Abstract
Free full text

The CD47-SIRPα Pathway in Cancer Immune Evasion and Potential Therapeutic Implications
Abstract
Multiple lines of investigation have demonstrated that that the immune system plays an important role in preventing tumor initiation and controlling tumor growth. Accordingly, many cancers have evolved diverse mechanisms to evade such monitoring. While multiple immune cell types mediate tumor surveillance, recent evidence demonstrates that macrophages, and other phagocytic cells, play a key role in regulating tumor growth through phagocytic clearance. In this review we highlight the role of tumor immune evasion through the inhibition of phagocytosis, specifically through the CD47-SIRPα pathway, and discuss how targeting this pathway might lead to more effective cancer immunotherapies.
Introduction
The initiation and perpetuation of cancer depends on several hallmark features including sustained proliferation, inhibition of growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion, and evading immune destruction [1]. The concept of tumor immune surveillance, the identification and elimination of cancer cells by the immune system, was first discussed over a century ago, and since then multiple immune system components have been implicated [2]. While the adaptive immune response is well-recognized to play an important role in anti-tumor immunity, the innate immune system, specifically the macrophage, has only recently been shown to play a prominent role in regulating tumor pathogenesis as well [3]. Macrophages exhibit functions including phagocytosis, antigen presentation, and cytokine production, which play roles in homeostatic cell clearance, pathogen defense, and inflammatory responses. Beginning in the 1970s, it was found that tumor growth could be promoted by tumor associated macrophages (TAMs) [4]. In the last two decades, these TAMs have been subdivided into two distinct macrophage subpopulations, which promote either a pro- or anti-tumorigenic environment depending on their capacity to present antigens, produce inflammatory cytokines, stimulate angiogenesis, and enable cytotoxic activity (reviewed in [5]). While cytokine production and antigen presentation by macrophages have been shown to impact tumor growth, the role of macrophage phagocytosis in tumor pathogenesis has been relatively unexplored. In physiologic settings, macrophage phagocytosis is crucial to programmed cell removal in clearing damaged and foreign cells. This phagocytic engulfment depends on the relative expression of pro- and anti-phagocytic signals on the target cell. Most notably, during apoptosis, expression of pro-phagocytic signals and loss of anti-phagocytic signals leads to engulfment (reviewed in [6]). Recent data have demonstrated that tumors evade macrophage phagocytosis through the expression of anti-phagocytic signals, including CD200 and CD47 [6]. This review will focus on the role of the CD47-SIRPα pathway in tumor pathogenesis and potential therapeutic strategies targeting this pathway.
The immunoregulatory role of CD47 in human malignancies
CD47 is a cell surface molecule in the immunoglobulin superfamily that binds several proteins including integrins [7] and thrombospondin-1 [8], and has been implicated in diverse physiologic processes including cell migration [9–11], T cell and dendritic cell (DC) activation [12], and axon development [13]. In addition, CD47 functions as an inhibitor of phagocytosis through ligation of signal-regulatory protein alpha (SIRPα) expressed on phagocytes, leading to tyrosine phosphatase activation and inhibition of myosin accumulation at the submembrane assembly site of the phagocytic synapse [14]. In this way, CD47 serves as a “don’t eat me signal” and a marker of self, as loss of CD47 leads to homeostatic phagocytosis of aged or damaged cells [15–17]. Indeed, CD47 is widely expressed on a majority of normal tissues [18], suggesting that its role in regulating phagocytosis is widespread. The existence of a programmed cell removal pathway that usually accompanies, but is independent of, programmed cell death [19] has implications for normal cell lifespan, aging of stem and progenitor cells, and pathways that must be defeated in the progression from normal cell to fully malignant cell clones [6].
CD47 was first identified as a tumor antigen on human ovarian cancer in the 1980s [20]. Since then, CD47 has been found to be expressed on multiple human tumor types including acute myeloid leukemia (AML) [10,21], chronic myeloid leukemia (CML) [10], acute lymphoblastic leukemia (ALL )[22], non-Hodgkin’s lymphoma (NHL) [23], multiple myeloma (MM) [24], bladder cancer [25], and other solid tumors (Willingham et al., submitted). While CD47 is ubiquitously expressed at low levels on normal cells, we found that multiple tumors express increased levels of CD47 compared to their normal cell counterparts [10,21–23]. We hypothesized that over-expression of CD47 enabled tumors to escape innate immune system surveillance through evasion of phagocytosis. This process occurs through binding of CD47 on tumor cells to SIRPα on phagocytes, thus promoting inhibition of phagocytosis and tumor survival. In support of this hypothesis, forced expression of CD47 in a CD47-deficient myeloid leukemia cell line facilitated aggressive dissemination and fulminant death in xenografted mice, in contrast to the minimal engraftment detected upon transplantation of the parental CD47-deficient cells [10]. Importantly, dissemination of the CD47-positive myeloid leukemia cells was directly due to evasion of phagocytosis.
Upregulation of CD47 expression in human cancers also appears to influence tumor growth and dissemination. First, increased expression of CD47 in several hematologic malignancies was found to be associated with a worse clinical prognosis, and in ALL to predict refractoriness to standard chemotherapies [21–23]. Second, CD47 was demonstrated to regulate tumor metastasis and dissemination in both MM and NHL [26,27]. In a mouse model of MM, tumor metastasis to bone was decreased in CD47-deficient mice compared to wild type controls [26]. Third, shRNA-mediated knock-down of CD47 in human NHL led to a dramatic reduction in hematogenous dissemination and spread to major organs in xenografted mice, in contrast to control NHL cells [27]. CD47 may mediate tumor dissemination through activation of integrin and chemokine-dependent cell migration or through inhibition of phagocytosis by ligation of SIRPα on phagocytes lining vascular entry sites that tumor cells must navigate past during the migratory process [27]. Additional mechanisms of tumor dissemination involving CD47 are likely and remain to be explored.
Therapeutic targeting of CD47 in human cancers
The increased expression of CD47 on many different human tumor types, and its known function as a “don’t eat me” signal, suggests the potential for targeting the CD47-SIRPα pathway as a common therapy for human malignancies. Efforts have been made to develop therapies inhibiting the CD47-SIRPα pathway, principally through blocking monoclonal antibodies directed against CD47, but also possibly with a recombinant SIRPα protein that can also bind and block CD47 (Figure 1). Anti-CD47 antibodies have demonstrated pre-clinical activity against many different human cancers both in vitro and in mouse xenotransplantation models. Administration of a blocking anti-human CD47 antibody to mice engrafted with primary human AML and ALL cells led to elimination of disease in both the peripheral blood and bone marrow, leading to long-term remissions in some cases [21,22]. Additionally, anti-CD47 antibody reduced tumor burden in vitro and/or in vivo for human NHL [23], MM [28], bladder cancer [25], and breast cancer [29].

Therapeutic targeting of the CD47-SIRPα pathway can cause elimination of cancer cells through multiple mechanisms. First, inhibition of the CD47-SIRPα interaction with a blocking anti-CD47 antibody, a blocking anti-SIRPα antibody, or a recombinant SIRPα protein (depicted here as a bivalent Fc-fusion protein) leads to phagocytic uptake of tumor cells by macrophages. Second, an anti-CD47 antibody can eliminate tumor cells through traditional antibody Fc-dependent mechanisms including NK cell-mediated ADCC and CDC. Third, anti-CD47 antibody may directly stimulate apoptosis of tumor cells through a caspase-independent mechanism. Fourth, anti-CD47 antibody may enable phagocytic uptake of tumor cells by DCs and subsequent antigen presentation to CD4 and CD8 T cells, thereby stimulating an anti-tumor adapative immune response. mAb=monoclonal antibody.
Anti-CD47 antibodies may facilitate elimination of tumor cells through a variety of mechanisms (Figure 1). First, anti-CD47 antibody can enable phagocytosis of tumor cells by blocking the binding of CD47 on tumor cells to SIRPα on phagocytes. Co-incubation of either a blocking anti-CD47 antibody or an antibody blocking SIRPα with tumor cells and macrophages led to rapid and efficient phagocytosis of multiple tumor cell types, while a non-blocking anti-CD47 antibody had no effect [10,21–23,25,30]. In vivo, anti-CD47 antibody-mediated elimination of tumor cells was dependent on phagocytes, as clodronate-mediated depletion of phagocytes in mice engrafted with human tumors abrogated the anti-tumor effect of the antibody [21,23]. Second, anti-CD47 antibody may eliminate tumor cells through Fc-dependent mechanisms including antibody-dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) (Figure 1). Indeed, one anti-CD47 antibody was found to induce NK-cell mediated cytotoxicity against head and neck cancer cell lines [31]. This effect was likely not due to blockade of the CD47-SIRPα interaction as human and mouse NK cells express little to no SIRPα [23]. Although anti-CD47 antibodies have not been reported to stimulate CDC, both ADCC and CDC mechanisms may be dependent on the specific anti-CD47 antibody IgG isotype, as IgG isotypes differ in their ability to stimulate ADCC and CDC. Third, anti-CD47 antibodies may eliminate tumor cells through direct induction of apoptosis (Figure 1). Several studies have shown that some anti-CD47 antibodies induced apoptosis of tumor cells in vitro and in vivo [28,29,32,33]. Anti-CD47 antibody-mediated apoptosis occurred independent of the caspase cell death pathway [33,34], and may also involve ligation and activation of thrombospondin, an additional ligand of CD47 [29]. Apoptotic effects varied as different anti-CD47 antibodies exhibited differing abilities to stimulate apoptosis of tumor cells [21,32,35]. These differences were most likely due to the specific CD47-targeting domain of each antibody, the IgG isotype, and involvement of antibody crosslinking, as apoptosis was not observed when anti-CD47 antibodies were administered in solution in vitro [21]. Fourth, analogous to macrophages, anti-CD47 antibody may also enable phagocytic uptake of tumor cells by dendritic cells leading to subsequent tumor antigen presentation to T cells and activation of the adaptive immune response (Figure 1). This may occur specifically through blockade of the CD47-SIRPα interaction. Indeed, DC and T cell subtypes express SIRPα (reviewed in [36]). In addition to negative regulation of DC phagocytosis, CD47-SIRPα ligation appears to partially negatively regulate DC activity. Activation of the CD47-SIRPα pathway suppressed DC maturation and inhibited cytokine production by mature DCs [37]; however, recent studies have also indicated an activating role of CD47-SIRPα signaling in DCs and T cells [36]. It is possible that binding of CD47 on tumor cells to SIRPα on DCs mediates immune evasion by both inhibiting phagocytosis and DC activity. This possibility, as well as the potential therapeutic benefit of activating the adaptive immune system through CD47-SIRPα blockade, remains to be formally explored.
In addition to CD47, SIRPα can also be targeted as a therapeutic strategy involving the CD47-SIRPα phagocytic signaling pathway. An antibody blocking SIRPα could inhibit binding of CD47 on tumor cells to SIRPα on phagocytes, enabling phagocytosis of tumor cells analogously to anti-CD47 antibody. Indeed, anti-SIRPα antibodies administered in vitro caused phagocytosis of tumor cells by macrophages [21,23,38].
CD47 is widely expressed on most normal tissues at low levels [18], while SIRPα expression is relatively restricted to myeloid and neural cells [36,39]. These observations raise the possibility of significant adverse effects resulting from targeting the CD47- SIRPα phagocytic pathway. The function of CD47 as a negative regulator of phagocytosis was determined by investigation of the CD47-knockout mice. Transfusion of red blood cells (RBC) or transplantation of leukocytes from the CD47-knockout mice into wild type mice resulted in elimination of the CD47-deficient cells due to phagocytosis in the recipients [15,16,40]. Furthermore, the CD47-SIRPα phagocytic pathway was found to modulate engraftment of human hematopoietic cells into immunodeficient mice, as the permissive NOD strain was found to have a germline polymorphism in SIRPα that facilitated enhanced binding to human CD47 [41]. Thus, it is possible that targeting the CD47-SIRPα phagocytic pathway may lead to significant toxicities, although such toxic effects are likely to be dependent on pro-phagocytic signals (discussed below). The possible toxic effects of targeting this pathway are an area of active investigation.
Selective targeting of tumor cells by anti-CD47 antibody can be mediated by the CD47-calreticulin phagocytosis axis
While CD47 is expressed on both tumor cells and many normal cell types, we showed that a blocking anti-CD47 antibody selectively eliminates tumor cells, but not their normal counterparts [21–23,30]. In addition, administration of anti-mouse CD47 antibodies to wild type mice for weeks did not result in any severe toxicity (data not shown) [21]. Moreover, inhibition of mouse CD47 with an antibody or morphilino conferred radioprotection to normal tissues [42]. These results suggest that it may be possible to safely target the CD47-SIRPα phagocytic pathway. Given that both tumor and normal cells express CD47, how does anti-CD47 antibody therapy selectively eliminate tumor cells while sparing normal cells? One possibility is that the increased expression of CD47 on tumor cells may lead to preferential binding of tumor cells providing a therapeutic window for anti-CD47 antibody. However, this possibility seems unlikely given the fact that the dose of anti-CD47 antibody utilized in the pre-clinical studies led to equal antibody coating of both tumor and normal cells [21–23,30], and CD47 expression on tumor cells did not correlate with anti-CD47 antibody-mediated phagocytosis since some tumor cells having similar CD47 expression to normal cell counterparts were still phagocytosed [21,22].
Another possible explanation is derived from the fact that while anti-CD47 antibody blocks a negative phagocytic signal, a positive phagocytic stimulus is still needed for phagocytosis. Thus, selective phagocytosis of tumor cells is dictated by expression of a pro-phagocytic signal(s) on tumor cells that is absent on normal cells. Indeed, we found that a wide array of human hematologic and solid tumors expressed the pro-phagocytic signal calreticulin (CRT) on the cell surface, while normal counterparts did not [30]. Calreticulin is an intracellular protein involved in calcium homeostasis in the endoplasmic reticulum that functions as a pro-phagocytic signal when translocated to the cell surface; this occurs during apoptosis where it binds its macrophage receptor, low-density lipoprotein-related protein (LRP), leading to engulfment of the target cell [17,43]. Anti-CD47 antibody-mediated phagocytosis of tumor cells was dependent on calreticulin as a CRT-blocking peptide, LRP antagonist, or shRNA knockdown of CRT completely abrogated phagocytosis by anti-CD47 antibody [30]. Moreover, phagocytic potency correlated with CRT expression on target cells, collectively indicating that anti-CD47 antibody targeting of tumor cells depended on both the blockade of anti-phagocytic (CD47) signals and exposure of pro-phagocytic (CRT) signals. In addition to calreticulin, dying cells and tumor cells often present other pro-phagocytic ligands, such as phosphatidyl serine, asialoglycoproteins, and attached antibodies with Fc isotypes that interact with phagocyte Fc-receptors (reviewed in [6]). The role of these pathways and calreticulin signaling in CD47-inhibitable phagocytosis remains to be determined for a number of cancers.
Combination strategies targeting CD47 in cancer
While monotherapies targeting CD47 were efficacious in several pre-clinical tumor models, combination strategies involving inhibition of the CD47-SIRPα pathway offer even greater therapeutic potential. Specifically, antibodies targeting CD47-SIRPα can be included in combination therapies with other therapeutic antibodies, macrophage- enhancing agents, chemo-radiation therapy, or as an adjuvant therapy to inhibit metastasis (Figure 2).
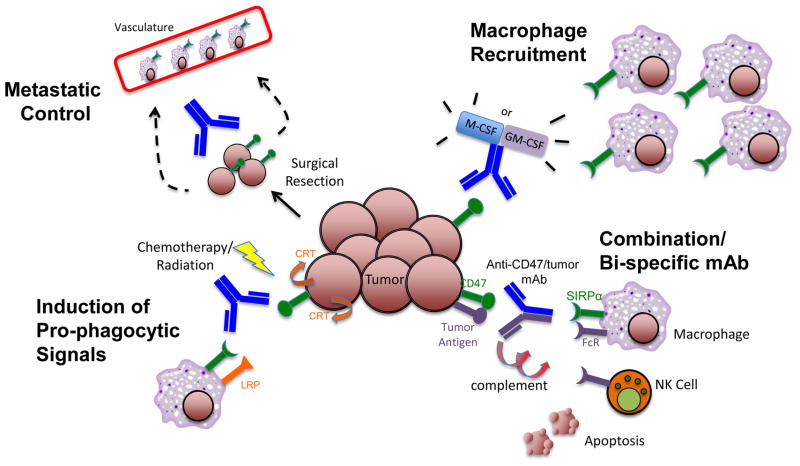
Anti-CD47 antibody may be utilized in several combination strategies to more effectively target tumor cells. First, anti-CD47 antibody may be combined with a second antibody against a tumor-specific antigen either separately or in a bi-specific format to recruit multiple cytotoxic mechanisms: macrophage-mediated phagocytosis, NK cell mediated-ADCC, and/or CDC. Second, anti-CD47 antibody may be combined with agents that augment macrophage effector cell number and function, including M-CSF or GM-CSF, to increase effector cells at tumor sites to enable phagocytic elimination. Third, chemotherapy and/or radiation may be combined with anti-CD47 antibody to induce pro-phagocytic signals (calreticulin) on tumor cells to augment anti-CD47 antibody potency. Fourth, given the ability of anti-CD47 antibody to inhibit tumor metastasis through phagocytosis by vascular-lining macrophages or direct inhibition of chemotaxis, this therapy can be administered systemically and/or infused locally at the time of surgical excision of the tumor mass to prevent metastatic spread.
First, a blocking anti-CD47 antibody can be combined with tumor-targeting antibodies utilizing distinct effector mechanisms for maximum efficacy. The primary mechanism of action of anti-CD47 antibody is the enabling of phagocytosis through specific blockade of the CD47-SIRPα interaction, which is independent of Fc-mediated functions [23]. This activity can be combined with a second therapeutic antibody capable of stimulating Fc-mediated functions such as ADCC and FcR-mediated phagocytosis to augment tumor cell killing (Figure 2). In this way, the combination therapy can stimulate phagocytosis of target cells by both blocking a negative signal and delivering a positive signal. Indeed, in several pre-clinical models of NHL, the combination of anti-CD47 antibody with the anti-CD20 antibody rituximab led to synergistic elimination and cure of mice engrafted with human NHL [23]. Similarly, anti-SIRPα antibody was found to potentiate ADCC mediated by the anti-Her2/Neu antibody trastuzumab against breast cancer cells [38]. These studies suggest that antibodies inhibiting the CD47-SIRPα pathway can be combined with other FcR-activating antibodies for the treatment of many tumor types. In addition to the utilization of complementary effector mechanisms, such combination antibody strategies have the additional advantage of being more likely to eliminate tumor cells with loss of a single antigen or pre-existing epitope variants which have been identified in resistant tumors treated with single antibody therapy [44,45]. Finally, dual antigen targeting can be combined into a single molecule in a bi-specific format, with one arm blocking CD47 and the other binding a tumor-specific antigen. Given that CD47 is expressed on both normal and tumor cells, such a bispecific antibody could reduce potential off-target toxicity and lead to specific elimination of tumor cells.
Second, anti-CD47 antibody may be combined with agents that augment macrophage effector cell number and function. In some pre-clinical xenograft models, anti-CD47 antibody efficacy was partially dependent on tumor burden. In human AML and ALL xenotransplant models, anti-CD47 antibody was not able to completely eliminate tumors in mice that had a bone marrow burden beyond 70% involvement [21,22]. However, these uncleared cells were able to be phagocytosed in vitro, suggesting that the lack of tumor elimination in these mice was likely due to insufficient macrophage effector cells in the bone marrow compartment. Indeed, efficacy of anti-CD47 antibody-mediated leukemic clearance in vivo correlated with the number of host macrophage effectors in the bone marrow. Thus, enhancing the activity and recruitment of macrophages to tumor sites may significantly augment anti-CD47 antibody efficacy. Enhanced macrophage number and function could be achieved through the co-administration of macrophage-stimulating cytokines including M-CSF and GM-CSF (Figure 2). In fact, concurrent administration of these cytokines with antigen-specific therapeutic antibodies can enhance anti-tumor activity against multiple tumor types [46–49].
Concurrent administration of chemo-radiation therapy with anti-CD47 antibody is a third combination strategy that may increase efficacy through two distinct mechanisms. First, chemo-radiation therapy administered prior to anti-CD47 antibody may lead to increased macrophage effector cells, as chemotherapy can induce an inflammatory response that attracts infiltrating macrophages to tumor sites [50–52]. Second, administration of chemo-radiation therapy may induce pro-phagocytic signals that might further augment anti-CD47 antibody-mediated phagocytosis. As described above, anti-CD47 antibody-mediated phagocytosis is dependent on tumor expression of the pro-phagocytic signal calreticulin. Interestingly, prior studies have shown that both chemotherapy and radiation lead to increased expression of cell surface calreticulin on tumor cells [53,54]. Thus, chemo-radiation therapy-mediated upregulation of cell surface calreticulin may potentially augment the activity of anti-CD47 antibody. However, this approach may also lead to increased toxicity as cell surface calreticulin is expressed on non-cancerous cells undergoing apoptosis, a principle effect of chemo-radiation therapy [17,53,54].
Lastly, anti-CD47 antibody may serve as an adjuvant in combination with local therapy to inhibit tumor dissemination. CD47 can facilitate migration of several normal cell types (reviewed in [7,12]), and was recently shown to play a role in the metastasis and dissemination of NHL and MM [26,27]. Furthermore, a blocking anti-CD47 antibody inhibited the dissemination of NHL cells to the peripheral blood and other secondary sites in vivo [27], and inhibited the metastasis of solid tumors (Willingham et al., submitted). CD47-mediated tumor dissemination likely occurs through either evasion of SIRPα expressing macrophages lining vascular entry points, direct inhibition of tumor chemotaxis, or other mechanisms [26,27]. To prevent tumor dissemination, anti-CD47 antibody could be administered systemically and/or infused locally at the time of surgical resection, thus eliminating tumor cells circulating in the vasculature (Figure 2).
Conclusion
Evasion of immune recognition is a major mechanism by which cancers establish and propagate disease. Recent data has demonstrated that the innate immune system plays a key role in modulating tumor phagocytosis through the CD47-SIRPα pathway. Therapeutic approaches inhibiting this pathway have demonstrated significant efficacy, leading to the reduction and elimination of multiple tumor types in pre-clinical models through several distinct mechanisms, most notably phagocytosis. While initial therapies targeting this pathway demonstrate significant potential for clinical translation, pre-clinical studies evaluating toxicity in non-human primates and subsequent Phase I human clinical trials will determine whether such potential can be realized.
Acknowledgments
This work is supported by the Ludwig Foundation and grants from the NIH (I.L.W.). R.M. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. M.P.C., R.M., and I.L.W. have filed U.S. Patent Application Serial No. 12/321,215 entitled ‘‘Methods For Manipulating Phagocytosis Mediated by CD47.’’ All authors contributed equally.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.coi.2012.01.010
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3319521?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.coi.2012.01.010
Article citations
Enzyme-armed nanocleaner provides superior detoxification against organophosphorus compounds via a dual-action mechanism.
J Nanobiotechnology, 22(1):593, 30 Sep 2024
Cited by: 0 articles | PMID: 39343894 | PMCID: PMC11440912
Circumventing resistance within the Ewing sarcoma microenvironment by combinatorial innate immunotherapy.
J Immunother Cancer, 12(9):e009726, 12 Sep 2024
Cited by: 0 articles | PMID: 39266215 | PMCID: PMC11404285
Pro-efferocytic nanotherapies reduce vascular inflammation without inducing anemia in a large animal model of atherosclerosis.
Nat Commun, 15(1):8034, 13 Sep 2024
Cited by: 1 article | PMID: 39271657 | PMCID: PMC11399336
Small molecule innate immune modulators in cancer therapy.
Front Immunol, 15:1395655, 10 Sep 2024
Cited by: 0 articles | PMID: 39318624 | PMCID: PMC11419979
Review Free full text in Europe PMC
CAR Macrophages: a promising novel immunotherapy for solid tumors and beyond.
Biomark Res, 12(1):86, 23 Aug 2024
Cited by: 1 article | PMID: 39175095 | PMCID: PMC11342599
Review Free full text in Europe PMC
Go to all (332) article citations
Other citations
Wikipedia
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy.
Trends Immunol, 39(3):173-184, 11 Jan 2018
Cited by: 213 articles | PMID: 29336991
Review
Blockade of CD47 or SIRPα: a new cancer immunotherapy.
Expert Opin Ther Targets, 24(10):945-951, 02 Sep 2020
Cited by: 9 articles | PMID: 32799682
The CD47-SIRPα Immune Checkpoint.
Immunity, 52(5):742-752, 01 May 2020
Cited by: 240 articles | PMID: 32433947 | PMCID: PMC7340539
Review Free full text in Europe PMC
The role of CD47-SIRPα immune checkpoint in tumor immune evasion and innate immunotherapy.
Life Sci, 273:119150, 01 Mar 2021
Cited by: 29 articles | PMID: 33662426
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: P01 CA139490
Grant ID: T32 CA009302
Grant ID: P01 CA139490-01





