Abstract
Free full text

Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats
Abstract
Hypertension in rats with chronic placental ischemia (reduced uterine perfusion pressure, RUPP) is associated with elevated inflammatory cytokines, agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) and CD4+ T cells; all of which are elevated in preclamptic women. Additionally, we have shown that adoptive transfer of RUPP CD4+ T cells increases blood pressure, inflammatory cytokines, and sFlt-1. The objective of this study was to determine the long-term effects of RUPP CD4+ T cells on AT1-AA, renal and systemic hemodynamics in pregnant rats. To answer this question CD4+ T splenocytes were magnetically isolated on day 19 of gestation from control RUPP and normal pregnant (NP) rats and injected into a new group of NP rats at day 13 of gestation. On day 19 of gestation mean arterial pressure (MAP) and renal function (glomerular filtration rates, GFR) were analyzed and serum collected for AT1-AA analysis. To determine a role for AT1-AA to mediate RUPP CD4+ T cell-induced blood pressure increases, MAP was analyzed in a second group of rats treated with AT1 receptor blockade losartan (10 mg·kg−1·day−1) and in a third group of rats treated with rituximab, a B cell-depleting agent (250 mg/kg) we have shown previously to decrease AT1-AA production in RUPP rats. MAP increased from 101 ± 2 mmHg NP to 126 ± 2 mmHg in RUPP rats (P < 0.001) and to 123 ± 1 mmHg in NP rats injected with RUPP CD4+ T cells (NP+RUPP CD4+T cells) (P < 0.001). Furthermore, GFR decreased from 2.2 ml/min (n = 7) in NP rats to 1.0 ml/min (n = 5) NP+RUPP CD4+T cell. Circulating AT1-AA increased from 0.22 ± 0.1 units in NP rats to 13 ± 0.7 (P < 0.001) units in NP+RUPP CD4+T cell-treated rats but decreased to 8.34 ± 1 beats/min in NP+RUPP CD4+ T cells chronically treated with rituximab. Hypertension in NP+RUPP CD4+T cell group was attenuated by losartan (102 ± 4 mmHg) and with B cell depletion (101 ± 5 mmHg). Therefore, we conclude that one mechanism of hypertension in response to CD4+ T lymphocytes activated during placental ischemia is via AT1 receptor activation, potentially via AT1-AA during pregnancy.
preeclampsia is characterized by new onset hypertension with proteinuria during pregnancy. Preeclampsia is also associated with immune activation and production of activating autoantibodies to the angiotensin II type I receptor (AT1-AA) (5, 6, 17–19). These autoantibodies were previously characterized and shown to be specific for a stretch of seven amino acids localized to the second extracellular loop of the AT1 receptor. Activation of this receptor is attenuated either by treatment with an AT1 receptor blocker or by epitope binding with the corresponding seven amino acid sequence. In addition, previous studies have demonstrated that women with preeclampsia have increased placental and plasma levels of inflammatory cytokines compared with women with normal pregnancies (3, 4). Recent data from the Xia laboratory (20) indicates that the severity of the disease strongly correlates to levels of the AT1-AA, suggesting the importance of the AT1-AA to mediate the pathophysiological phenotype observed in preeclamptic women. Furthermore, studies from ours and other laboratories have shown that chronic administration of the AT1-AA to pregnant rats or mice induces a preeclamptic-like state, characterized by increased endothelin-1, sFlt-1, and oxidative stress (12, 16, 22, 24, 25). Data from the Xia laboratory have shown that AT1-AA administration has profound pathophysiological effects on the kidney and fetal development, both of which are normalized by administration of AT1 receptor blockade or administration of the seven amino acid epitope binding sequence, illustrating the specific effects of the AT1-AA during pregnancy (22–25).
We have recently shown that adoptive transfer of CD4+ T helper 1 cells from a rat model of preeclampsia (reduced uterine perfusion pressure, RUPP) causes significant increases in blood pressure, circulating inflammatory cytokines tumor necrosis factor-α (TNF-α), IL-6, and IL-17 as well as sFlt-1 in normal pregnant recipient rats (21). However, adoptive transfer of RUPP CD4+ T cells had no effects on nonpregnant rats. Previous studies from other laboratories have also demonstrated that adoptive transfer of activated Th1-like splenocytes into pregnant mice elicited high blood pressure, protienuria, and an inflammatory response similar to that observed in preeclamptic women (23). One important function of CD4+ T helper cells is to assist other immune cells, such as B lymphocytes in their routine function, including production of immunoglobulin (1). For full activation of B lymphocytes, interaction with activated T lymphocytes via the CD4 receptor and several costimulatory molecules must occur. While CD4+T cells increase blood pressure, it is unknown whether AT1-AA are produced, moreover, the importance of the AT1-AA in mediating the increase in blood pressure in response to RUPP CD4+ T helper cells remains unclear. Therefore, the first objective of the current study was to determine whether hypertension caused by adoptive transfer of CD4+ T cells activated in response to placental ischemia is associated with B lymphocyte production and secretion of AT1-AA from normal pregnant (NP) recipient rats. The second objective of the study was to determine whether the stimulated AT1-AA from NP recipient rats of RUPP CD4+ T cells play a role in the hypertension by activation of the AT1 receptor. To achieve these goals, AT1-AA was measured from serum of NP recipient rats of RUPP CD4+ T cells. To determine a role for activation of the AT1-receptor, the adoptive transfer was repeated in NP rats treated chronically with an AT1-recptor antagonist. To determine a role for endogenous B cell activation and AT1-AA secretion in CD4+ T cell-mediated hypertension, the adoptive transfer was again repeated into a new group of rats treated with rituximab, a B cell-depleting agent, that we have previously shown to be efficient in suppressing endogenous AT1-AA production in RUPP rats (15).
METHODS
All studies were performed in 250 g timed-pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN). Animals were housed in a temperature-controlled room with a 12:12 light:dark cycle. All experimental procedures in this study were in accordance with the National Institutes of Health guidelines for use and care of animals and were approved by the Institutional Animal Care and Use Committee at the University of MS Medical Center.
Reduction in uterine perfusion pressure.
The RUPP procedure that reduces the uterine perfusion pressure was performed on day 14 under isoflourane anesthesia by the application of a constrictive silver clip (0.203 mm) to the aorta superior to the iliac bifurcation performed via a midline laparotomy. Ovarian collateral circulation to the uterus is reduced with restrictive clips (0.100 mm) to the bilateral uterine arcades at the ovarian end (2, 11, 14, 15).
Protocol 1: effect adoptive transfer of RUPP CD4+ T helper cells on AT1-AA, blood pressure, and renal function in NP rats.
This protocol was designed following that of previous investigators and previous studies performed in our laboratory demonstrating an important role for T cells to mediate hypertension during pregnancy (21, 23). Although, we have previously demonstrated the importance of CD4+ T helper cells stimulated in response to RUPP to mediate hypertension during pregnancy, this study was designed to determine the effect of RUPP CD4+ T cells to decrease renal function and stimulate AT1-AA production from naïve endogenous B cells resident to normal pregnant recipient rats.
Spleens from RUPP and NP rats were isolated at the time of euthansia and immediately placed in ice-cold phosphate-buffered saline, pH 7.0, homogenized in culture dishes with RPMI medium containing 10% FBS, and filtered through a 100-μm cell strainer to obtain single cell suspensions. CD4+ T lymphocytes were isolated from the splenocytes via magnetic separation using Dynabeads CD4 according to the manufacturer's recommended protocol (Invitrogen, Carlsbad, CA). Once released from the Dynabeads, CD4+ T cells were washed in PBS and cultured in RPMI containing 25 mM HEPES, 2 mM glutamine, 100 U/ml Pen/Strep, 1.022 ng/ml IL-2, and 4 ng/ml IL-12 for 24 h at 5% CO2 at 37°C in a humidified atmosphere to maintain cellular viability and integrity.
We utilized flow cytometric analysis to determine 90% purity of CD4+ T cell populations on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). For flow cytometry analysis equal numbers of leukocytes (1 × 106) were incubated for 30 min at 4°C with antibodies against mouse CD4 (BD Biosciences, San Jose, CA). After being washed, cells were labeled with the secondary FITC antibody (Southern Biotech, Birmingham, AL) for 30 min at 4°C.
After centrifugation, cell pellets were washed with saline and adjusted to 1 × 106 cells/100 μl saline at >90% purity and injected intraperitoneally into recipient normal pregnant rats on gestational day 13.
Measurement of arterial pressure and renal hemodynamics in conscious chronically instrumented pregnant rats.
During isoflurane anesthesia, rats at day 18 of pregnancy were surgically instrumented with catheters (PE-50 tubing) in the jugular vein and carotid artery for blood sampling and blood pressure monitoring (2, 11, 12–14, 16, 21). A midline lower abdominal incision was made and the bladder was cannulated with flare-tipped PE-90 tubing for urine collection. All catheters were tunneled to the back of the neck and exteriorized. The groups examined for blood pressure differences were as follows: NP rats, RUPP rats, NP rats injected with NP CD4+ T cells (NP+NPCD4+T cells), and NP rats injected with RUPP CD4+ T cells (NP+RUPP CD4+T cells). Renal function in RUPP rats was previously shown, but this is the first study to illustrate significant differences in renal function results from RUPP-activated CD4+ T cells. For renal function the groups compared were NP and NP recipients of RUPP CD4+T cells.
Protocol 2: effect of B cell depletion on hypertension in response to adoptive transfer of RUPP CD4+ T helper cells.
This protocol was designed to determine a role for NP recipient endogenous B cells stimulated in response to adoptive transfer of RUPP CD4+ T cells to produce AT1-AA as a mediator of hypertension. To do so we utilized the technique of B cell depletion implemented by chronic administration of rituximab beginning on day 13 of gestation, the time of adoptive transfer. Rituximab inhibits activation of the B cell costimulatory molecule CD20 from interacting with activated CD4+ T cells, thereby interfering with B cells entering the circulation for antibody secretion. We have recently shown that B cell-depleted RUPP rats have suppressed circulating B cells and AT1-AA, indicating efficacy for B cell depletion in pregnant rats (22). Minosmotic pumps infusing rituximab (250 mg/kg) were implanted into NP recipient rats of RUPP CD4+ T cells (NP+RUPP CD4+T cells+R). On day 19 of gestation arterial blood pressure was analyzed as described previously (2, 11–16, 21).
Protocol 3: effect of AT1 receptor blockade on hypertension in response to adoptive transfer of RUPP CD4+ T helper cells.
This protocol was designed to examine the role of AT1 R activation in mediating hypertension in response to RUPP CD4+ T lymphocytes. For this objective, adoptive transfer of RUPP CD4+ T lymphocytes occurred on day 13 of gestation was performed in pregnant rats chronically treated with losartan (10 mg/day) in their drinking water, provided ad libitum, beginning on day 13 of gestation (NP+RUPPCD4+T cells+L). On day 19 of gestation arterial blood pressure was analyzed as described previously (2, 11–16, 21).
Determination of AT1-AA production.
Antibodies were detected by the chronotropic responses to AT1 receptor-mediated stimulation of cultured neonatal rat cardiomyocytes coupled with receptor-specific antagonists as previously described (5, 6, 12, 15, 16). Briefly, on day 18 of gestation blood was collected and immunoglobulin was isolated from 1 ml of serum by specific anti-rat IgG column purification. Subsequently, AT1-AA was purified by the seven amino acid epitope binding from the column-purified rat IgG. AT1-AA activity was measured, as previously described, utilizing a bioassay that we have previously published in which the chronotropic response of rat neonatal cardiomyocytes in culture is detected and counted (5, 6, 12, 15, 16). Increased chronotropic response is counted and expressed as the change in beats per minute (Δbpm) of the rat neonatal cardiomyocytes in culture, therefore, units of AT1-AA are expressed as Δbpm over basal levels.
Statistical analysis.
All data are expressed as means ± SE. Differences between control and experimental groups were analyzed using the Student's t-test for renal function. Blood pressure analysis and AT1-AA levels were analyzed using one-way ANOVA for comparison among multiple groups. Values were of P < 0.05 were considered significant.
RESULTS
Protocol 1: effect of adoptive transfer of RUPP CD4 ± T helper cells on blood pressure, AT1-AA, and renal hemdynamics.
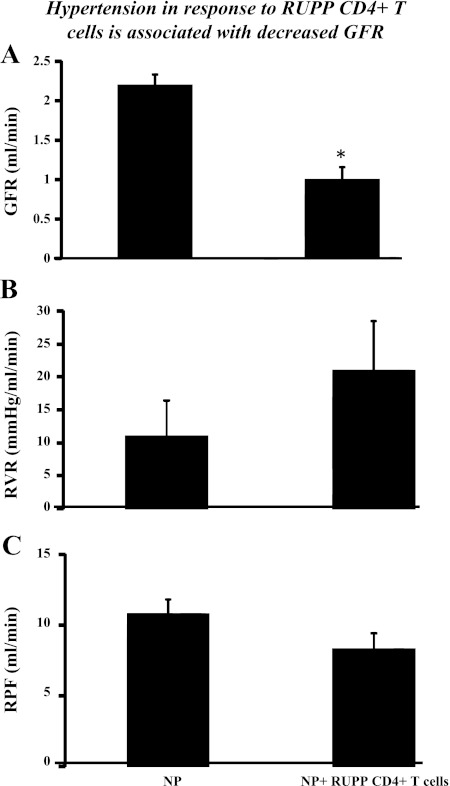
We have recently shown that adoptive transfer of RUPP CD4+ T helper cells to NP rats causes ~20 mmHg increase in mean arterial pressure (MAP) (21). Importantly, this model is reproducible, and again, in this study, we show that blood pressure increased 20 mmHg with adoptive transfer of RUPP CD4+ T helper cells to NP rats. MAP increased from 103 ± 3 mmHg in NP rats (n = 16) to 126 ± 2 mmHg in RUPP rats (n = 20, P < 0.001) and increased to 123 ± 4 mmHg NP+RUPPCD4+ T cells (n = 7, P < 0.001) (Fig. 1). MAP was unchanged in NP recipients of NP CD4+ T cells (104 ± 3 mmHg). Although significantly less in RUPP rats compared with NP rats, pup and placental weight was unchanged by adoptive transfer of NPCD4+ T cells or RUPP CD4+ T cells into NP recipient rats. Pup weights were 2.3 ± 0.11 g in NP rats and were 1.9 ± 0.04 g in RUPP rats (P < 0.05). Pup weights were 2.3+ ± 0.04 g in NP+NP CD4+ T cells and were 2.2 ± 0.09 g in NP+RUPP CD4+ T cells. Placental weights were unchanged among the groups; NP was 0.58 ± 0.03 g, RUPP was 0.53 ± 0.2 g, NP+NPCD4+ T cells was 0.57 ± 2 g, and NP+RUPPCD4+ T cells was 0.53 ± 0.11 g. Circulating AT1-AA is stimulated in placental ischemic RUPP rats (14.8 bpm) compared with NP control rats (1.1 bpm) (Fig. 2). Interestingly, AT1-AA activity increased from 0.22 ± 0.1 bpm in NP recipient rats of NP CD4+ T lymphocytes to 13 ± 0.7 (P < 0.001) bpm in NP recipient rats of RUPPCD4+T lymphocytes (Fig. 2). We have previously shown that glomerular filtration rates (GFR) significantly decreases in response to placental ischemia and infusion of other inflammatory factors, such as TNF-α and IL-6. In this study we demonstrate the renal function greatly declines with adoptive transfer of RUPP CD4+ T cells into NP rats (Fig. 3). Since there is no change in blood pressure, cytokine levels, nor AT1-AA production in NP recipients of NP CD4+ T lymphocytes compared with NP controls, renal function was not analyzed in this group. Importantly, renal function was compared between NP controls and NP recipients of RUPP CD4+ T cells that display elevated blood pressure, circulating inflammatory cytokines, and AT1-AA. GFR was 2.2 ml/min in NP rats (n = 7) and was significantly decreased to 1.0 ml/min in NP+RUPP CD4+ T cells (n = 5) (Fig. 3). Albeit not significantly different, renal vascular resistance increased twofold, from 11 ± 6 in NP to 21 ± 8 in NP recipients of RUPP CD4+ T cells. Renal plasma flow decreased slightly, 10.7 ± 2 in NP to 8.3 ± 2 in NP recipients of RUPP CD4+ T cells.
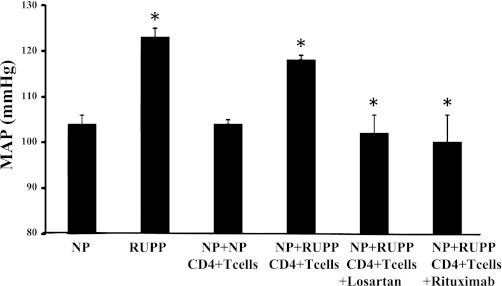
Blood pressure (MAP) increases in response to reduced uterine perfusion pressure (RUPP) (n = 20) during pregnancy compared with normal pregnant (NP) rats (n = 10) (P < 0.05). Blood pressure is significantly increased in response to adoptive transfer of RUPP CD4+ T cells into NP recipient rats (n = 11) compared with blood pressure in NP recipient rats of NP CD4+ T cells (n = 5) (P < 0.05). Hypertension in response to RUPP CD4+ T cells is attenuated with chronic administration of the AT1-R antagionist losartan (n = 11) or by B cell depletion with administration of rituximab (n = 4). *P < 0.05.
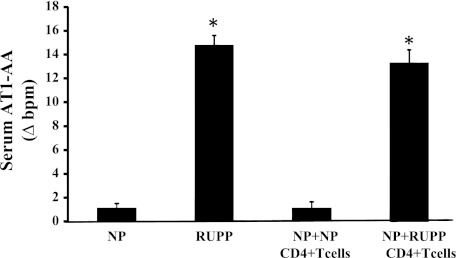
AT1-AA is significantly increased in RUPP rats (n = 7) compared with NP control rats (n = 7) (P < 0.05). AT1-AA is significantly increased in response to adoptive transfer of RUPP CD4+ T cells into normal pregnant recipient rats. (n = 4) compared with NP controls or NP recipients of NP CD4+ T cells (n = 4) (*P < 0.05).
Protocol 2. effect of B cell depletion on hypertension in response to adoptive transfer of RUPP CD4+ T helper cells.
We have previously shown that B cell depletion by rituximab administration decreased circulating B lymphocytes, AT1-AA, and blood pressure in response to placental ischemia in pregnant rats (15). To determine a role for the endogenous B cell activation in mediating hypertension in response to RUPP T cells, CD4+ T cells were isolated from splenocytes obtained from new groups of RUPP rats and administered to NP rats treated with rituximab (NP+RUPPCD4+ T cells+R) (n = 5). MAP in NP+RUPP CD4+T cells +R was 101 ± 5 mmHg, which was significantly less than that of NP+RUPPCD4+ T cells (123 ± 4 mmHg; P < 0.01, Fig. 1). Administration of rutiximab completely attenuated the increase in blood pressure of NP rats treated with adoptive transfer of CD4+ T cells from RUPP rats. Furthermore, administration of rituximab significantly decreased AT1-AA in response to RUPP CD4+ T cells; AT1-AA levels were 8.34 ± 1 bpm in NP+RUPP CD4+ T cells chronically treated with rituximab. This is a similar decrease to what was achieved with RUPPs chronically treated with rituximab.
Protocol 3: effect of AT1 receptor blockade on hypertension in response to adoptive transfer of RUPP CD4+ T helper cells.
To determine a role for the AT1-R activation in mediating hypertension in response to RUPP T cells, CD4+ T cells were isolated from splenocytes obtained from new groups of RUPP rats and administered to NP rats treated with losartan (NP+RUPPCD4+T cells+L). Administration of losartan completely attenuated the increase in blood pressure of NP rats treated with adoptive transfer of CD4+ T cells from RUPP rats (n = 10). MAP in NP+RUPP CD4+T cells+L was 102 ± 4 mmHg, which was significantly less than that of NP+RUPPCD4+ T cells (123 ± 4 mmHg; P < 0.01; Fig. 1).
DISCUSSION
Our laboratory recently demonstrated that placental ischemia (RUPP) serves as a stimulus for a dysregulation among CD4+ T helper cells (14). Furthermore, we have shown that hypertension in response to placental ischemia decreases renal excretory function, which is associated with activating autoantibodies of the angiotensin II type I receptor (2, 12–15). We previously showed that, like RUPP, adoptive transfer of RUPP CD4+ T cells increased blood pressure, TNF-α, sFlt-1, with a trend for an increase in IL-6 and IL-17 seen in NP recipients of RUPP CD4+ T cells verses NP recipients of NPCD4+ T cells (21). In this new study we demonstrate that hypertension in response to adoptive transfer of RUPP CD4+ T lymphocytes results in AT1-AAs in NP recipient rats. We have previously shown that NP rats do not produce or secrete active AT1-AA without stimulation by either an inflammatory cytokine such as TNF-α or IL-6, or here, CD4+ T cells stimulated by placental ischemia. Furthermore, this hypertension is associated with significantly decreased glomerular filtration with a trend toward a decrease in renal plasma flow as well as an increase in renal vascular resistance during pregnancy. As with inflammatory cytokines IL-6 and IL-17 and intrauterine growth restriction, neither renal plasma flow nor renal vascular resistance was significantly altered, thereby suggesting that although activated CD4+ T cells and AT1-AA play an important role in the pathophysiology of this disease, there are still many other factors equally important in the ramifications of hypertension in response to placental ischemia that deserve further investigation.
Importantly, hypertension in response to RUPP CD4+ T lymphocytes is attenuated by administration of AT1 receptor antagonist losartan, suggesting the importance of AT1-AA activation of AT1 receptors to mediate hypertension when CD4+ T cells are elevated during pregnancy. Administration of losartan also inhibits ANG II activation of AT1 receptors, therefore, this study cannot rule out the role of ANG II in mediating hypertension in response to adoptive transfer of RUPP CD4+ T cells. However, the endogenous RAS is not activated in response to placental ischemia, therefore, the attenuation of the hypertension in this study is likely due to blockade of AT1-receptor activation by endogenous AT1-AA produced by NP recipient rats of RUPP CD4+ T cells.
It is presumed that the adoptively transferred T cells from RUPP rats stimulated B cells in the NP recipient rats to produce the AT1-AA (15). To test this theory, we administered rituximab to NP rats the same day RUPP T cells were injected. Rituximab is a chemotherapeautic agent that has shown efficacy in treatment of patients with autoimmune diseases. Rituximab binds to the CD20 costimulator molecule inhibiting B cells from entering the circulation and secreting antibody. Recent data from our laboratory demonstrated that adminstration of rituximab was not only effective in B cell depletion but also significantly suppressed AT1-AA activity in response to placental ischemia in RUPP pregnant rats (15). As a result, B cell-depleted, AT1-AA-suppressed RUPP rats had lower blood pressure than control RUPP rats. In this study, administration of rituximab to NP recipient rats of RUPP CD4+ T cells significantly decreased blood pressure to levels similar to NP control rats. In addition, administration of rituximab significantly decreased AT1-AA in response to adoptive transfer of RUPP CD4+ T cells. Although the AT1-AA was nonattenuated, it was decreased to levels seen in women that were previously preeclamptic during pregnancy but are no longer hypertensive, thereby indicating the AT1-AA could be an underlying mechanism for cardiovascular disease and hypertension that develop in these women with age. Collectively, these data indicate that similar to other immune mediators, moderately high circulating concentration of the AT1-AA is essential to cause hypertension during pregnancy. These data support the theory that adoptive transfer of CD4+ T cells stimulated in response to placental ischemia causes blood pressure increases by stimulating B cells to produce and secrete active AT1-AA. Although there may be other factors stimulated in response to adoptive transfer of RUPP CD4+ T cells, the focus of this current study was the resulting AT1-AA and the increase in blood pressure caused by AT1-AA and AT1-R activation.
It is important to note that we have previously demonstrated an important affect TNF-α, sFlt-1, IL-6 to increase blood pressure and decrease renal hemodynamics in pregnant rats (8–10, 12, 14, 16). Furthermore, studies from the Xia laboratory have shown an important role for the AT1-AA to cause renal pathology and fetal demise, which can be attenuated by AT1 receptor blockade. In this study we did not perform histological examinations of kidneys nor placentas to determine pathology caused by RUPP CD4+ T cells. Future studies examining a role for antigen presentation in either kidney or placenta in NP rats treated with RUPP T cells would provide explanation of antigen localization that may be involved in AT1-AA generation in this model. Furthermore, we did not determine the effects of CD4+ T cells to activate the ET-1 system, which we have shown to be involved in hypertension in response to elevated AT1-AA, TNF-α, as well as sFlt-1 during pregnancy. All of these systems play an important role in the pathophysiology of preeclampsia and therefore are equally worthy of further investigation in this and other animal models of preeclampsia. It is from these types of studies we can learn more about the mechanisms causing hypertension, proteinuria, intrauterine growth restriction, and inflammation triggered in response to placental ischemia that will help us define better diagnostic and treatment parameters for women with this disease.
GRANTS
This work was supported by American Heart Association Grant SDG0835472N and by National Heart, Lung, and Blood Institute Grants HL-78147 and HL-51971, and NIH/NICHD R01 HD067541. R. Dechend is supported by the German Research Foundation (DFG 631/7-1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R.N., K. Wallace, J.H., J.M., P.D., A.W., G.W., F.H., K. Wenzel, R.D., and B.L. performed experiments; S.R.N., J.H., J.M., G.W., F.H., R.D., and B.L. analyzed data; S.R.N., F.H., and B.L. interpreted results of experiments; S.R.N. and B.L. drafted manuscript; S.R.N., J.N.M., R.D., and B.L. edited and revised manuscript; K. Wallace and B.L. prepared figures; K. Wallace, P.D., A.W., G.W., F.H., K. Wenzel, J.N.M., R.D., and B.L. approved final version of manuscript; R.D. and B.L. conception and design of research.
REFERENCES
Articles from American Journal of Physiology - Regulatory, Integrative and Comparative Physiology are provided here courtesy of American Physiological Society
Full text links
Read article at publisher's site: https://doi.org/10.1152/ajpregu.00623.2011
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3362148
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1152/ajpregu.00623.2011
Article citations
Placental ischemia-upregulated angiotensin II type 1 receptor in hypothalamic paraventricular nucleus contributes to hypertension in rat.
Pflugers Arch, 476(11):1677-1691, 31 Aug 2024
Cited by: 0 articles | PMID: 39215834
Reduced uterine perfusion pressure as a model for preeclampsia and fetal growth restriction in murine: a systematic review and meta-analysis.
Am J Physiol Heart Circ Physiol, 327(1):H89-H107, 17 May 2024
Cited by: 1 article | PMID: 38758122 | PMCID: PMC11380978
Review Free full text in Europe PMC
AT1-AA Is Produced in Offspring in Response to Placental Ischemia and Is Lowered by B-Cell Depletion Without Compromising Overall Offspring Health.
J Am Heart Assoc, 13(4):e031417, 14 Feb 2024
Cited by: 0 articles | PMID: 38353227 | PMCID: PMC11010106
The role of immune cells and mediators in preeclampsia.
Nat Rev Nephrol, 19(4):257-270, 12 Jan 2023
Cited by: 37 articles | PMID: 36635411 | PMCID: PMC10038936
Review Free full text in Europe PMC
The Role of Different Lymphoid Cell Populations in Preeclampsia Pathophysiology.
Kidney360, 3(10):1785-1794, 12 Aug 2022
Cited by: 2 articles | PMID: 36514732 | PMCID: PMC9717666
Review Free full text in Europe PMC
Go to all (52) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats.
Hypertension, 52(6):1168-1172, 13 Oct 2008
Cited by: 123 articles | PMID: 18852381 | PMCID: PMC2782765
Blockade of CD40 ligand for intercellular communication reduces hypertension, placental oxidative stress, and AT1-AA in response to adoptive transfer of CD4+ T lymphocytes from RUPP rats.
Am J Physiol Regul Integr Comp Physiol, 309(10):R1243-50, 26 Aug 2015
Cited by: 17 articles | PMID: 26310940 | PMCID: PMC4666934
Hypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system.
Am J Physiol Regul Integr Comp Physiol, 303(2):R144-9, 30 May 2012
Cited by: 36 articles | PMID: 22647295 | PMCID: PMC3404637
Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia.
Curr Opin Pharmacol, 11(2):175-179, 01 Apr 2011
Cited by: 49 articles | PMID: 21317038 | PMCID: PMC3075337
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (3)
Grant ID: HL-51971
Grant ID: F32 HL108558
Grant ID: HL-78147
NICHD NIH HHS (1)
Grant ID: R01 HD067541
 1
1