Abstract
Free full text

Oscillatory activity of single units in a somatosensory cortex of an awake monkey and their possible role in texture analysis.
Abstract
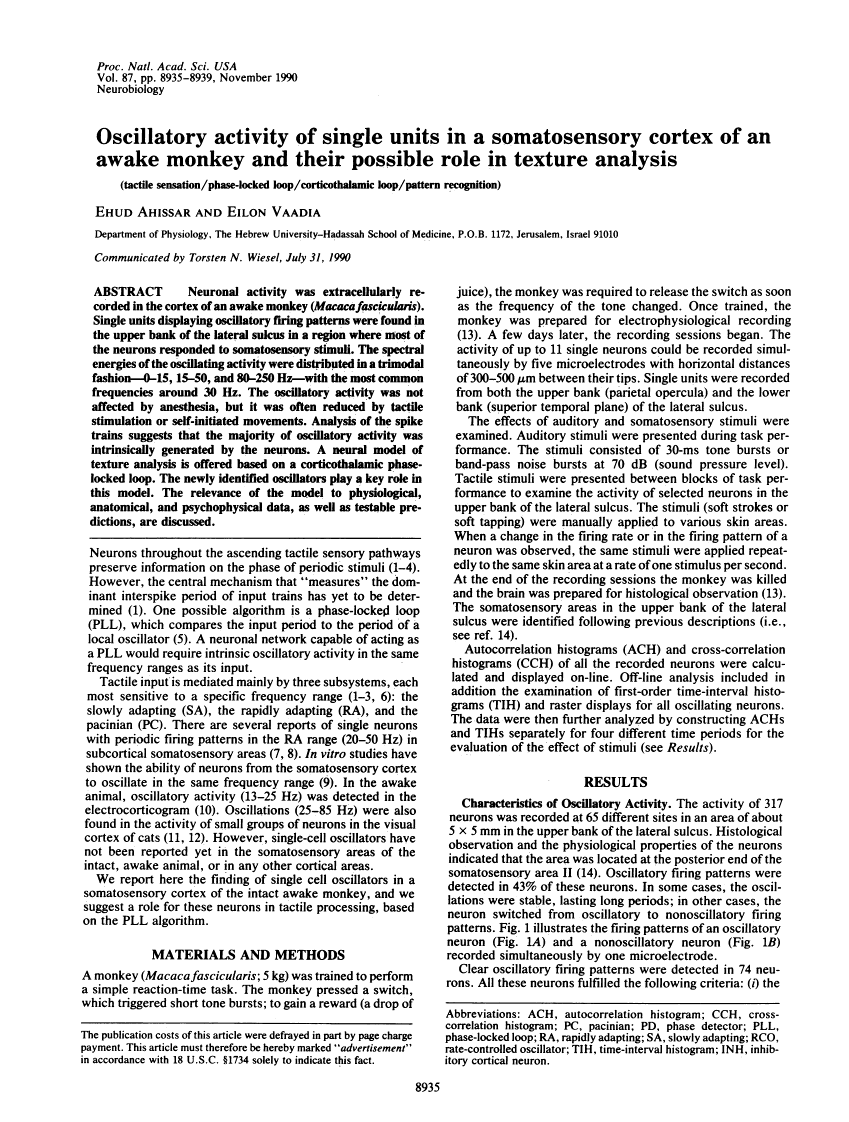
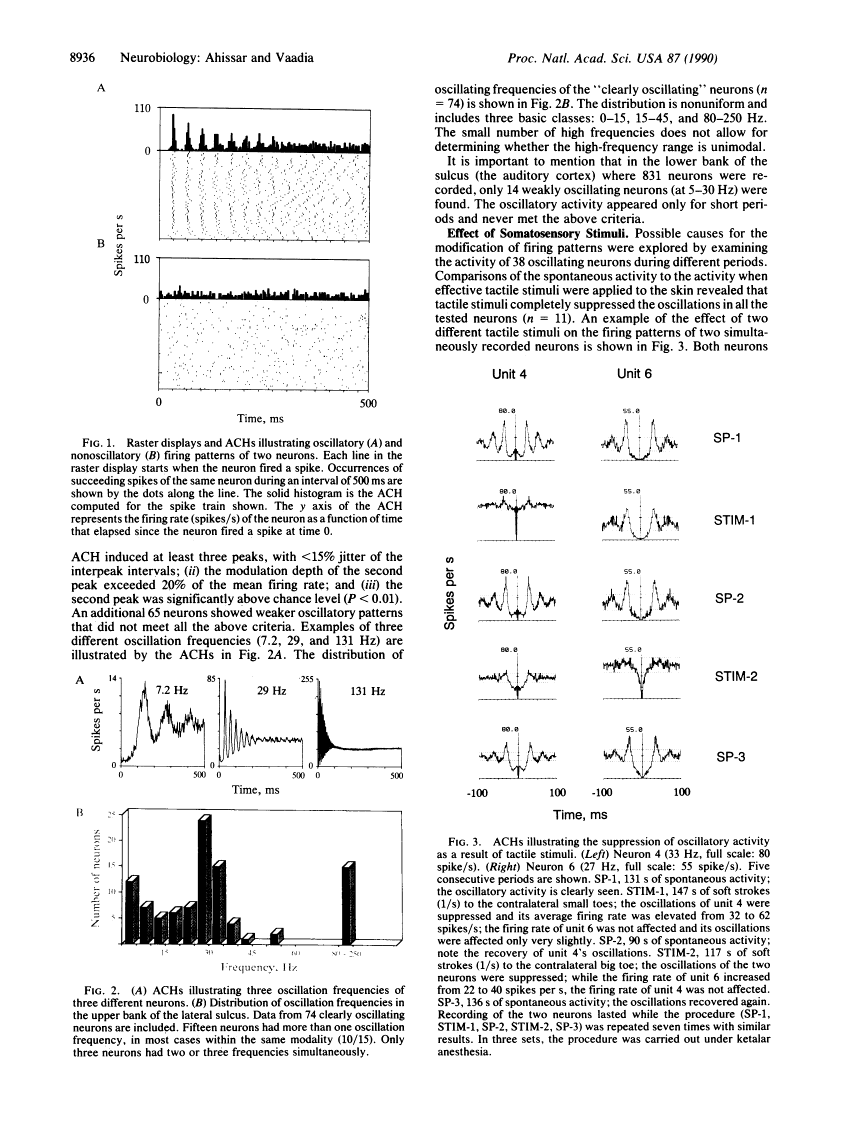
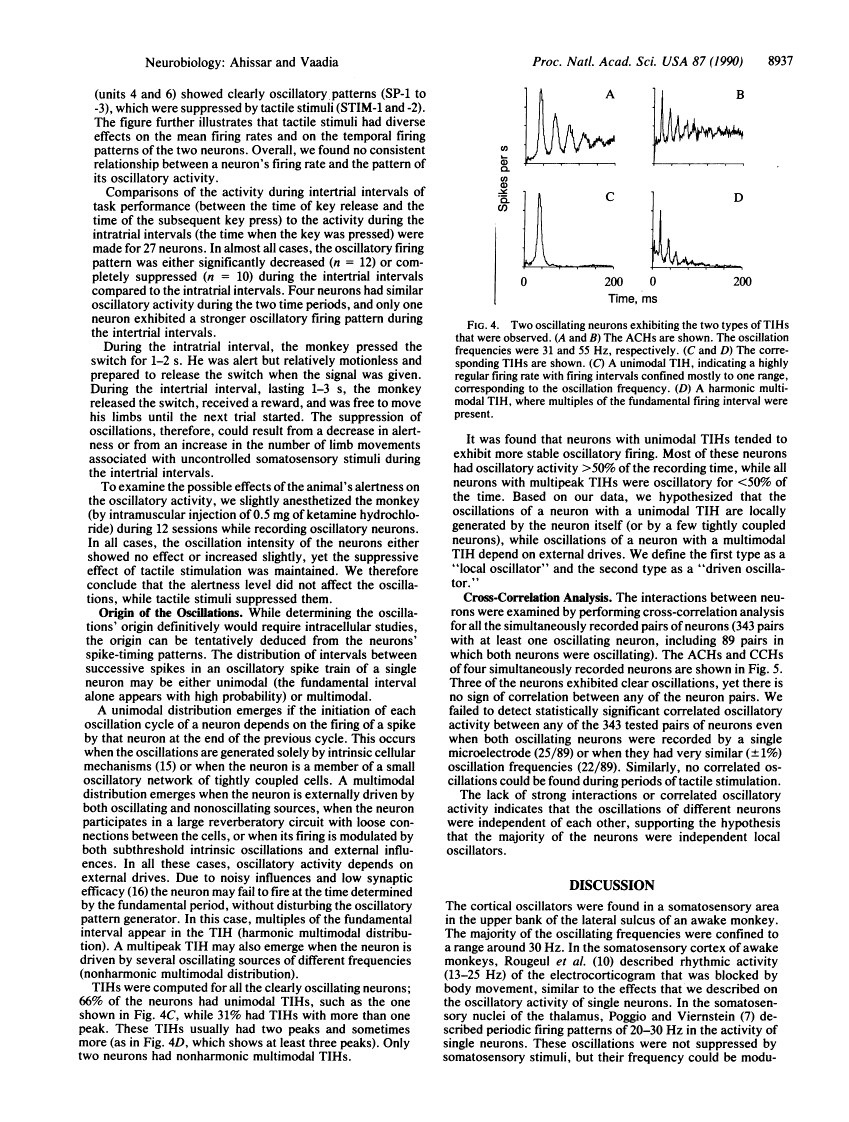
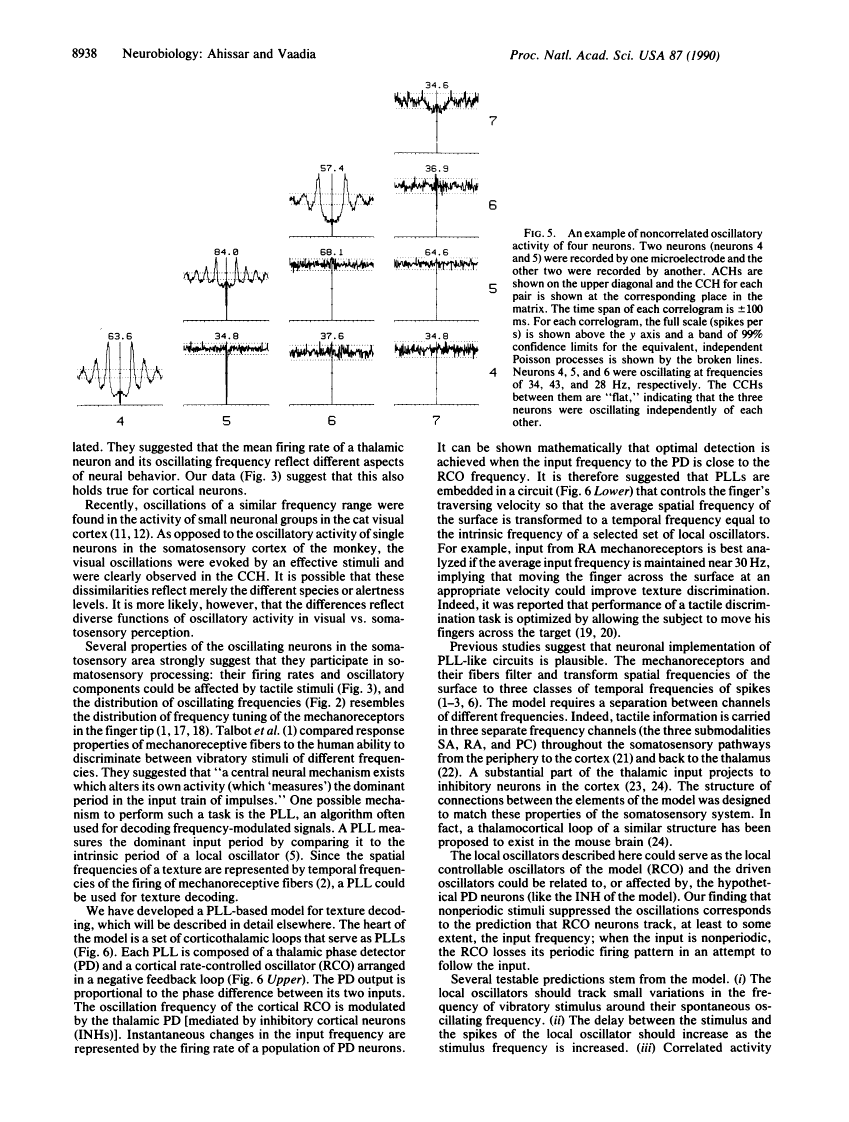
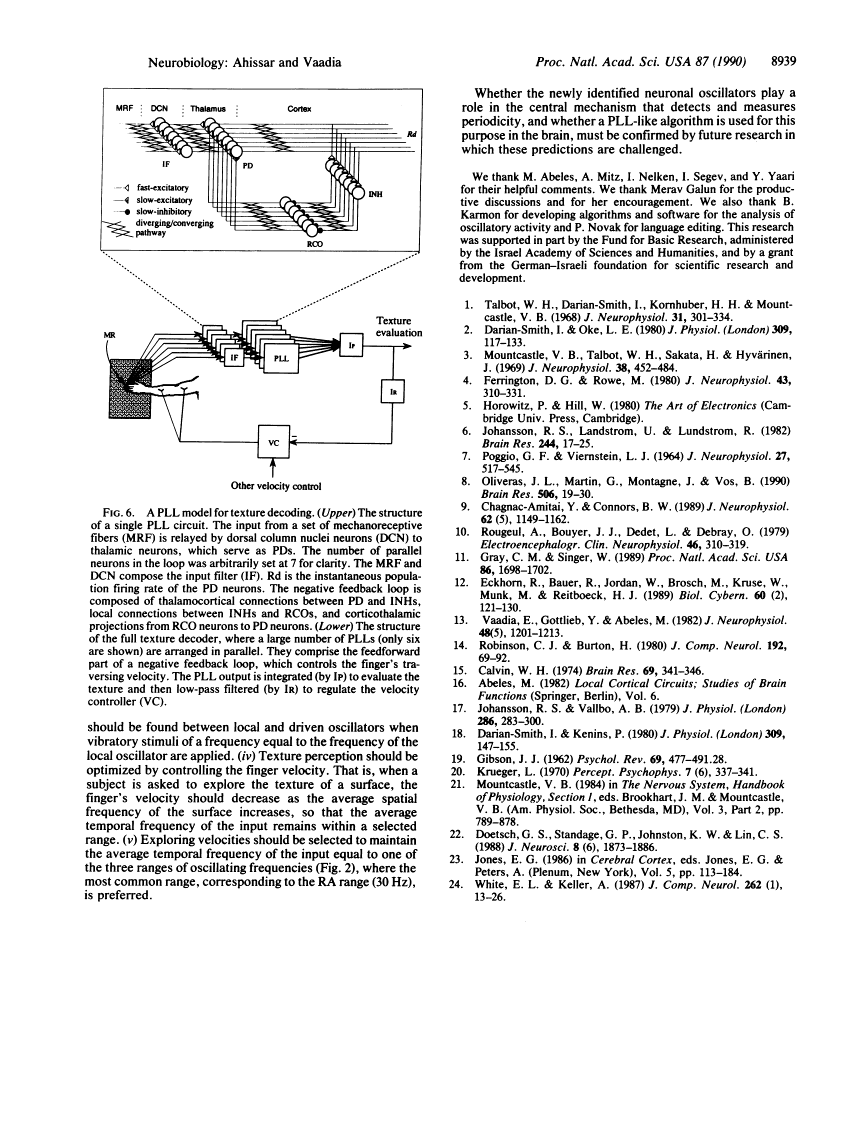
Neuronal activity was extracellularly recorded in the cortex of an awake monkey (Macaca fascicularis). Single units displaying oscillatory firing patterns were found in the upper bank of the lateral sulcus in a region where most of the neurons responded to somatosensory stimuli. The spectral energies of the oscillating activity were distributed in a trimodal fashion--0-15, 15-50, and 80-250 Hz--with the most common frequencies around 30 Hz. The oscillatory activity was not affected by anesthesia, but it was often reduced by tactile stimulation or self-initiated movements. Analysis of the spike trains suggests that the majority of oscillatory activity was intrinsically generated by the neurons. A neural model of texture analysis is offered based on a corticothalamic phase-locked loop. The newly identified oscillators play a key role in this model. The relevance of the model to physiological, anatomical, and psychophysical data, as well as testable predictions, are discussed.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. [Abstract] [Google Scholar]
- Darian-Smith I, Oke LE. Peripheral neural representation of the spatial frequency of a grating moving across the monkey's finger pad. J Physiol. 1980 Dec;309:117–133. [Abstract] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. [Abstract] [Google Scholar]
- Ferrington DG, Rowe M. Differential contributions to coding of cutaneous vibratory information by cortical somatosensory areas I and II. J Neurophysiol. 1980 Feb;43(2):310–331. [Abstract] [Google Scholar]
- Johansson RS, Landström U, Lundström R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res. 1982 Jul 22;244(1):17–25. [Abstract] [Google Scholar]
- POGGIO GF, VIERNSTEIN LJ. TIME SERIES ANALYSIS OF IMPULSE SEQUENCES OF THALAMIC SOMATIC SENSORY NEURONS. J Neurophysiol. 1964 Jul;27:517–545. [Abstract] [Google Scholar]
- Oliveras JL, Martin G, Montagne J, Vos B. Single unit activity at ventromedial medulla level in the awake, freely moving rat: effects of noxious heat and light tactile stimuli onto convergent neurons. Brain Res. 1990 Jan 1;506(1):19–30. [Abstract] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989 Nov;62(5):1149–1162. [Abstract] [Google Scholar]
- Rougeul A, Bouyer JJ, Dedet L, Debray O. Fast somato-parietal rhythms during combined focal attention and immobility in baboon and squirrel monkey. Electroencephalogr Clin Neurophysiol. 1979 Mar;46(3):310–319. [Abstract] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1698–1702. [Europe PMC free article] [Abstract] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. [Abstract] [Google Scholar]
- Vaadia E, Gottlieb Y, Abeles M. Single-unit activity related to sensorimotor association in auditory cortex of a monkey. J Neurophysiol. 1982 Nov;48(5):1201–1213. [Abstract] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol. 1980 Jul 1;192(1):69–92. [Abstract] [Google Scholar]
- Calvin WH. Three modes of repetitive firing and the role of threshold time course between spikes. Brain Res. 1974 Apr 5;69(2):341–346. [Abstract] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979 Jan;286:283–300. [Abstract] [Google Scholar]
- Darian-Smith I, Kenins P. Innervation density of mechanoreceptive fibres supplying glabrous skin of the monkey's index finger. J Physiol. 1980 Dec;309:147–155. [Abstract] [Google Scholar]
- GIBSON JJ. Observations on active touch. Psychol Rev. 1962 Nov;69:477–491. [Abstract] [Google Scholar]
- Doetsch GS, Standage GP, Johnston KW, Lin CS. Thalamic connections of two functional subdivisions of the somatosensory forepaw cerebral cortex of the raccoon. J Neurosci. 1988 Jun;8(6):1873–1886. [Abstract] [Google Scholar]
- White EL, Keller A. Intrinsic circuitry involving the local axon collaterals of corticothalamic projection cells in mouse SmI cortex. J Comp Neurol. 1987 Aug 1;262(1):13–26. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.87.22.8935
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc55075?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Thalamocortical loops as temporal demodulators across senses.
Commun Biol, 6(1):562, 26 May 2023
Cited by: 1 article | PMID: 37237075 | PMCID: PMC10220046
Review Free full text in Europe PMC
Idiosyncratic selection of active touch for shape perception.
Sci Rep, 12(1):2922, 21 Feb 2022
Cited by: 0 articles | PMID: 35190603 | PMCID: PMC8861104
Active sensory substitution allows fast learning via effective motor-sensory strategies.
iScience, 24(1):101918, 11 Dec 2020
Cited by: 2 articles | PMID: 33392481 | PMCID: PMC7773576
An Oscillator Ensemble Model of Sequence Learning.
Front Integr Neurosci, 13:43, 20 Aug 2019
Cited by: 0 articles | PMID: 31481883 | PMCID: PMC6710383
Temporal Coding of Visual Space.
Trends Cogn Sci, 22(10):883-895, 01 Oct 2018
Cited by: 38 articles | PMID: 30266148 | PMCID: PMC6179437
Review Free full text in Europe PMC
Go to all (67) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys.
J Neurophysiol, 76(6):3968-3982, 01 Dec 1996
Cited by: 263 articles | PMID: 8985893
Tactile representation in somatosensory thalamus (VPL) and cortex (S1) of awake primate and the plasticity induced by VPL neuroprosthetic stimulation.
Brain Res, 1625:301-313, 05 Sep 2015
Cited by: 6 articles | PMID: 26348987
Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli.
J Neurosci, 20(14):5503-5515, 01 Jul 2000
Cited by: 200 articles | PMID: 10884334 | PMCID: PMC6772326
Response patterns in second somatosensory cortex (SII) of awake monkeys to passively applied tactile gratings.
J Neurophysiol, 84(2):780-797, 01 Aug 2000
Cited by: 27 articles | PMID: 10938305