Abstract
Free full text

7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz Syndrome
Abstract
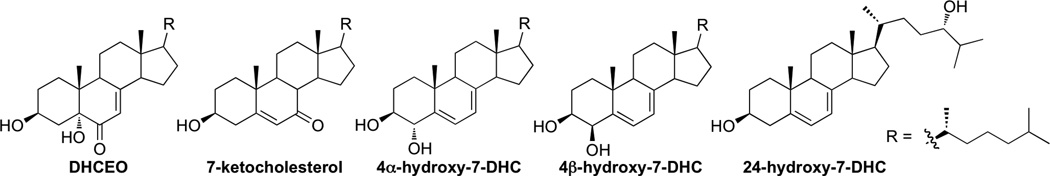
Smith-Lemli-Opitz syndrome (SLOS) is a recessive disease characterized by markedly elevated levels of 7-dehydrocholesterol (7-DHC) and reduced levels of cholesterol in tissues and fluids of affected individuals, due to defective 3β-hydroxysterol-Δ7-reductase (Dhcr7). Treatment of Sprague-Dawley rats with AY9944 (an inhibitor of Dhcr7) leads to similar biochemical features as observed in SLOS. Eighteen oxysterols previously have been identified as oxidation products of 7-DHC (most of them distinct from cholesterol (Chol)-derived oxysterols) in solution, in cells, and in brains obtained from Dhcr7-KO mice and AY9944-treated rats, formed either via free radical oxidation (peroxidation) or P450-catalyzed enzymatic oxidation. We report here the identification of five 7-DHC-derived oxysterols, including 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), 4α- and 4β-hydroxy-7-DHC, 24-hydroxy-7-DHC and 7-ketocholesterol (7-kChol, an oxysterol that is normally derived from Chol), in the retinas of AY9944-treated rats by comparing the retention times and mass spectrometric characteristics with corresponding synthetic standards in HPLC-MS analysis. Levels of 4α- and 4β-hydroxy-7-DHC, DHCEO, and 7-kChol were quantified using d7-DHCEO as an internal standard. Among the five oxysterols identified, only 7-kChol was observed in retinas of control rats, but the levels of 7-kChol in retinas of AY9944-rats were >30-fold higher. Intravitreal injection of 7-kChol (0.25 µmol) into a normal rat eye induced panretinal degeneration within one week; by comparison, contralateral (control) eyes injected with vehicle alone exhibited normal histology. These findings are discussed in the context of the potential involvement of 7-DHC-derived oxysterols in the retinal degeneration associated with the SLOS rat model and in SLOS patients.
1. Introduction
SLOS is an autosomal recessive metabolic disorder caused by mutations in the gene encoding 3β-hydroxysterol-Δ7-reductase (DHCR7; EC 1.3.1.21), the enzyme that catalyzes the reduction of 7-dehydrocholesterol (7-DHC) to cholesterol in the last step of cholesterol biosynthesis (reviewed in [1, 2]). This defect results in reduced levels of cholesterol and significantly elevated levels of 7-DHC in bodily tissues and fluids of affected individuals, who may present with a spectrum of phenotypic and functional abnormalities, including congenital dysmorphologies, neurological and behavioral defects, and moderate to severe mental retardation. Visual function deficits and other associated ophthalmologic abnormalities defects also have been observed in SLOS patients [3–5].
Both cholesterol deficiency and/or the buildup of 7-DHC may contribute to the pathobiology of SLOS, but therapeutic approaches toward SLOS mainly target the former. Cholesterol supplementation therapy is the routine standard of care for SLOS patients, but the therapeutic efficacy of this approach tends to be inconsistent and modest, at best [6–9]. Simvastatin therapy has been used to block the formation and steady-state accumulation of 7-DHC, but the efficacy of this treatment also is variable and may even aggravate the symptoms in severely affected patients [10–12], possibly via off-target (pleiotropic) pharmacological effects [13].
Our recent reports suggest that 7-DHC-derived oxysterols might play an important role in the pathophysiology of SLOS. 7-DHC was found to be the most reactive lipid molecule toward free radical oxidation (e.g., >200 times more reactive than cholesterol and >7 times more reactive than docosahexaenoic acid (DHA)) [14], and fourteen novel 7-DHC-derived oxysterols have been identified as products of the free radical oxidation of 7-DHC in solution [15]. One of these oxysterols, 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), also was identified in Dhcr7-deficient cultured Neuro2a cells, SLOS human fibroblasts, and Dhcr7-KO mouse brains by HPLC-MS [16, 17].
Oxysterols exert a broad spectrum of biological functions in cells and tissues, such as promoting cell apoptosis, activating inflammation, regulating cholesterol homeostasis and hedgehog signaling pathways, and modulating immune system responses [18–20]. The oxysterol mixture derived from free radical oxidation of 7-DHC in solution can reduce cell proliferation, induce cell differentiation, and lead to morphological changes in Neuro2a cells [16]. In addition, in normal cells, the oxysterol mixture also has been shown to induce gene expression changes that are similar to those observed in Dhcr7-deficient cells [15, 16]. DHCEO also is biologically active, inducing changes in Ki67, Egr1, and lipid-biosynthesis-related gene transcripts [16].
Dhcr7-knockout mice, a genetic model of SLOS, die shortly after birth [21, 22]. Since the vertebrate retina undergoes postnatal maturation and, in rodents, does not fully differentiate until at least four postnatal weeks (see: http://webvision.med.utah.edu/book/), the knockout mouse models are not suitable for studying retinal degeneration. Animal models of SLOS also have been developed by treating rats with pharmacological inhibitors of Dhcr7, such as AY9944 (trans-1,4-bis(2-chlorobenzylaminomethyl) cyclohexane dihydrochloride) and BM15766 (4-[2-[4-[3-(4-Chlorophenyl)-2-propenyl]-1-piperazinyl]ethyl]benzoic acid sulfate) [23, 24]. We improved the AY9944-induced SLOS rat model so that postnatal viability was extended to at least three months [25–27]. This allowed us to characterize the onset and progression of the biochemical, morphological, and electrophysiological features of the retinal degeneration that occurs in this model [26]. While the retina appears structurally and functionally normal up to the first postnatal month, within three postnatal months, significant deterioration of retinal histology, including progressing thinning of the outer nuclear layer (ONL), increased ONL pyknosis, reduction of photoreceptor outer segment (OS) length, with concomitant associated electrophysiological deficits (i.e., reduction in rod and cone ERG wave amplitudes and increase in implicit times) were observed in AY9944-treated rats, relative to age-matched controls [25, 26]. We subsequently reported that the levels of lipid hydroperoxides in retinas of non-photo-damaged AY9944-treated rats were comparable to those found in photo-damaged retinas of control rats [28]. We also found that exposure of AY9944-treated rats to intense visible (green) light greatly accelerated the build-up of such lipid peroxides in the retina [28], which correlates with markedly increased severity and geographic extent of the associated retinal degeneration in these rats [29].
The high oxidizability of 7-DHC provides a suitable explanation for the vulnerability of the retinas of AY9944-treated rats to oxidation. Recently, we isolated and characterized four new 7-DHC-derived oxysterols in addition to DHCEO in brains from AY9944-treated rats, including 4α- and 4β-hydroxy-7-DHC, 24-hydroxy-7-DHC, and 7-ketocholesterol (7-kChol) [30]. With the exception of 24-hydroxy-7-DHC, all oxysterols also were identified in liver and blood samples of these rats. Evidence supports the notion that both free radical and enzymatic oxidation mechanisms contribute to the formation of these oxysterols [30, 31].
We sought to identify and quantify the oxysterols formed in the retina in the AY9944-induced SLOS rat model, as we have hypothesized that such molecules may play a central role in the retinal degeneration observed in this model [32]. Herein, we report the following: 1) the elucidation of the oxysterol profile in the retinas of AY9944-treated vs. control rats using HPLC-MS; 2) the quantification of four oxysterols in the retinas of control and AY9944-treated rats by HPLC-MS-MS using a deuterated (d7) standard of DHCEO; and 3) the effect of intravitreally injected 7-kChol on the histology of the retinas of normal rats.
2. Materials and methods
2.1. Materials
AY9944 ((trans-1,4-bis [2-dichlorobenzylamino-ethyl] cyclohexane dihydrochloride) was custom synthesized and recrystallized to homogeneity (A.H. Fauq, Chemistry Core, Mayo Clinic, Jacksonville, FL). Purity was verified by HPLC and LC-MS, and the structure was verified in comparison to an authentic sample of AY9944 (a gift from Wyeth-Ayerst Research, Princeton, NJ), using NMR, UV-VIS spectroscopy, and MS. Unless specified otherwise, all biochemical and chemical reagents were of analytical reagent grade or higher purity and used as obtained from Sigma-Aldrich (St. Louis, MO). HPLC grade solvents (hexanes and 2-propanol) were purchased from Thermo Fisher Scientific Inc. Syntheses of [25,26,26,26,27,27,27-d7]-7-DHC, [25,26,26,26,27,27,27-d7]-DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 7-ketocholesterol were described elsewhere [17,30]. NH2-SPE cartridges (55 µm, 70Å, 500 mg/3mL) were purchased from Phenomenex, Inc.
2.2 Methods
2.2.1. The SLOS rat model
The SLOS rat model was generated as previously described [27]. All procedures involving animals were approved by the Buffalo VA IACUC, and were in accordance with the ARVO Resolution on the Use of Animals in Research and with the NIH Guide for the Care and Use of Laboratory Animals. In brief, a concentrated solution of AY9944 (dissolved in 0.1X strength PBS, pH 7.4) was administered via Alzet osmotic pumps (model 2ML4; Durect Corp., Cupertino, CA) to pregnant Sprague-Dawley rats (Harlan Bioproducts for Science, Indianapolis, IN; 6 d sperm-positive), so as to deliver a dosage of 0.37 mg AY9944/kg/d at an infusion rate of 2.5 µL/h. Rats were fed cholesterol-free chow (Purina Mills Test Diet, Richmond, IN) and maintained on a 12-h light/12-h dark cyclic lighting regimen (20–40 lux) at 22–25°C. Control dams were fed the same diet and maintained under the same ambient conditions, but were given no other treatment. Pups from AY9944-treated dams were injected subcutaneously with AY9944 (30 mg/kg, in PBS) three times per week on alternating days, starting at postnatal day one (P1) and continuing until termination (up to 3 month), under the same dietary and ambient environmental conditions as described above. Retinas from age-matched control and AY9944-treated animals were harvested under dim red light (to avoid photooxidation of lipids), flash frozen in liquid N2 and stored in darkness at −80°C until processed for HPLC-MS analysis.
2.2.2. Intravitreal injection of 7-kChol
Adult (300–350 g, ca. 3-mo old), male Sprague Dawley rats (Harlan; N=3) were anesthetized by intraperitoneal injection with a mixture of ketamine/xylazine mixture (75 mg/kg and 15 mg/kg, respectively). Body temperature was maintained using a heating pad. Eyes were dilated with 1–2 drops per eye of 1% cyclopentoate solution (Cyclogyl®; Alcon, Ft. Worth, TX), followed by application of 1–2 drops of topical anesthetic (0.5% proparacaine hydrochloride solution, Ophthaine®; Alcon). After cutting a window of temporal conjunctival to reveal bare sclera, an initial injection hole was created ca. 1–2 mm posterior to the limbus with a disposable #27 gauge sterile needle. Intravitreal injections were then performed using a 10-µL Hamilton syringe fitted with a disposable #30 gauge sterile needle. A DMSO solution of 7-kChol was made, such that 0.25 µmole would be delivered in 5 µL. A 1-µL air bubble was first drawn into the syringe, followed by 5 µL of oxysterol solution; this was then injected into one eye per animal (the air bubble can be visualized in the vitreous via the dilated pupil, intraoperatively, indicating complete delivery of the solution). The contralateral eye received 5 µL of DMSO alone (vehicle control). After allowing 5 seconds to elapse (holding the syringe stationary), the syringe was withdrawn, and Triple Antibiotic Ophthalmic Ointment® (Bausch & Lomb, Rochester, NY) was applied to the eyes. Animals were allowed to recuperate postoperatively in cages on heating pads in dim room light until awake, and then returned to the vivarium. All animals were inspected daily for any signs of eye infection or discomfort; no such complications were observed.
2.2.3. Histology
One week post-injection of oxysterol (or DMSO vehicle alone), animals were euthanized by intraperitoneal injection with an overdose of sodium pentobarbital (Beuthanasia®; Schering-Plough Animal Health Corporation, Union, NJ). Eyes were enucleated, fixed by immersion in a buffered glutaraldehyde-paraformaldehyde solution, processed for epoxy resin embedment, and retinal sections were stained and examined by light microscopy as described in detail elsewhere [26].
2.2.4. HPLC-MS-MS analysis of sterols in retinas of control and AY9944-treated rats
Each rat retina was homogenized in Folch solution (5 mL, CHCl3/MeOH, 2/1, containing 0.001M (each) BHT and PPh3) by blade homogenizer in the presence of appropriate amount of d7-DHCEO and d7-7-DHC standards. The resulting mixture was left under Ar at room temperature for 30 min. NaCl aqueous solution (0.9 %, 1 mL) was then added and the resulting mixture was vortexed for one minute and centrifuged for five minutes. The bottom organic layer was collected, dried under a stream of nitrogen, re-dissolved in methylene chloride (400 µL), and subject to NH2-SPE chromatographic separation (500 mg cartridge; the cartridge was conditioned with 4 mL of hexanes and the neutral lipids containing oxysterols were eluted with 4 mL of chloroform/2-propanol (2/1)). The desired fraction was then dried under nitrogen, re-constituted in methylene chloride (300 µL), and stored at −80 °C until NP-HPLC-APCI-MS-MS analysis. Analyses of oxysterols and sterols were carried out following previously established methods using d7-DHCEO and d7-7-DHC as the internal standards, respectively [15–17]. Briefly, oxysterol was analyzed by monitoring the dehydration process of the ion [M+H]+ or [M+H-H2O]+ in the mass spectrometry [17]. For quantification of 7-DHC and cholesterol, dehydration ions [7-DHC+H+-H2O] (m/z = 367) and [Chol+H+-H2O] (m/z = 369), along with [d7-7-DHC+H+-H2O] (m/z 374), were monitored [17]. Under the HPLC-MS condition employed, the response factors of 4α-hydroxy-7-DHC (m/z 383 → m/z 365), 4β-hydroxy-7-DHC (m/z 383 → m/z 365), and 7-kChol (m/z 401 → m/z 383) relative to d7-DHCEO (m/z 406 → m/z 388) were 1.34, 1.86, and 0.24, respectively. The response factor of Chol (m/z 369) relative to 7-DHC (m/z 367) was 0.75.
3. Results
3.1. Oxysterol profile of the retinas of AY9944-treated rats
We have documented the levels of 7-DHC and cholesterol (Chol) in retina and other tissues of AY9944-treated rats, in comparison with age- and sex-matched controls: typically, 7-DHC/Chol mole ratios reach ≥ 4/1 in retina, brain, liver, and serum by one postnatal month of AY9944 treatment, and the ratios increase to >11/1 in liver and serum and up to 7/1 in retina by three postnatal months; the ratios in the specimens of the control rats remain close to zero throughout the same time period [25, 26, 32, 33]. We also demonstrated previously that elevated levels of 7-DHC lead to accumulation of oxysterols in brain, liver, and serum of AY9944-treated rats [30]. Hence, we expect the same to occur in the retina.
In a typical procedure, retinas were extracted by the Folch procedure, using a chloroformmethanol solution containing BHT and PPh3 to minimize ex vivo oxidation of lipids. Neutral lipids that contain sterols then were separated from other lipid classes using NH2-SPE cartridge chromatography and were analyzed by normal phase HPLC-APCI-MS-MS, as described previously [17, 30].
In comparing the chromatograms of retinas from AY9944-treated rats relative to matched controls (Figure 1), a number of chromatographic peaks were observed that had masses consistent with those expected of 7-DHC-derived compounds containing one or two additional oxygen atoms. A number of authentic oxysterol standards, including DHCEO, 4α- and 4β-hydroxy-7-DHC, and 7-kChol, were chemically synthesized in the course of our previous study, and 24-hydroxy-7-DHC was isolated and identified from the brains of AY9944-treated rats [17, 30]. By comparing the MS and retention time (RT) characteristics of the chromatographic peaks from the biological specimens against those of the authentic standards, these five oxysterols were readily identified in the chromatograms of AY9944-treated rat retinas even though other unknown peaks remain to be characterized (Figures 1B and and2).2). The overall oxysterol profile of retina is similar to that of brains from AY9944-treated rats; however, a minor peak that corresponds to the mass of 7-DHC plus three oxygen atoms was not observed in retina, although this oxysterol was observed in both brain and liver specimens from AY9944-treated rats (but not controls).
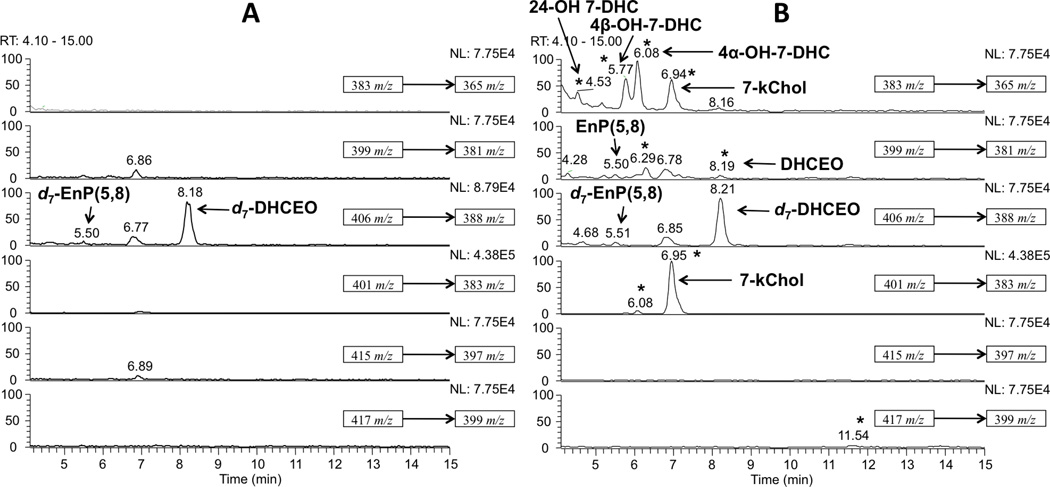
Normal phase HPLC-APCI-MS-MS (Silica 4.6 mm × 25 cm column; 5µ; 1.0 mL/min; elution solvent: 10% 2-propanol in hexanes) analysis of the oxysterols from retinas of two-month old (A) control and (B) AY9944-treated rats. New peaks observed in AY9944-rats relative to control rats are indicated by asterisks (*). An internal standard of d7-DHCEO was included in all samples for quantification purposes.
To differentiate oxysterols formed ex vivo from those generated in vivo, each retina was “spiked” with a known amount of d7-7-DHC standard immediately after harvesting the tissues and prior to flash freezing in liquid nitrogen. A minor peak that corresponds to one of the photooxidation products of 7-DHC, 5α,8α-epidioxy-cholest-6-en-3β-ol (EnP(5,8)), was observed in retinas from AY9944-treated rats even though the samples were processed under dim red light. However, this oxysterol was apparently formed by ex vivo oxidation of 7-DHC, since we also observed the formation of the deuterated (d7) analogue of this compound (derived from the exogenously introduced d7-7-DHC standard).
3.2. Quantification of Chol, 7-DHC and oxysterols in the retinas of control and AY9944-treated rats
Chol, 7-DHC, and four of the five known oxysterols were quantified using d7-7-DHC and d7-DHCEO as internal standards; the results are summarized in Table 1. As shown, 4α- and 4β-hydroxy-7-DHC, as well as 7-kChol, were the major oxysterols observed in retinas while DHCEO was only a minor product, being observed at a level ca. 1/30th of the other three oxysterols. With the exception of 7-kChol, all oxysterols are formed exclusively in AY9944-treated (not control) rats. DHCEO (a known oxysterol that can be formed from 7-DHC via free radical oxidation) has been observed previously in Dhcr7-deficient cell lines and in brain tissues from Dhcr7-KO mice, and has been proposed as a useful biomarker for 7-DHC peroxidation in biological systems [17]. Consistent with our prior studies using this model, AY9944 treatment resulted in a four-fold reduction in the steady-state level of Chol in the retina, relative to age-matched controls, and the 7-DHC/Chol mole ratio was nearly 5:1 (in controls, this ratio is zero) [25, 26].
Table 1
Quantification of oxysterols, cholesterol, and 7-DHC in retinas of two-month old control and AY9944-treated rats.a
| Control | AY9944 | |
|---|---|---|
| 4α-OH-7-DHC (ng) | 0 | 64 ± 10 |
| 4β-OH-7-DHC (ng) | 0 | 60 ± 6 |
| 7kChol (ng) | 1.7 ± 0.6 | 57 ± 16 |
| DHCEO (ng) | 0 | 2.0 ± 0.6 |
| Chol (µg) | 37.2 ± 2.4 | 9.0 ± 1.6 |
| 7-DHC (µg) | 0 | 42 ± 8 |
| 7-DHC/Chol | 0 | 4.7 ± 0.9 |
3.3. Effect of intravitreally injected 7-kChol on retinal histology in normal rats
The levels of 7-kChol in the retinas of two-month old AY9944-treated rats (ca. 60 ng per retina) are approximately 30 times those found in control rats (Table 1). Lipids contribute to ca. 20% of the dry weight of the retina, while the proteins contribute to the remaining 80%, and the water content comprises ca. 87% of the wet weight of the adult retina [34]. As a result, the wet weight of the adult rat retina is estimated to be ca. 10 mg, based on the above information in conjunction with the fact that the protein content of the adult rat retina is ca. 1 mg. Thus, assuming the density of the wet retina is 1 g/cm3, the level of 7-kChol in AY9944-treated retina can be converted to a molar concentration of 15 µM, a concentration that is toxic to a number of cell lines, including Neuro2a cells and human macrophages [16, 35].
To investigate the direct effects of 7-kChol on retinal structure and cellular viability in vivo, we injected 7-kChol (0.25 µmol, ≈ 100 µg, in 5 µL of DMSO) intravitreally into one eye each of normal, adult Sprague-Dawley rats (N=3), while the contralateral eye was injected with the same volume of DMSO alone (vehicle control). The results obtained from one animal, representative of the group, are shown in Fig. 3. One week after the treatment, extensive degenerative changes were observed in retinas exposed to 7-kChol, including massive death and dropout of photoreceptors, regional loss of histological stratification of cellular layers, and extensive gliosis, while the retinal histology of vehicle-injected (control) eyes (Fig. 3D) remained normal. In the most extensively affected regions (see Fig. 3C), remnant cells in the former outer nuclear layer (ONL) of retinas from 7-kChol-treated eyes consisted largely of nuclei surrounded by sparse cytoplasm; no recognizable photoreceptor outer segments (OS) or cell bodies were identified. At regions more distal to the site of oxysterol deposition in the vitreous, histological stratification of the retina was maintained; however, proximal to the site of greatest degeneration, the normal lamellar organization of the ONL was disrupted, exhibiting undulations and incipient folds (Fig. 3B) with loss of photoreceptors and reduction in OS length. The peripheral retinal regions (Fig. 3A) were thinner than normal and exhibited diminished OS length, but had normal ONL lamellar organization. It is important to note that observation of these degenerative changes in the retina were made far away from the injection site, so as to avoid any possible contributions from wound responses due to tissue disruption caused by the injection itself. Also, the vehicle-alone control injections did not provoke any such degenerative changes. Both the rapidity and geographic extent of this 7-kChol-induced degeneration were considerably more pronounced than are typically observed in other models of hereditary or toxicity-induced retinal degenerations [36, 37].
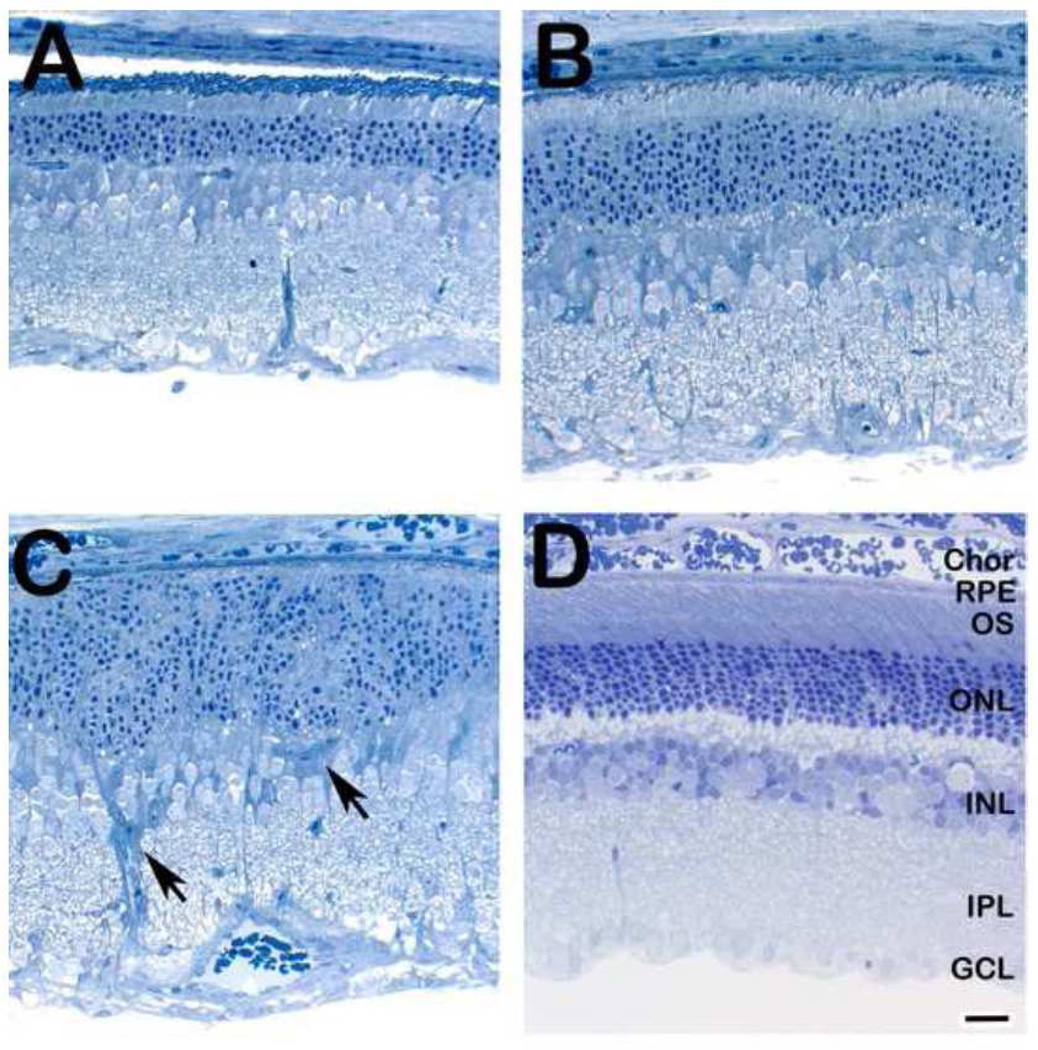
Histological analysis of retinas from an adult rat one week after a single intravitreal injection with (A–C) 7-kChol (0.25 µmol, in 5 µl DMSO) or (D) DMSO alone (5 µl; vehicle control). Note the normal histological appearance of the retina from the vehicle control eye, compared to the marked retinal degeneration observed in the oxysterol-treated eye. Arrowheads (panel C) indicate gliotic elements in the degenerating retina. Spurr’s resin embedment, 1-µm thick sections, Toluidine blue stain. Abbreviations: Chor, choroid; RPE, retinal pigment epithelium; OS, photoreceptor outer segment layer; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar (all panels), 25 µm.
4. Discussion
In the present study, we demonstrated for the first time the accumulation of 7-DHC-derived oxysterols in the retina of the AY9944-induced rat model of SLOS, including DHCEO, 4α- and 4β-hydroxy-7-DHC, 24-hydroxy-7-DHC, and 7-kChol. Some or all of these oxysterols have recently been found in other tissues of this same animal model, including brain, liver, and serum [30]. The observation of DHCEO validates this oxysterol as a biomarker of 7-DHC free radical peroxidation. C4-Hydroxylation of 7-DHC could occur via either cytochrome P450 (CYP) 3A4-catalyzed or free radical oxidation, but no evidence is yet available to support either mechanism [30, 38]. 24-Hydroxy-7-DHC is presumably formed via the catalysis of CYP 46A1 (cholesterol 24-hydroxylase), the enzyme that catalyzes the oxidation of cholesterol to 24(S)-hydroxy-cholesterol in brain [39]. The formation and accumulation of 7-kChol most likely can be explained by the results from another recent study, which established that CYP 7A1 (cholesterol 7α-hydroxylase; the rate-limiting enzyme in bile acid biosynthesis) can catalyze the conversion of 7-DHC to 7-kChol [31]. Although CYP 7A1 is generally considered to be a liver-specific enzyme [40], there are no published reports to date that demonstrate directly either the presence or the absence of this enzymatic activity in the retina. However, CYP 7B1, an oxysterol and steroid 7α-hydroxylase, is abundant in the nervous system (although, again, neither directly demonstrated to be present in or absent from retina) [41, 42]. Thus, the possibility remains that either CYP 7A1 or 7B1 or both may be responsible, in part, for the conversion of 7-DHC to 7-kChol in the mammalian retina.
It is not clear from this finding, however, whether these oxysterols were formed in the retina in situ from local 7-DHC (de novo), or whether they arose from oxysterol-laden lipoproteins delivered to the retina via the blood. Using intravitreally-injected radiolabeled precursors, it has been demonstrated previously that the rat retina has the capacity to synthesize cholesterol de novo, and that blocking the penultimate step in cholesterol biosynthesis with a systemically administered inhibitor causes the formation of the radiolabeled substrate of the blocked enzyme (the immediate precursor of cholesterol) in the retina [43–46]. Yet, additional evidence, derived subsequently by studying the time-dependent uptake by the retina of intravenously injected lipoproteins containing a fluorescent sterol probe, indicates that the retina also has the capability to take up sterols from blood-borne lipoproteins [47]. The relative contributions of de novo synthesis vs. exogenous uptake of sterols to the overall maintenance of sterol steady-state levels in the retina have yet to be resolved [32, 33]. Regardless of the exact source(s) of sterol, it is well documented that 7-DHC itself accumulates in the retina in this rat model of SLOS [25–27]. The facts that the retina contains free (unbound) as well as ferritin-bound iron, and is exposed continuously to an extremely high oxygen tension [48] and that 7-DHC is the most reactive known lipid molecule susceptible to free radical oxidation [14] suggest that in all likelihood the 7-DHC-derived oxysterols are generated in situ from 7-DHC in the retina.
Recent studies from our lab have shown that some primary 7-DHC-derived oxysterols formed in free radical oxidation in solution are extraordinarily toxic to cells [16]. For example, two of these oxysterols (5,9-endoperoxy-cholest-7-en-3β,6α-diol and the corresponding 3β,6β-diol) are approximately 10-fold more cytotoxic to Neuro2a cells in culture (LD50 = 5 µM) than is 7-kChol (LD50 = 50 µM). However, as the primary oxysterols may be metabolized in cells and in vivo, it is the metabolically stable 7-DHC-derived oxysterols that are ultimately accumulated in these biological systems, such as DHCEO and 7-kChol. DHCEO can affect cell viability, induce gene expression changes, and accelerate differentiation and arborization of cortical neurons [16, 17, 49], while 7-kChol exerts a spectrum of biological activities and is one of the most studied oxysterols [18–20, 50, 51].
Moderate to severe neurological defects have been observed in SLOS patients, including microcephaly, holoprosencephaly, hypoplastic or absent corpus callosum, frontal lobe and cerebellar hypoplasia [1, 2]. Impaired cognitive function, developmental delay, behavior problems, and autism are also typical non-physical phenotypes [2]. The biological effects of 7-DHC-derived oxysterols, such as DHCEO, on primary cortical neurons suggest that they may play some important roles in nervous system abnormalities [49]. Previous work by Chattopadhyay and co-workers suggests that substitution of cholesterol with 7-DHC in membranes does not restore the function of the hippocampal serotonin1A receptor [52, 53]. The authors subsequently demonstrated that the signaling function of the serotonin1A receptor was impaired in an AY9944-induced cell model of SLOS [54]. Since oxysterols are known to modify the structures of lipid domains in membranes, which are critical to cell signaling [55, 56], it is possible that retinal serotonergic neuronal activity may be altered in the AY9944-treated rat model of SLOS, compared to controls. However, the extent to which this may contribute to electrophysiological dysfunction or retinal degeneration is not clear, especially since it is the amacrine cells (located in the inner retina, which exhibits no degenerative features), rather than the photoreceptors (which are the cells that degenerate and die in this model), that are serotonergic (see: http://webvision.med.utah.edu/book/part-iv-neurotransmitters-in-the-retina/).
Since 7-DHC-derived oxysterols have been observed in tissues and fluids in both genetic and AY9944-induced SLOS animal models, their biological effects could potentially contribute to the broad spectrum of phenotypic abnormalities observed in SLOS [1, 2]. 7-KChol, in particular, has been implicated in the pathobiology of various human diseases [19, 50, 51, 57], and thus is considered a “benchmark” cytotoxic oxysterol [18–20]. Normally, under conditions where there is no appreciable steady-state accumulation of 7-DHC, any 7-kChol found in tissues is thought to be derived from oxidation of Chol. However, very recently, it has been demonstrated that 7-kChol can form directly from 7-DHC [31]. Hence, in situations where 7-DHC is the overwhelmingly dominant sterol, such as in the rat model of SLOS employed in the present study, the occurrence of 7-kChol as a major oxysterol in tissues suggests that it was most likely derived predominantly, if not solely, from 7-DHC, instead of Chol. Such is the case in the present study, where 7-kChol was found to be one of the three most prevalent oxysterols in retinas from AY9944-treated rats (57 ng/retina, i.e., > 30-fold higher than in control retinas). The fact that retinas from age-matched control rats (where Chol is almost the exclusive sterol present, and could have served as a precursor to 7-kChol) contained minimal levels of 7-kChol (1.7 ng/retina) further strengthens this conclusion. The level of 7-kChol is < 0.005% that of Chol in control retinas, while the level is ca. 0.6% of Chol in retinas of AY9944-treated rats, suggesting that Chol is not the major precursor to 7-kChol. Note that 7-kChol is normally detected at a minimum level in tissues and fluids of healthy individuals; for example, 7-kChol is virtually undetectable in normal human blood vessels. Even in advanced atherosclerotic plaques, the 7-kChol level is < 0.4% of the level of Chol [50]. Under normal conditions, the very low steady-state levels of 7-kChol that exist in the retina are apparently well tolerated by the retina, since no degeneration takes place [48]. However, if one exposes albino rats to intense, constant green light (1700 lux, 490–580 nm) for several hours, the levels of 7-kChol increase several fold, with concomitant retinal degeneration [48]. It is of interest that the steady-state levels of lipid hydroperoxides in retinas from AY9944-treated rats (not exposed to intense constant light) were found to be comparable to those in retinas of normal albino rats exposed to this “retinal light damage” paradigm [28].
While it is not known whether or not the C-4-hydroxylated derivatives of 7-DHC are cytotoxic, a host of studies have clearly demonstrated that 7-kChol is [19, 35, 51, 58]. The presence of 7-kChol in the retina of the SLOS rat model suggests its possible implication in the mechanism underlying the associated retinal degeneration. Consistent with this hypothesis, we found that a single intravitreal injection of as little as 0.25 µmol of 7-kChol in the rat eye caused massive, panretinal degeneration within one week post-injection, while the contralateral eye injected with vehicle alone (equivalent volume of DMSO) exhibited normal histology. The actual concentration of 7-kChol that reached the retina is not known, but must have been only a minute fraction of what was injected, since the majority of the sterol appeared to precipitate within the vitreous (which, although composed largely of water, is by no means a homogenous aqueous medium) almost immediately after injection and the residual soluble 7-kChol may be further diluted within the bulk vitreal volume and diffuse radially (and perhaps asymmetrically) from the point of initial oxysterol deposition in the eye. In earlier experiments (S.J. Fliesler, unpublished), using equivalent amounts of intravitreally-injected cholesterol dissolved in DMSO, sterol precipitation in the vitreous also was observed, but without subsequent retinal degeneration within one week post-injection (the longest time point examined). The involvement of 7-DHC-derived oxysterols as key players in triggering the cascade of events that ultimately results in the observed retinal degeneration in the SLOS rat model has been proposed previously [32]. At this point we cannot say with certainty which oxysterols, alone or in combination, are involved in the degenerative process. The present findings, however, give further credence to this hypothesis and now warrant the systematic testing of the various oxysterols found in situ in the retina in the SLOS rat model for the cytotoxic potency against retinal cells, both in vitro (where conditions can be better controlled) and in vivo.
Acknowledgments
Supported, in part, by U.S.P.H.S. grants EY007361 (SJF), ES013125 (NAP), HD064727 (NAP), and by an Unrestricted Grant from Research to Prevent Blindness (SJF). The technical assistance of Ms. Barbara A. Nagel is gratefully acknowledged.
Abbreviations
| APCI | atmospheric pressure chemical ionization |
| BHT | butylated hydroxytoluene |
| Chol | cholesterol |
| Chor | choroid |
| CYP | cytochrome P450 |
| 7-DHC | 7-dehydrocholesterol |
| DHCEO | 3β,5α-dihydroxycholest-7-en-6-one |
| Dhcr7 or DHCR7 | 7-dehydrocholesterol reductase |
| DMSO | dimethylsulfoxide |
| EnP(5,8) | 5α,8α-epidioxy-cholest-6-en-3β-ol |
| GCL | ganglion cell layer |
| 7-kChol | 7-ketocholesterol |
| MeOH | methanol |
| NP | normal phase |
| 4α-OH-7-DHC | 4α-hydroxy-7-DHC |
| 4β-OH-7-DHC | 4β-hydroxy-7-DHC |
| 24-OH-7-DHC | 24-hydroxy-7-DHC |
| INL | inner nuclear layer |
| IPL | inner plexiform layer |
| IS | inner segment layer |
| ONL | outer nuclear layer |
| OPL | outer plexiform layer |
| OS | outer segment layer |
| PPh3 | triphenylphosphine |
| SLOS | Smith-Lemli-Opitz syndrome |
| SRM | selective reaction monitoring |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bbalip.2012.03.001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3340457?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.bbalip.2012.03.001
Article citations
The drug release of PLGA-based nanoparticles and their application in treatment of gastrointestinal cancers.
Heliyon, 10(18):e38165, 19 Sep 2024
Cited by: 0 articles | PMID: 39364250 | PMCID: PMC11447355
Review Free full text in Europe PMC
Chemical Inhibition of Sterol Biosynthesis.
Biomolecules, 14(4):410, 28 Mar 2024
Cited by: 1 article | PMID: 38672427 | PMCID: PMC11048061
Review Free full text in Europe PMC
Inhibition of 7-dehydrocholesterol reductase prevents hepatic ferroptosis under an active state of sterol synthesis.
Nat Commun, 15(1):2195, 12 Mar 2024
Cited by: 4 articles | PMID: 38472233 | PMCID: PMC10933264
Oxy- and Phytosterols as Biomarkers: Current Status and Future Perspectives.
Adv Exp Med Biol, 1440:353-375, 01 Jan 2024
Cited by: 0 articles | PMID: 38036889
Cholic acid increases plasma cholesterol in Smith-Lemli-Opitz syndrome: A pilot study.
Mol Genet Metab Rep, 38:101030, 28 Nov 2023
Cited by: 1 article | PMID: 38077958 | PMCID: PMC10698565
Go to all (34) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome.
J Lipid Res, 52(10):1810-1820, 04 Aug 2011
Cited by: 52 articles | PMID: 21817059 | PMCID: PMC3173002
Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome.
Exp Eye Res, 82(3):496-504, 19 Dec 2005
Cited by: 27 articles | PMID: 16360150 | PMCID: PMC2844790
Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome: Implications for an Improved Therapeutic Intervention.
Molecules, 23(10):E2720, 22 Oct 2018
Cited by: 8 articles | PMID: 30360379 | PMCID: PMC6222618
Review Free full text in Europe PMC
An efficient synthesis of 4α- and 4β-hydroxy- 7-dehydrocholesterol, biomarkers for patients with and animal models of the Smith-Lemli-Opitz syndrome.
Chem Phys Lipids, 175-176:73-78, 03 Aug 2013
Cited by: 3 articles | PMID: 23920082
Funding
Funders who supported this work.
NEI NIH HHS (3)
Grant ID: EY007361
Grant ID: R01 EY007361
Grant ID: R01 EY007361-18
NICHD NIH HHS (2)
Grant ID: HD064727
Grant ID: R01 HD064727
NIEHS NIH HHS (2)
Grant ID: P01 ES013125
Grant ID: ES013125