, and broncholavage, can be biologically active. They include nitrite (NO2−), nitrate (NO3−), iron nitrosyls (FeNO), and S-nitrosothiols (SNO) such as SNO-hemoglobin. Hemoglobin allostery can regulate interconversion of nitrogen oxide forms and their biological activity [8–11]. NO metabolism also leads to nitration of tyrosine in proteins in tissue and blood [17]. Methods to measure nitrogen oxides have been well described [11, 18–22]. Methods to measure gas phase NO are discussed in some detail because measures of gases require instruments validated for use at altitude.
Materials and Methods
Selection of studies for review
Database searches linking altitude and nitric oxide and human identified 32 published articles [Table 1]. The databases were Annual Reviews, Article First, BIOSIS, CINAHL, ClinicalTrials.gov, Dissertation Abstracts, PubMed (Medline), Science Direct, SCOPUS, SPORTDiscus, TOXNET, Worldcat, and Web of Science. Abstract screening eliminated articles using other organisms or conducted in hypoxia chambers or tents. Studies at altitudes of 2500m were included based on the evidence that many responses to acute exposure are first manifest above that altitude [23] and on convention in the field of high altitude human biology [24]. Table 1 summarizes the altitude exposure, sample, and measurement method of the studies retained for review.
Table 1
Summary of studies reviewed
| Source | Altitude and length of exposure | Sample composition | Measurement techniques (footnotes indicate sensor or chemical analysis) | |||||
|---|---|---|---|---|---|---|---|---|
| Ethnicity or Country | Health Status | Number of Males | Number of Females | Total Number of People | Average Age or Age Range | |||
| ACUTE EXPOSURE < 24 hours | ||||||||
| Bailey et al. (2010). The Journal of Physiology 588 (Pt 23): 4837–4847. | 3 and 20 hours, after travel from 110 to 4559m | Ethnicity unspecified, Germany | Healthy, non-smoking | 32 | 6 | 38 | 37 years | NO-derived products [NOx] in plasma and red blood cells a |
| Berger et al. (2009). High Altitude Medicine & Biolog 10(1): 17–24. | 3 and 20 hours, after travel from 110 to 4559m | Ethnicity unspecified, Germany | Healthy, non-smoking, including some HAPE-S | 29 | 5 | 34 | 37 years | plasma NOx a |
| Brown et al (2006). Am J Hum Biol 18(2): 196–202. | 4200m, 3 hours | Ethnicity unspecified, U.S. | Healthy, non-smoking | 24 | 23 | 47 | ~45 years | offline exhaled breath a |
| ACUTE EXPOSURE 24–48 HOURS | ||||||||
| Duplain et al. (2000). American Journal of Respiratory and Critical Care Medicine 162(1): 221–224. | 4559m, 48 hours | Ethnicity unspecified, Switzerland | HAPE-S mountaineers | 18 | 10 | 28 | 40 years | offline, exhaled NO a |
| Ethnicity unspecified, Switzerland | HAPE-R mountaineers | 15 | 9 | 24 | 38 years | |||
| Mansoor et al. (2005). High Altitude Medicine & Biology 6(4): 289–300. | 4342m, 24 hours | Ethnicity unspecified, U.S | Healthy, active | 7 | 0 | 7 | 37 years | L-arginine supplement, Breath condensate NO2−, NO3− a |
| Sartori et al (1999). Lancet Infectious Diseases 353(9171): 2205–2207. | 4559m for 48 hours | Ethnicity unspecified, Switzerland | experienced perinatal insult | 10 | 21 years | Breathed 40 ppm NO for 20 minutes, change in pulmonary artery pressure [ΔPAP] | ||
| Ethnicity unspecified, Switzerland | No history of perinatal complicatons | 10 | 21 years. | |||||
| Scherrer et al. (1996). New England J Med 334(10): 624–629. | 4559m, 48 hours | Ethnicity unspecified, Switzerland | HAPE-S mountaineers | 15 | 3 | 18 | 42 years | Breathed 40 ppm NO for 15 minutes, ΔPAP |
| Ethnicity unspecified, Switzerland | HAPE-R mountaineers | 13 | 5 | 18 | 42 years | |||
| Schneider et al. (2001). European Respiratory J 18(2): 286–292. | 4350m, 36 hours after travel from 35m | Ethnicity unspecified, France | Healthy | 8 | 3 | 11 | 28 years | L-arginine infusion, Δ L-citrulline and cGMP d |
| Swenson et al. (2002). JAMA 287(17): 2228–2235. | 4559m, 48 hours | Lowlanders, Switzerland | HAPE-S | 12 | 24–52 years | Bronchoalveolar lavage [BAL], NOx a | ||
| Lowlanders, Switzerland | 6 HAPE-R | 10 | 24–52 years | |||||
| ACUTE EXPOSURE 2 to 30 days | ||||||||
| Donnelly et al. (2011). Respiratory Physiology & Neurobiology, 177(3), 213–7. | 0 to 5050m over an estimated 20 days | New Zealand | Healthy nonsmokers | 7 | 4 | 11 (9 analyzed) | 32 years | online, exhaled NO c |
| Janocha et al., (accepted for publication). New England J Medicine. | 1300 to 5050m over 19 days | Italy, US | Not stated | 10 | 8 | 18 | Not stated | serum, saliva, urine NOx a |
| Levett et al. (2011). Scientific Reports 1 | 75m to 5300m over 30 days | European | Healthy | 18 | 6 | 24 | 19–59 years | plasma NOx a,g |
| Liu et al. (1999) Chinese Journal of Applied Physiology 15(1): 47–49. | 3658m, 5 days compared with lowlanders | Ethnicity unspecified, China | Healthy | 60 | 18–21 years | plasma NOx b, k | ||
| Ethnicity unspecified, China | Healthy | 70 | 18–21 years | |||||
| Schena et al (2002). Journal of Sports Medicine and Physical Fitness 42(2): 129–134. | 3100m, 8 days | European lowlanders: trained skiers | Active, physically trained, nonsmokers | 9 | 0 | 9 | 26 years | plasma NOx b |
| European lowlanders: untrained men | Active nonsmoker | 6 | 0 | 6 | 32 years | |||
| Vinnikov et al. (2011). Respiratory Physiology & Neurobiology 175(2): 261–264. | 4000m, 14 or 21 days | Kyrgyz and European lowlanders, Kyrgyzstan | 73 | 81 | 126 (81 analyzed) | 32 years | online, exhaled NO c | |
| ACUTE EXPOSURE UNSPECIFIED TIME, patients | ||||||||
| Ahsan et al. (2006). Chest 130(5): 1511–1519. | 3500m, unspecified and sea level | Ethnicity unspecified, India | HAPE patients | 72 | 0 | 72 | 30–40 years | plasma NOx b |
| Same unstated ethnicity, India | HAPE-R | 60 | 0 | 60 | 30–40 years | |||
| Anand et al. (1998). Circulation 98(22): 2441. | 3600m, unspecified | India | HAPE | 14 | 0 | 14 | 29 years | Breathed NO at 15 ppm for 30 mins, ΔPAP |
| HIGH ALTITUDE RESIDENTS & NATIVES | ||||||||
| Ahsan et al. (2005). Annals of Human Genetics 69(Pt 3): 260 | 3400m and low, native residents | Ethnicity unspecified Highlander monks, India | 131 | 131 | 46 years | Plasma NOx b | ||
| Ethnicity unspecified Highlander, India | control | 136 | 136 | 21 years | ||||
| Ethnicity unspecified Lowlanders at low, India | Control | 170 | 170 | 30 years | ||||
| Ahsan et al (2004). Thorax 59(11): 1000–1002. | 3400m, native residents | Ethnicity unspecified lowlanders, India | HAPE | 59 | 59 | 30–40 years | Plasma NOx b | |
| Ethnicity unspecified highlanders, India | control | 136 | 136 | 30–40 years | ||||
| Ethnicity unspecified Lowlanders same ethnicity, India | control | 170 | 170 | 30 years | ||||
| Appenzeller et al. (2006). Stroke 37: 1754–1758. | 3622 m residents and 794m for 24 hrs in | High-altitude Amhara, Ethiopia | No history of chronic disease | 9 | 9 | 36 years | Sublingual pharmaceutical NO donor: isosorbide dinitrate in Peru, nitroglycerin in Ethiopia | |
| Ethiopia; 4388m residents and 150m for 24 hrs in Peru | High-altitude residents, Peru | 5 healthy, 4 with CMS | 9 | 9 | 37 years | |||
| Beall et al. (2001). Nature 414:411–412. | 4200m in Tibet, 3900m in Bolivia, 272m in U.S., native residents | Tibetan | Healthy, non-smokers | 45 | 62 | 105 | 8–56 years | offline, exhaled NO a |
| Bolivian Aymara U.S. | Healthy, non-smokers | 67 | 78 | 144 | 8–56 years | |||
| U.S. | Healthy, non-smokers | 13 | 20 | 33 | 32/31? years | |||
| de Bisschop et al. (2010). Nitric Oxide 23(3): 187–193 | 4000m lifelong, sea level lifelong, 3750m 4 days | Highlanders, Bolivia | 10 with CMS, 8 healthy | 14 | 4 | 18 | 45 years | pulmonary NO diffusing capacity, NO/CO transfer technique |
| European | healthy | 8 | 8 | 16 | 36 years | |||
| Droma et al. (2006) High Altitude Medicine and Biology 7(3): 209–220. | 3440m and 1300m lifelong | Sherpas, Nepal | Normal blood pressure | 44 | 61 | 105 | ~31 years | serum NOx d |
| Non-Sherpa Nepalis | Normal blood pressure | 53 | 58 | 111 | 30 years | |||
| Erzurum et al. 2007.PNAS 104(45):17593. | 4200m, lifelong, indigenous population | Tibetan | Healthy h | 25 | 63 | 88 | 31 years | plasma and Red blood cells, NO2−, NO3−, FeNO and nitrosoproteins [RXNO] b, i |
| U.S. lowlanders | 23 | 27 | 50 | 37 years | ||||
| Gao et al. (2005). Comparative Biochemistry and Physiology, 141: 93–100. | 3658 lifelong and long-term residents | Tibetan and Han Chinese | Women at full-term delivery | 0 | Not stated | Not stated | Not stated | umbilical vein endothelial cells, HIF-1A, VEGF, eNOS and iNOS mRNA e |
| Ge et al. (2011). American Journal of Physiology. 300(4): H1427–1433. | 4300m, some lifelong, some 10 years | Tibetan | Chronic Mountain Sickness (CMS) | 8 | 42 years | serum eNOS f | ||
| Han Chinese | 16 | 42 years | ||||||
| Tibetan | Healthy | 40 | 39 years | |||||
| Han Chinese | 10 | 39 years | ||||||
| Gonzales et al. (2011). Endocrine. E pub | 4340m and 150m | high-altitude residents for 37–38 years, Peru | excessive erythrocytosis | 33 | 33 | 43 years | serum NOx b | |
| Healthy | 29 | 29 | 45 years | |||||
| Low-altitude residents for 34 years, Peru | Healthy | 30 | 30 | 43 years | ||||
| Hoit et al. (2005). J Appl Physiol 99:1796–1801. | 4200m, lifelong, indigenous population | Tibetan | Healthy h | 20 | 37 | 57 | 31 years | online, exhaled NO a |
| U.S. lowlanders | Healthy | 7 | 13 | 20 | 47/39 years | |||
| Hoit et al. (2011). Am J Hum Biol 23:168–176. | 3700m, 1200m, 282m lifelong | Highland Amhara, Ethiopia | Healthy h | 53 | 22 | 76 | 31 years | Urine a |
| Lowland Amhara, Ethiopia | Healthy h | 45 | 9 | 54 | 30 years | |||
| U.S. lowlanders | Healthy h | 21 | 25 | 46 | 35 years | |||
| Jayet et al. (2010). Circulation 122(5): 488–494. | 3600m lifetime | Aymara highlanders, Bolivia | Mothers were pre-eclamptic | 24 | 24 | 48 | 13 years | inhaled 40 ppm NO for 20 minutes, ΔPAP |
| Mothers had normal pregnancy | 55 | 35 | 90 | 14 years | ||||
| Julian et al. (2008) Am J Physiol Regul Integr Comp Physiol 295(3): R906–915. | 3100m, 1600m, resident | high-altitude resident, U.S. | Pregnant and post-partum | 0 | 25 | 25 | 26 years | serum NO, NO2−, NO3−, and nitrosothiol a |
| Low-altitude resident, U.S. | Pregnant and post-partum | 0 | 18 | 18 | 28 years | |||
| Schwab et al. (2008). High Altitude Medicine & Biology 9(4): 295. | 3600m–4000m lifelong residents or lowlanders with 6 years of residence | Aymara highlanders, Bolivia | healthy | 24 | 10 | 34 | 36 years | online, exhaled NO c |
| European lowlanders, Bolivia | healthy | 21 | 13 | 34 | 38 years | |||
| Stuber et al. (2008). Chest 134(5): 996. | 3600m–4000m lifelong residents or upward migrants with 2+ years of residence and 450m | Aymara highlanders, Bolivia | Healthy and free of infirmity | 105 | 95 | 200 | 9.5 years | online, exhaled NO c |
| European highlanders, Bolivia | Healthy and free of infirmity | 42 | 35 | 77 | 9.5 years | |||
| Lowlanders, Switzerland | Healthy and free of infirmity | 16 | 13 | 21 | 8.8 years | |||
| Teran et al. (2009). “International Journal of Gynecology & Obstetrics 104(2): 140–142 | 2800m, resident | High-altitude resident, Ecuador | Healthy pregnant term | 0 | 30 | 30 | ~20 years | plasma, placenta NOx a |
| High-altitude resident, Ecuador | Pre-eclamptic | 0 | 33 | 33 | ~20 years | |||
| Low-altitude resident, Ecuador | Healthy pregnant term | 0 | 30 | 60 | ~20 years | |||
| Pre-eclamptic | 0 | 30 | ||||||
Study outcomes are summarized for comparison using a calculated variable called an effect size, d. The advantage of this approach is that ‘d’ can compare studies with different designs, outcome variables, and sample sizes to estimate the magnitude of the treatment effect (altitude, for example) on the outcome variable (total lung nitric oxide or pulmonary artery pressure, for example). Cohen’s ‘d’ is calculated as the difference between two means divided by the “square root of the mean of the two variances” [25 p. 44]. That is, ‘d’ quantifies the size of the difference between two means in terms of the number of their pooled standard deviations. For example, an effect size ‘d’ of 1.0 describes a situation where fewer than half the observations in two samples overlap whereas an effect size ‘d’ of 0.2 describes a situation where more than 80% of the observations overlap. By convention, d’s of 0.8 or more are considered large, d’s of 0.5 are considered medium effect and those of 0.2 are considered small effects. Because there are no conventions for effect sizes in the range from 1 to more than 5 that are encountered here, this review will provide those effect size values. Positive effects indicate that the characteristic was higher at high altitude; negative effects indicate that the characteristic was lower at high altitude. An effect size calculator is available at http://www.uccs.edu/~faculty/lbecker/ (accessed October 1, 2011).
Technical considerations for measurement of NO in exhaled gas at high altitudes
Detecting gas phase molecules requires instruments that provide valid measurements at the generally lower humidity, temperature and barometric pressure found at altitudes above 2500m in the low-altitude laboratory or clinic for which most instruments are designed. Ambient conditions at morning calibrations in one of our studies illustrate the contrast: 14.4°C average temperature, 52% relative humidity, and 464 Torr barometric pressure at 4200m in the field laboratory in Tibet as compared with warmer and dryer conditions of 23°C, 29% relative humidity, and 754 Torr barometric pressure in a climate-controlled room in Cleveland [26, 27]. These environmental contrasts can confound interpretations.
Five sensor technologies detect gas phase molecules of NO: chemiluminescence, optical spectroscopy, mass spectrometry, chromatography, and electrochemistry. Two technologies are commercially available and have been used to measure NO at high altitude: the Sievers NOA (GE Analytical, Boulder, CO) and the NIOX MINO® (Aerocrine, New Providence, NJ). The Sievers NOA uses a chemiluminescence sensor and the NIOX MINO® uses disposable electrochemical sensors. The Sievers NOAi analyzer is based on a gas phase chemiluminescent photomultiplier tube that detects photon emission from electronically excited nitrogen dioxide produced from the reaction between nitric oxide and ozone in the reaction chamber. While the design of the photomultiplier housing system and reaction chamber compensates for any change in external barometric pressure, variation in the atmospheric pressure can influence the sampling flow rate through the restrictor of the sensor and thus lead to lower levels of the NO signal. To avoid this artifact, calibration at the prevailing barometric pressure with standard NO gas is required so that any change in the sampling flow rate is accounted for in the collection and measure of NO at altitude. The Sievers NO analyzer measures gas phase NO with a sensitivity of less than 1 part per billion (ppb) and liquid phase NO-derived molecules with a sensitivity of 1 picomole [28].
The NIOX MINO® sensor is electrochemical. Details of the electrochemistry are not available, however this type of sensor reacts with gas phase molecules that diffuse into a container where electrodes sit in a solution designed to detect NO. Because electrochemical sensor technology depends on diffusion to drive the gas into the reaction chamber, changes in atmospheric pressure influence NO detection. Lower air pressure at altitude results in less gas diffusion into the reaction chamber, and reduces the accuracy of the measurement. The technical specifications of the instrument list an operating range of barometric pressures from 700 to 1060 hPa or from about 3100m to below sea level. Yet a 2009 paper identified factors that cause the readings to be inaccurate at high altitudes and warned against using the device above 1000m [29]. That paper suggested using two correction factors based on measurement of nine people in a temperature-controlled hypobaric chamber. The manufacturer describes accuracy as ± 5 ppb or max 10% (http://www.aerocrine.com/en-us/niox-mino/Specifications/ accessed June 27, 2011). In addition, relative humidity and temperature can affect accuracy of some electrochemical reactions.
Calibration is essential when equipment is moved from one altitude to another. The Sievers NOA calibration and measurement of NO occurs in the reaction chamber containing the photomultiplier tube maintained at constant pressure, humidity and temperature. The manufacturer recommends two point calibrations using air free of NO (obtained with a filter that removes NO) and a high NO concentration chosen by the investigator (such as 45ppm). In contrast, the NIOX MINO® does not calibrate. The company recommends an external quality control using an individual with steady and stable exhaled nitric oxide levels. This is problematic for investigations designed to test hypotheses about changes in exhaled NO at different altitudes. Thus, the NIOX MINO® is not recommended for studies across altitudes.
Because of these considerations, this review reports only gas phase measures obtained by chemiluminescence. For the future, it will be important to a) publish details of electrochemical analyses and their sensitivities to temperature and other relevant environmental factors and b) to simultaneously compare electrochemical and chemiluminescence measures under field conditions.
Sample collection for NO analysis
NO is present in the exhaled breath of humans (3). The use of chemiluminescence analyzers allowed for the detection of NO in exhaled breath in the early 1990s and ultimately the use of exhaled NO as a clinical test [30–32]. The methods and equipment for measuring NO in exhaled breath were standardized for clinical pulmonary function laboratories. The standardized ‘online’ measure of NO is useful for clinical testing for airway disease, but other experimental methods remain in place and are optimal for study of NO at high altitudes.
The online technique measures airway NO in the early part of exhalation during a ten-second exhalation at 50 mL/sec that is the standard method recommended for clinical pulmonary function labs. hus the measurements represent the level of NO in the first 500–750 mL of exhaled gases from the conducting airways [33]. In contrast, the technique of offline collection of exhalate in non-permeable mylar balloons measures exhaled NO in the vital capacity lung volume, including NO derived from alveolar spaces and vascular bed, and is recommended for evaluation of NO at altitude. A breath hold maneuver increases the sensitivity for detection of vital capacity NO [34, 35].
The online-offline distinction is important because the two methods inform about different locations. Studies may reach opposite conclusions depending on the method of collection. Figure 1A compares the cumulative frequency distributions of exhaled NO measured online at a rate of 50 mL/s for a US sample at low altitude and two Tibetan samples at different high altitudes. Roughly half the low altitude sample exhaled 15nmHg or more as compared with about 20% of the Tibetans at 4200m and none of the Tibetans at 4700m. The data reflect less NO production in the conducting airway of the respiratory tract among high altitude Tibetans that decreases with altitude. Data on low altitude Tibetans are needed to determine airway production in normoxia.

A. A low altitude U.S. sample exhaled more NO and a wider range of values from the conducting airways (measured online at a flow rate of 50 ml/s into the NO analyzer) than a Tibetan sample at 4200m that in turn exhaled more than a Tibetan sample at 4700m. The x-axis marks intervals of 5nm Hg NO and the y-axis the percent of observations in the interval or lower.
B. A Tibetan sample at 4200m contained more NO in the vital capacity volume collected from exhaled breath and a wider range of values in the total lung exhalate (measured in the total volume of breath exhaled into a mylar collection bag after a 15s breathhold, i.e. offline) than a Tibetan sample at 4700m that in turn exhaled more than a low-altitude U.S. sample.
The relative positions of the samples are reversed in Figure 1B comparing offline collection of exhaled breath NO measurements after a 15-second breath hold. Offline measurements represent the equilibration of the pools of NO in the pulmonary vascular bed, alveolar space and in the conducting airways and therefore provide insight into the total NO of the lung [34, 35], and its conduction and transfer [36]. Roughly half the Tibetans at 4200m exhaled more than 10 nmHg NO as compared with roughly 10% of the Tibetans at 4700m and none of the lowlanders at low altitude. The lower values for the Tibetan sample at 4700m reflect oxygen sensitivity of the NOS or substrate limitation [37].
The contrast in Figures 1A and B starkly illustrates that sample collection techniques can lead to different conclusions. This paper uses the term ‘airway NO’ for measures obtained using online techniques and the term ‘vital capacity NO’ for measures obtained using offline techniques. Airway NO among Tibetans at 4200m is lower than among low-altitudes controls while vital capacity NO is higher. Regional heterogeneity of NO levels in the lung probably accounts for these findings [36].
Units of measurement and reporting
NO analyzers report measures in parts per billion (ppb) in the exhaled breath. However, converting to and reporting exhaled NO as the partial pressure of exhaled gas is appropriate for comparing levels across altitudes where barometric pressures affect the measure of gas in a volume. For example, we published concentration in parts of NO per billion (ppb) in a paper comparing Tibetan and Andean highlanders with low altitude [37]. Because the Tibetan and Andean samples were collected at roughly the same altitude (4200m and 3900m with similar barometric pressures of 467 and 484 mmHg, respectively) the Tibetan-Andean comparison of vital capacity NO reported there had a straightforward interpretation: Tibetans had higher vital capacity NO than Andean highlanders. The barometric pressure differences confound interpretation of high and low altitude differences. While Tibetans’ vital capacity NO in mm Hg was higher than lowlanders, Bolivian Aymara exhaled significantly more than lowlanders in ppb but there was only a trend toward differences in nmHg. Figures 2A and B illustrate this with a comparison of plots in units of ppb and nmHg.
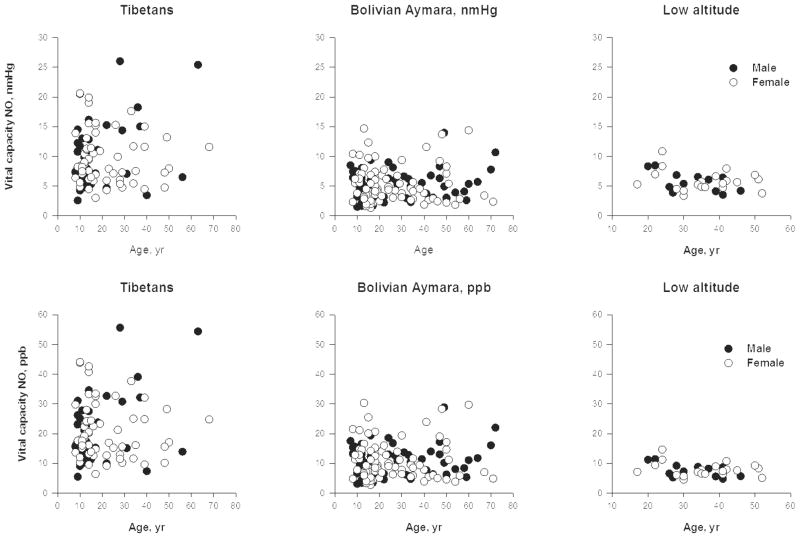
Top. Tibetans at 4200m exhaled more NO than Bolivian Aymara at 3900m who did not differ significantly from a U.S. low-altitude sample when reported as partial pressure of NO in vital capacity exhalate. The respective geometric means were 8.7 nmHg with a range of 2.6 to 26 among Tibetans, 4.6 nmHg with a range of 1.3 to 14.7 nmHg among Aymara, and 5.5 nmHg with a range from 3.3 to 10.8 nmHg among lowlanders.
Bottom. Tibetans at 4200m exhaled more NO than Bolivian Aymara at 3900m who in turn exhaled more than a U.S. low-altitude sample when reported as the concentration of NO in exhalate. The respective geometric means were 18.6 ppb with a range of 5.5 to 55.7 among Tibetans, 9.5 ppb with a range of 2.7 to 30.3 ppb among Aymara, and 7.5 ppb with a range from 4.5 to 14.6 ppb among lowlanders. Redrawn from [1] with permission of the publishers.
This review converts values published in ppb to nm Hg if the authors reported barometric pressure. Partial pressure of NO in nm Hg is calculated as follows: reported NO in ppb multiplied by ambient barometric pressure in mmHg and divided by1000. Using this calculation, a reading of 10 ppb obtained at sea level with barometric pressure 760 mm Hg and at 4200m with barometric pressure 464 mm Hg corresponds to a partial pressure of NO of 7.6 nm Hg at sea level and 4.6 nm Hg at 4200m, i.e. there are fewer NO molecules in the measured volume at high altitude owing to the lower barometric pressure. Reports of studies at high altitude should include barometric pressure [38].
Sources of NO apart from synthesis in the body
Oral bacteria can produce nitrate (NO3−) while food and drink may contribute nitrite (NO2−) and NO3− to circulating levels of biologically active compounds [39]. Guidelines for exhaled NO collection recommend that study participants refrain from food and drink an hour before providing samples [32].
These two potential sources of NO could contribute to total NO measures yet are rarely taken into account [40, 41]. Dietary sources of NO among Tibetans at 4200m were evaluated by collecting dietary information in a rural agropastoral district of the Tibet Autonomous Region [27, 37, 42]. Five men and five women volunteered to provide samples of exhaled breath and urine. They also reported their dietary intake in the previous 24 hours and provided aliquots of the foods, when available, and of the household water supply. Beverages — water, tea and home-brewed barley beer — from volunteers’ own meals contained levels ranging from undetectable to <15 uM NO2− and < 70 uM NO3−. None of the food contained green vegetables or other sources of NO3− such as cured meat; typical meals contained low levels of NO2− [< 0.4 mg/kg] and NO3− [< 125 mg/kg]. Overall, the average daily consumption of NO3− was not at a level expected to significantly alter circulating NO3− or NO2− levels [43]. Excluding dietary sources for high levels of NO strengthens the inference of high endogenous production in this high altitude sample with a diet typical of highland Tibet.
Results
Adaptation is a concept with many meanings sharing the sense of response that improves function under a stress. Modes of adaptation may be distinguished on a time scale ranging from rapid and reversible acclimatization to evolutionary adaptation in the gene pool over generations [44, 45]. Samples of people with different high altitude exposures, for example acute exposure for hours or chronic over lifetimes or generations, can offer insights into the different modes of adaptation to high altitude. Studies of NO response to high altitude hypoxia have evaluated function many ways, for example the presence or absence of acute or chronic mountain sickness or high altitude pulmonary edema. Because NO is a vasodilator and intimately involved in oxygen delivery to tissues, some studies have quantified pulmonary artery pressure, blood flow in the brain, lungs, uterus and forearm, and exercise capacity. Genotype, experience at altitude, physical fitness, ethnicity and other individual factors can modify NO response.
Acute exposure
NO therapy for failure to acclimatize
Acute exposure to hypoxia engages short term responses. Acclimatization is usually successful in the sense of preserving function although not always at normal low altitude baseline levels. However, some people do not acclimatize successfully. Evidence suggests that failure to acclimatize may be related to insufficient NO production. A potentially fatal response is high altitude pulmonary edema (HAPE) characterized by an exaggeration of the normal constriction of pulmonary blood vessels in response to hypoxia that causes an extreme elevation of pulmonary blood pressure. For example, pulmonary artery systolic pressure increased by an average of 13 mmHg in a healthy low-altitude sample at 4559m for 20 hours; it increased twice as much (27 mmHg) in a sub-sample that developed HAPE [46].
NO can counteract hypoxic pulmonary vasoconstriction by causing vasodilation. Inhaling a gas mixture of 15–40 ppm NO for 15–40 minutes lowers pulmonary artery pressure in every sample reported. Figure 3A shows extremely large effect sizes ranging from −2 to −5 pooled standard deviations (d) among six samples of people acutely exposed to altitudes ranging from 3600m to 4559m. The average fall in pulmonary artery pressure was 15 ± 7 mmHg or 28%. Pre- and post-NO treatment pressures were highly correlated (Figure 3B, r = + 0.93). The falls show that high altitude pulmonary hypertension was vasoreactive and related to insufficient levels of NO to maintain pulmonary vasodilation [47].
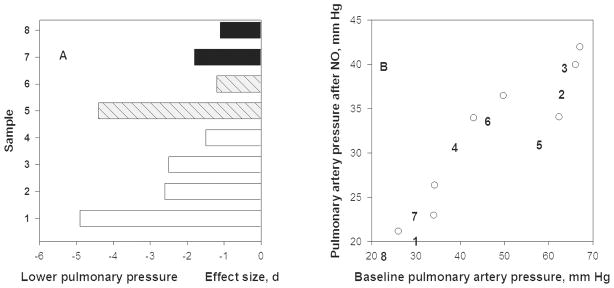
A. Pulmonary artery pressure (systolic except for case #1 that is mean arterial pressure) fell after breathing 30–40 ppm NO for 15–40 minutes at 3600–4665m altitude [2–5]. Sample #1 was composed of Indian soldiers at 3600m with HAPE (high-altitude pulmonary edema) [6], #2 were HAPE susceptible lowlanders with HAPE, #3 were HAPE susceptible lowlanders without HAPE, #4 were HAPE resistant lowlanders all at 4559m [5], #5 are lowlanders, age 21 years, who suffered perinatal hypoxia and #6 were those who did not at 4559m [7], and #7 were Bolivian Aymara highlanders, age 13–14 years, whose mothers had pre-eclampsia and #8 are those mothers had normal pregancies at 3600m [4]. Effect size, d, of NO on pressures is shown.
B. Pulmonary artery pressure after NO inhalation at 3600–4559m (shown on y-axis) declines from high altitude baseline (shown on x-axis). The post-treatment levels are directly associated with pre-treatment levels and the decline across studies is 28% based on linear regression analyses.
Perinatal stress, post-natal hypoxia or gestation by a mother with pre-eclampsia, was associated with stronger hypoxic pulmonary vasoconstriction later in life. The top two bars of Figure 3A compare 13–14 year old Bolivian Aymara highlanders at 3600m whose mothers had normal pregnancies with those whose mothers had mild-to-moderate pre-eclampsia [48]. The next two bars contrast 21-year-old low-altitude Europeans who had a normal transition to extra-uterine life with those who received oxygen therapy for transient perinatal hypertension. Samples 5 and 7 suffered an insult and had higher baseline pulmonary artery pressure at altitude and larger falls after breathing NO than their controls (samples 6 and 8). This occurred among individuals exposed chronically (Bolivian Aymara) and those exposed acutely (21-year old Europeans). There may be perinatal origins of vulnerability to HAPE later in life that are NO-dependent.
Acute exposure of 3 to 48 hours
Gas phase measurements of NO
A fall in NO due to less synthesis of NO due to oxygen substrate limitation or oxygen sensitivity of the NOS could explain the immediate rise in pulmonary artery pressure upon arrival at altitude as a consequence of a reduced capacity to vasodilate. Reports of lowlanders’ first 3 to 48 hours of exposure to altitudes of 4200m and 4559m reveal a dynamic situation.
Figure 4 shows that vital capacity NO fell slightly at three hours and substantially at 12 and 24 hours at 4559. HAPE-resistant study volunteers returned to baseline during the second 24 hours. HAPE-sensitive did not; instead their vital capacity NO fell by more than 2 standard deviations at 12 hours and remained at least 1 standard deviation below baseline for the full 48 hours of exposure [49].
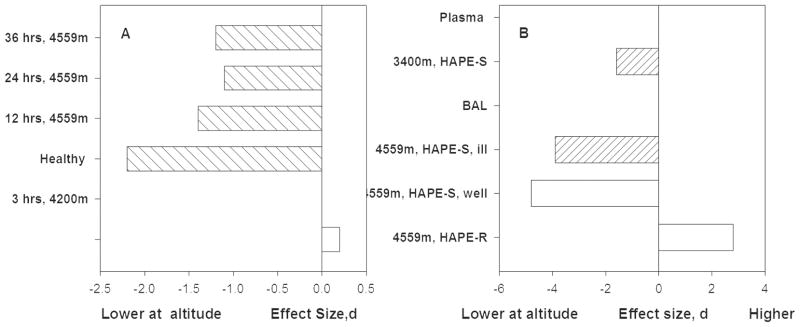
Figure 4A. Total lung NO falls in the first hours at altitudes above 4000m and then trends slightly toward baseline values among HAPE susceptible individuals and returns to baseline by 38 hours among HAPE resistant individuals [8, 9].
Figure 4B. Healthy HAPE resistant individuals showed increased nitrogen oxides in bronchoalveolar lavage (BAL) fluid at 12 hours. In contrast HAPE susceptible individuals had reductions of nitrogen oxides at altitude regardless of whether or not they suffered from HAPE during this particular study [10].
NO is synthesized from oxygen, in short supply at high altitude, and L-arginine. Therefore one study provided additional L-arginine substrate before and during exposure to 4342m in an attempt to drive greater NO synthesis. Lung NO trended upward during 12 hours but not significantly so, perhaps because the time was too short, as suggested by Figure 4, or the changes in NO occurred in locations that do not accrue benefit, or oxygen limitation could not be overcome [50]. Exercise capacity did not change, and unfortunately, L-arginine supplementation increased high altitude headache. The latter suggests NO and/or NO-derived species might have increased in locations of no benefit to acclimatization, and if so, perhaps added to adverse effects.
Circulating Nitrogen Oxides
NO gas is a free radical that diffuses into the blood where it may form NO2−, NO3−, and S-nitrosoproteins (RSNO), all of which are measurable in the plasma and serum. Allostery of hemoglobin determines formation of many of the NO products. Oxyhemoglobin reacts with NO to form NO3−. Deoxyhemoglobin reacts with NO2− to form NO, RSNO and iron nitrosyls in heme (FeNO) [51]. Because deoxyhemoglobin levels are much higher at high altitude, this almost certainly leads to changes in NO-hemoglobin reactions and levels of NO-derived products. For example, Tibetan men at 4200m with an oxygen saturation of 85% had 1.6 mmol/L deoxyhemoglobin as compared with men at 282m with an oxygen saturation of 97% who had 0.3 mmol/L deoxyhemoglobin. The influence of deoxyhemoglobin on circulating nitrogen oxides has received little consideration in studies despite accumulating evidence of the fundamental role of the NO-hemoglobin interactions on blood flow and oxygen delivery.
Intravascular levels of plasma NO2−, RSNO and red blood cell NO in arterial and venous blood during the first 20 hours at altitude provide insight into the changes in NO production and chemistry that comprise the acclimatization response. Figure 5A shows large and unequal falls of arterial and venous NO2− resulting in a smaller arterial-venous difference by 20 hours at 4559m. Reporting only those measures would have been misleading because a very large increase in arterial nitrosoproteins also occurred. Both arterial and venous red blood cell nitrogen oxides increased more than a standard deviation and resulted in a larger arterial-venous difference. The finding of increases of nitrogen oxides in the blood was in contrast to lower bioavailable NO in the lung [46]. The large increase in red blood cell associated nitrogen oxides was interpreted as evidence of reapportionment of NO to longer-lived forms [46 p. 4844]. Red blood cell nitrogen oxides accounted for about 22% of total arterial NO at high altitude as compared with about 11% at low. The red cell associated NO could be released locally to cause vasodilation, greater blood flow and improved oxygen delivery.

A. Arterial and venous plasma NO2− and venous S-nitrosothiol (RSNO) concentrations among healthy people generally fell at 3 and 20 hours at 4559m but arterial RSNO values increased strikingly, which may reflect synthesis and/or greater hemoglobin-NO interactions [11]. Arterial-venous differences uniformly decreased.
B. Arterial and venous erythrocyte (RBC)-nitrogen oxides [(nitrite, nitrosyl hemoglobin (FeNO) and S-nitrosohemoglobin (SNO:Hb)] and total nitrogen oxides (sum of those in plasma plus those associated with the erythrocytes) at 3 and 20 hours at 4559m among healthy people [11]. Erythrocyte-associated nitrogen oxides increased while plasma NO2− fell. The arterial-venous differences in erythrocyte nitrogen oxides increased, suggesting greater offloading of NO to tissues at altitude.
Another study reported that plasma nitrogen oxides obtained retrospectively at low altitude from individuals who had been resistant to HAPE were much higher than levels among individuals with HAPE at 3400m [52]. The meaning of that finding is difficult to interpret because the samples differ in health, altitude and response. Further, other studies reported that HAPE-resistant and HAPE-sensitive individuals did not differ prior to exposure [49, 53]. Two prospective comparisons of HAPE-Sensitive and HAPE-Resistant individuals compared pre-exposure baseline measures and found no differences in total lung NO [49] or NO2− or NO3− in bronchoalveolar lavage fluid (BAL) [53]. However, BAL from a single sample of HAPE-resistant individuals at altitude showed a very large increase in nitrogen oxides (Figure 4B) [53], while BAL from HAPE-sensitive individuals had marked decreases in nitrogen oxides of nearly 4 standard deviations. Fitness or activity may also play a role in the size of the response to altitude. Plasma nitrogen oxides increased more among untrained men than among athletes who continued to train for eight days at 3100m [54]. Thus, the magnitude of the NO acclimatization response may partly depend on physical fitness.
Acute exposure of 2 to 30 days
Two studies of trekkers and climbers describe processes over days of hypoxia. One provided values at a 1300m baseline, 3400m on day five and 5050m on day 13 of the trek [55]. The other plotted trends over roughly the same route and schedule [21]. Both reported a moderate increase in serum or plasma NO2− at 3440m and then a slight decrease at 5050m. In contrast, hemoglobin associated nitrogen oxides including S-nitroso-hemoglobin (SNO:Hb) and iron nitrosyl (FeNO) hemoglobin increased moderately at 3440m and more at 5050m. Higher levels of SNO:Hb and hemoglobin both associated directly with exercise performance. Thus, hemoglobin associated nitrogen oxides and NO2− are part of the biological response to altitude.
While details of study design varied enormously and NO was measured in different locations, overall, studies of acute exposure reported that higher NO levels associated with less illness and better function at altitude, confirming that NO is important in the acclimatization to altitude.
Chronic exposure
Less is known about NO levels among long-term high altitude residents and natives. High altitude native refers to someone from a population on the Tibetan, Andean, or East African plateau with a long history and pre-history of high altitude residence and the opportunity for natural selection to work. Most of the information was provided by Tibetan highlanders. While acutely exposed lowlanders usually serve as their own controls, so far studies of highlanders compare them with low altitude samples of low-altitude ancestry. This confounds altitude and ancestry. There is evidence that both influence NO location in the body and the relative amounts of NO-derived products.
Gas phase measurements of NO
Bolivian Aymara had a small elevation and Tibetan highlanders had a very large elevation of vital capacity NO [Figure 6]. Relieving hypoxia with 20 minutes of 50% oxygen did not elicit a response in the Bolivian Aymara sample, but raised vital capacity NO by more than 1 nm Hg among the Tibetans at 4200m [37]. That result plus lower vital capacity NO among more hypoxic Tibetans at a higher altitude suggested that greater NO production was due to greater NO synthesis, but maximal production of NO was limited by oxygen availability.

Tibetan and Andean highlanders show higher vital capacity NO levels compared with sea level. Tibetans at the same altitude have lower airway NO levels. Among Tibetans, samples at 4700m have lower NO values in both compartments than those at 4200m. The lower values of airway NO may be related to decreased local production of NO by airway epithelial type 2 NO synthase due to limitation of oxygen substrate to the enzyme while higher total lung NO may be related to increasing values of circulating nitrogen products. Source [1, 12]
Circulating Nitrogen Oxides
Tibetans had much higher levels of circulating nitrogen oxides than low-altitude samples at low altitude [Figure 7]. High altitude natives and residents of India at 3400m and Peru at 4350m had small to large elevations. Peruvians with more symptoms of a maladaptation (such as palpitations, cyanosis and headaches) known as Chronic Mountain Sickness had lower levels of serum nitrogen oxides than their healthy counterparts [56]. The lower levels of circulating nitrogen oxides in the Nepalese Sherpa sample is puzzling because they emigrated from eastern Tibet beginning in the early 1500s [57] and would therefore be expected to resemble Tibetans. Consistent with Figure 7 is the finding that red blood cell associated nitrogen oxides were more than two hundred times higher among Tibetans at 4200m as compared with lowlanders at low altitude [27].
The whole-body production of NO quantified as urinary nitrogen oxides [58] was higher in Tibetans than low altitude populations, and consistent with the total lung and nitrogen oxide measurements, suggesting greater synthesis of NO. Tibetans at 4200m had a ten-fold elevation of urinary nitrate as compared with a US low-altitude sample [27]. Interestingly, Tibetans have slightly more deoxyhemoglobin than other populations at comparable altitudes. For example, Tibetan men at 4200m have an average of 1.6 mmol/L deoxyhemoglobin as compared with 1.2m mmol/L among Andean Aymara men at 3900m. The comparable figures for Tibetan and Andean Aymara women are 1.3 and 1.1 mmol/L deoxyhemoglobin. Perhaps NO-deoxyhemoglobin interactions contribute to the greater circulating nitrogen oxides of Tibetans. However, Ethiopian Amhara at 3700 m had a three-fold elevation of urinary nitrate, but so did Amhara at 1200m, suggesting that hypoxia did not account for the elevated NO levels in that case [59]. Perhaps genetic variation predisposes some populations to greater NO production or perhaps dietary differences play a role.
In this context, responsible NOS or NOS pathway gene or chemical reaction is not known. Candidate gene analyses focused on endothelial NOS, the constitutively expressed form. Highlanders from Ladakh, India and Sherpas from Nepal had more than 10% higher allele frequencies at two polymorphic NOS3 sites associated with higher nitrogen oxide levels [52, 60–62]. Selecting appropriate control samples is difficult because allele frequencies of low-altitude populations vary widely [63]. A genome scan for signals of natural selection found preliminary evidence of selection on the inducible NOS (NOS2) in an Andean sample but not a Tibetan sample [64, 65].
Regardless of the mechanisms leading to greater NO, functional benefits accompany the Tibetans’ high levels. Higher levels of total lung NO were associated with lower pulmonary artery systolic pressure, more pulmonary blood flow and oxygen delivery at 4200m [26]. The higher plasma and red cell nitrogen oxides among Tibetans accompanied more than 2-fold higher forearm blood flow and oxygen delivery [27]. Notably, gas phase measurements did not associate with forearm blood flow and plasma measurements did not associate with pulmonary artery systolic pressure. Those findings emphasize the importance of studying local concentrations and effects.
However, findings in Tibetans cannot be uniformly applied. Different high altitude native populations have unique NO responses. For example, cerebral blood vessels normally dilate in response to hypoxia and in response to NO [66]. Neither Peruvian nor Ethiopia residents of 4340m or 3700m had the expected fall in cerebral blood flow when acutely removed from hypoxia by visiting low altitude, implying that neither had hypoxic cerebral vasodilation at altitude. However, responses to pharmaceutical NO donors among Peruvian Quechua and Ethiopian Amhara highlanders identified population differences in NO-mediated vasoreactivity of cerebral blood vessels. Among Peruvians, pharmaceutical NO donors caused a medium fall in cerebral blood flow at altitude and a large fall at sea level. That is, Peruvians’ cerebral blood vessels had greater NO mediated cerebral vasoreactivity under normoxia. Among Ethiopian Amhara, pharmaceutical NO donors caused a large fall in cerebral blood flow at altitude and a medium fall at low altitude, i.e. more pronounced NO-mediated under hypoxia. The authors concluded that these findings reflect acclimatization among the Peruvians and genetic adaptation among Amhara [67]. Alternatively, these results may be interpreted to reflect differences in reactive oxygen species production, which will impact on NO availability [21].
Pregnancy adds to oxygen requirements and the stress of high altitude hypoxia, which offers another opportunity to understand the role of NO for thriving under conditions of hypoxia. A study of pre-eclamptic women at 2800m in Ecuador found higher plasma and placental nitrogen oxides than healthy pregnant women. That was one of the few instances when higher NO levels associated with poorer function. In comparison, pre eclamptic women at sea level had lower plasma and placental nitrogen oxides than healthy pregnant women [68]. Another study considered balance of vasoconstrictors and NO-derived vasodilators on blood flow to the uterus. Pregnant high altitude residents at 3100m in Colorado had lower serum nitrogen oxides than their counterparts at 1600m, and the ratio of the vasoconstrictor endothelin relative to nitrogen oxides was higher at 3100m. The higher ratios associated with lower uterine artery diameter and blood flow and lower birthweight [69]. These studies point out the importance of studying NO in the context of other counteractive biologic mediators of altitude effects.
Overall, Tibetans had exceptionally high levels of NO at all locations except for the conducting airways. High levels of circulating nitrogen oxides associated with high blood flow and oxygen delivery. The few measurements available for Andean Aymara and Ethiopian Amhara showed trends in the same direction suggesting that some degree of elevated NO is important for all populations at high altitude.
Conclusion
Studies of NO in humans at high altitude, where all individuals are unavoidably exposed to low ambient oxygen, cumulatively identify a role for NO in many beneficial adaptive responses. Unfortunately, no single study provides comprehensive information about all biological locations, forms of NO, or their functional outcomes, which is needed to provide a clear view to mechanisms of production and effect. This review begins to outline a model of high levels of pulmonary NO and NO-derived molecules providing functional benefits including limiting the extent of hypoxic pulmonary hypertension and sustaining high systemic blood flow for greater oxygen delivery. Much of this review deals with measurement and data reporting issues. Studies at high altitudes have the challenges of appropriate sample collection and measurement in field conditions very different from those of low-altitude laboratories and research institutes. Chemiluminescence sensors are appropriately designed for the conditions, although they are heavy and bulky. Electrochemical sensors are convenient, but their accuracy at high altitudes and cold temperatures has not been established. There are reasons to doubt the validity of their measurements and they cannot be calibrated. Until that information is available, chemiluminescence sensors should be used. Otherwise, scientists and study volunteers will continue to devote resources to unproven technology.
Early studies simply asked if NO is higher or lower at high altitude. Studies affirm that high levels of NO are associated with function benefits and the avoidance of illness. However, many questions remain to be answered. Questions include whether NO remains elevated in lowlanders who successfully acclimatize, or if the increases are transient, i.e. is NO an early but unsustained response? Similarly, information on Tibetans at low altitude is necessary to understand whether the high levels of NO at altitude are maintained when hypoxia is removed. Other questions remain to be answered regarding the chemistry of NO-derived products. Which nitrogen oxide(s) are important for functional benefits and what are the mechanisms? Functional benefits come to those with high nitrogen oxides, however we do not yet know how the high levels are achieved. Genetic polymorphisms in the many pathways that influence NO synthesis are unstudied.
Future human studies that integrate samples of lung, plasma/serum, and red cell nitrogen oxides along with relevant functional measures will begin to answer some of these questions.
Acknowledgments
SCE received support from NIH HL60917. CMB received support from NSF 0924726, 0452326, and 021547. DL has consulted for GE Analytics. We thank our colleagues for their insights and supportive enthusiasm, our study participants for their willingness to volunteer, and our reviewers for their helpful suggestions.
Abbreviations
| cGMP | cyclic guanosine monophosphate |
| d | effect size |
| FAD | flavin adenine dinucleotide |
| FeNO | iron nitrosyl |
| FMN | Flavin mononucleotide |
| HAPE | high altitude pulmonary edema |
| HIF | hypoxia inducible factor |
| m | meters |
| ml/s | milliliters per second |
| mmHg | millimeters of mercury |
| nmHg | nanometers of mercury |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NOS | nitric oxide synthase |
| NO | Nitric oxide |
| NO2− | nitrite |
| NO3− | nitrate |
| NOx | nitrogen oxides |
| ppb | parts per billion |
| psi | pounds per square inch |
| RSNO | S-nitrosoprotein |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References for Figures
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.freeradbiomed.2011.12.028
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3295887?pdf=render
Citations & impact
Impact metrics
Article citations
Micro- and macrovascular function in the highest city in the world: a cross sectional study.
Lancet Reg Health Am, 38:100887, 25 Sep 2024
Cited by: 0 articles | PMID: 39381083 | PMCID: PMC11459627
Association of altitude and frailty in Chinese older adults: using a cumulative frailty index model.
Front Public Health, 12:1321580, 06 Mar 2024
Cited by: 1 article | PMID: 38510346 | PMCID: PMC10951379
Differential splenic responses to hyperoxic breathing at high altitude in Sherpa and lowlanders.
Exp Physiol, 109(4):535-548, 05 Jan 2024
Cited by: 2 articles | PMID: 38180087 | PMCID: PMC10988702
Bone mineral metabolism and different indices of skeletal health of Ladakhi women living at high altitude.
Osteoporos Sarcopenia, 9(4):131-136, 29 Nov 2023
Cited by: 0 articles | PMID: 38374823 | PMCID: PMC10874723
Association between Plasmodium Infection and Nitric Oxide Levels: A Systematic Review and Meta-Analysis.
Antioxidants (Basel), 12(10):1868, 16 Oct 2023
Cited by: 0 articles | PMID: 37891947 | PMCID: PMC10604424
Review Free full text in Europe PMC
Go to all (81) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
High altitude adaptation in Tibetans.
High Alt Med Biol, 7(3):193-208, 01 Jan 2006
Cited by: 128 articles | PMID: 16978132
Review
Comparative human ventilatory adaptation to high altitude.
Respir Physiol, 121(2-3):257-276, 01 Jul 2000
Cited by: 57 articles | PMID: 10963780
Review
Down-Regulation of EPAS1 Transcription and Genetic Adaptation of Tibetans to High-Altitude Hypoxia.
Mol Biol Evol, 34(4):818-830, 01 Apr 2017
Cited by: 74 articles | PMID: 28096303 | PMCID: PMC5400376
Sleep Architecture in Partially Acclimatized Lowlanders and Native Tibetans at 3800 Meter Altitude: What Are the Differences?
High Alt Med Biol, 16(3):223-229, 06 Aug 2015
Cited by: 5 articles | PMID: 26248036
Funding
Funders who supported this work.
NHLBI NIH HHS (6)
Grant ID: R01 HL060917
Grant ID: R01 HL069170-10
Grant ID: R37 HL060917
Grant ID: U10 HL109250
Grant ID: R01 HL069170
Grant ID: HL60917
 1
1 





