Abstract
Background
To examine the rates and predictors of deep periprosthetic infections after primary total shoulder arthroplasty (TSA).Methods
We used prospectively collected data on all primary TSA patients from 1976-2008 at Mayo Clinic Medical Center. We estimated survival free of deep periprosthetic infections after primary TSA using Kaplan-Meier survival. Univariate and multivariable Cox regression was used to assess the association of patient-related factors (age, gender, body mass index), comorbidity (Deyo-Charlson index), American Society of Anesthesiologists class, implant fixation, and underlying diagnosis with risk of infection.Results
A total of 2,207 patients, with a mean age of 65 years (SD, 12 years), 53% of whom were women, underwent 2,588 primary TSAs. Mean follow-up was 7 years (SD, 6 years), and the mean body mass index was 30 kg/m(2) (SD, 6 kg/m(2)). The American Society of Anesthesiologists class was 1 or 2 in 61% of cases. Thirty-two confirmed deep periprosthetic infections occurred during follow-up. In earlier years, Staphylococcus predominated; in recent years, Propionibacterium acnes was almost as common. The 5-, 10-, and 20-year prosthetic infection-free rates were 99.3% (95% confidence interval [CI], 98.9-99.6), 98.5% (95% CI, 97.8-99.1), and 97.2% (95% CI, 96.0-98.4), respectively. On multivariable analysis, a male patient had a significantly higher risk of deep periprosthetic infection (hazard ratio, 2.67 [95% CI, 1.22-5.87]; P = .01) and older age was associated with lower risk (hazard ratio, 0.97 [95% CI, 0.95-1.00] per year; P = .05).Conclusions
The periprosthetic infection rate was low at 20-year follow-up. Male gender and younger age were significant risk factors for deep periprosthetic infections after TSA. Future studies should investigate whether differences in bone morphology, medical comorbidity, or other factors are underlying these associations.Free full text

Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective
Abstract
Background
To examine the rates and predictors of deep periprosthetic infections after primary total shoulder arthroplasty (TSA).
Methods
We used prospectively collected data on all primary TSA patients from 1976-2008 at Mayo Clinic Medical Center. We estimated survival free of deep periprosthetic infections after primary TSA using Kaplan-Meier survival. Univariate and multivariable Cox regression was used to assess the association of patient-related factors (age, gender, body mass index), comorbidity (Deyo-Charlson index), American Society of Anesthesiologists class, implant fixation, and underlying diagnosis with risk of infection.
Results
A total of 2,207 patients, with a mean age of 65 years (SD, 12 years), 53% of whom were women, underwent 2,588 primary TSAs. Mean follow-up was 7 years (SD, 6 years), and the mean body mass index was 30 kg/m2 (SD, 6 kg/m2). The American Society of Anesthesiologists class was 1 or 2 in 61% of cases. Thirty-two confirmed deep periprosthetic infections occurred during follow-up. In earlier years, Staphylococcus predominated; in recent years, Propionibacterium acnes was almost as common. The 5-, 10-, and 20-year prosthetic infection–free rates were 99.3% (95% confidence interval [CI], 98.9-99.6), 98.5% (95% CI, 97.8-99.1), and 97.2% (95% CI, 96.0-98.4), respectively. On multivariable analysis, a male patient had a significantly higher risk of deep periprosthetic infection (hazard ratio, 2.67 [95% CI, 1.22-5.87]; P =.01) and older age was associated with lower risk (hazard ratio, 0.97 [95% CI, 0.95-1.00] per year; P =.05).
Conclusions
The periprosthetic infection rate was low at 20-year follow-up. Male gender and younger age were significant risk factors for deep periprosthetic infections after TSA. Future studies should investigate whether differences in bone morphology, medical comorbidity, or other factors are underlying these associations.
Total shoulder arthroplasty (TSA) is a common surgery that is associated with significant improvements in pain, function, and quality of life in patients with end-stage shoulder joint arthritis.1,6,9 Infection is an uncommon complication after TSA, with the reported incidence ranging between 0.4%5 and 2.9%.15 However, infection is a catastrophic complication of TSA and, despite treatment, is associated with unsatisfactory results.10,11,13,14
One previous study reported that hematoma formation after shoulder arthroplasty was associated with deep periprosthetic infection risk.4 To our knowledge, none of the published studies have systematically examined the patient characteristics as risk factors for infection after TSA. In this study our aims were to examine the incidence of deep periprosthetic infection after TSA and to examine whether patient characteristics, implant fixation, and underlying diagnosis are associated with infection risk after TSA.
Methods
We used the Mayo Clinic Medical Center Total Joint Registry, which prospectively captures data on every shoulder arthroplasty since the surgery was performed at the Mayo Clinic, Rochester, Minnesota, USA, beginning in 1976. Our study cohort consisted of every adult aged 18 years or older with primary TSA performed at the Mayo Clinic Medical Center, Rochester, in a 33-year period from 1976-2008.
We identified all patients with periprosthetic infections (deep or superficial) using the prospectively collected data from the Mayo Clinic Medical Center Total Joint Registry. The Total Joint Registry provided data on patient characteristics (age at surgery, gender), implant fixation (uncemented vs cemented), and underlying diagnoses (osteoarthritis, rheumatoid arthritis, rotator cuff disease, trauma, tumor, and so on). The Mayo Clinic Medical Center electronic databases provided data on American Society of Anesthesiologists (ASA) class (1-4, where a higher class indicates worse physical status), body mass index (BMI) in kilograms per square meter, and Deyo-Charlson index, a validated comorbidity measure.7 The Deyo-Charlson index is the most commonly used comorbidity scale, consisting of a weighted scale of 17 comorbidities (including cardiac, pulmonary, renal, and hepatic disease; diabetes; cancer; and human immunodeficiency virus), expressed as a summative score2,3; a higher score indicates more comorbidity load.
Definitions for periprosthetic infection were constructed a priori in discussions with 2 experienced orthopaedic surgeons, who have worked closely with infectious disease consultants in managing these infected cases over the last 4 decades (J.W.S. and R.H.C.) and a rheumatologist-epidemiologist (J.A.S.). We defined infected TSA by the presence of one or both of the following: (1) positive joint fluid culture from needle aspiration, arthroscopic procedure, fluid obtained at surgery, or fluid draining from a wound communicating with the humerus; or (2) positive synovial or bone tissue culture. In those patients without a positive joint fluid culture, the presence of a clinical infection was defined when the treating orthopaedic surgeon believed an infection was present based on clinical presentation (history and physical examination), documented in the surgeon’s note or in the operating room (OR) note, and one or both of the following were found: (1) operative findings including purulent joint fluid, thick serosanguineous joint fluid, or the presence of necrotic synovial tissue; or (2) a positive blood culture. Infections that were limited to the skin and subcutaneous tissue without any extension beyond the fascial planes were categorized as superficial infections.
Summary statistics are reported for patient demographics as mean (standard deviation) or proportions. All analyses are presented by joints, with models accounting for correlated joints within the same patient. Kaplan-Meier survival was used to obtain infection-free survival estimates at 5, 10, and 20 years after TSA (with 95% confidence intervals [CIs]). Univariate Cox regression analyses assessed the association of patient and implant characteristics of interest (age, gender, BMI, comorbidity as assessed by Deyo-Charlson index [per unit increase], cemented vs uncemented implant), ASA class, and the underlying diagnosis. Similarly, multivariable analyses used Cox regression, by use of a backward-selection method, confirmed by use of forward selection. The α level was set at .05 for statistical significance.
Results
During the study period 1976-2008, 2,207 patients underwent 2,588 primary TSAs. The mean age was 65 years (SD, 12 years), and 53% of patients were women. Mean follow-up was 7 years (SD, 6 years; range, 1 day to 31 years), with follow-up censored at revision arthroplasty (patients were followed up only until the occurrence of revision surgery). The mean BMI was 30 kg/m2 (SD, 6 kg/m2); the ASA grade was 1 or 2 in 61% of cases and 3 or 4 in 39%. Other characteristics of the population are shown in Table I.
Table I
Clinical and demographic characteristics of study population
| All TSA cases (2,207 patients [2,588 shoulders]) | TSA with confirmed infection | |
|---|---|---|
| Age at surgery [mean (SD) (range)] (y) | 65 (12) (19-91) | 60 (12) (22-77) |
| Male/female | 47%/53% | 75%/25% |
| Deyo-Charlson index [mean (SD) (range)] | 0.8 (1) (0-13) | 0.6 (1) (0-6) |
| Implant fixation* | ||
 Cemented Cemented | 96% | 97% |
 Uncemented Uncemented | 4% | 3% |
| Diagnosis | ||
 Rheumatoid Rheumatoid | 17% | 16% |
 Trauma Trauma | 15% | 12% |
 Tumor Tumor | 1% | 0% |
 Osteoarthritis Osteoarthritis | 63% | 53% |
 Rotator cuff disease Rotator cuff disease | 2% | 3% |
 Other† Other† | 2% | 16% |
| BMI [mean (SD) (range)] | 30 (6) (16-60) | 31 (6) (20-40) |
| ASA class | ||
 1 or 2 1 or 2 | 61% | 55% |
 3 or 4 3 or 4 | 39% | 45% |
| Follow-up duration [mean (SD) (range)] | 7 y (6 y) (1 d to 31 y)‡ | 8 y (6 y) (2 mo to 21 y) |
| Median Deyo-Charlson index (IQR) | 0 (0-3) | 0 (0-3) |
| Deyo-Charlson index group | ||
 Heart disease (MI, CHF) Heart disease (MI, CHF) | 3.7% | 3.1% |
 Peripheral vascular disease Peripheral vascular disease | 2.4% | 0% |
 Cerebrovascular disease (stroke, hemiplegia) Cerebrovascular disease (stroke, hemiplegia) | 3.7% | 3.1% |
 Moderate-severe renal disease Moderate-severe renal disease | 3.4% | 0% |
 Peptic ulcer disease Peptic ulcer disease | 2.7% | 6.3% |
 Chronic obstructive pulmonary disease Chronic obstructive pulmonary disease | 6.8% | 6.3% |
 Diabetes (with or without organ damage) Diabetes (with or without organ damage) | 6.9% | 9.4% |
 Connective tissue disease Connective tissue disease | 18.3% | 18.8% |
 Cancer Cancer | 7.0% | 6.3% |
 Other (dementia, liver disease, AIDS) Other (dementia, liver disease, AIDS) | 1.0% | 0% |
IQR, interquartile range; CHF, congestive heart failure; MI, myocardial infarction.
There were 46 prosthetic infections recorded during the follow-up in the Mayo Clinic Total Joint Registry after TSA. Of these, 32 deep infections were confirmed on medical record review (Table II). In all confirmed cases, either an organism was identified on joint fluid/tissue gram stain or culture (n = 24) or the patient had clinical septic arthritis (n = 8) and 1 of the following: positive blood culture and/or presence of purulent or serosanguineous joint fluid or necrotic joint tissue (culture negative or culture not performed). Of these 8 cases, 6 required revision surgery or debridement, several with evidence of frank pus (n = 2), serosanguineous fluid (n = 1), or necrotic tissue (n = 1) obtained from the joint during joint surgery. Of the 14 unconfirmed infections, records were not available for 2 patients and 7 patients had superficial infection or hematoma. For 5 patients with no chart documentation of evidence of definite infection, the registry personnel entered the possibility of a periprosthetic infection being present when the surgeon in charge of the case noted a strong belief that an infection would be identified. However, in these cases an infection was later excluded, as documented by the surgeon in the medical record.
Table II
Periprosthetic infections in patients after TSA and shoulder hemiarthroplasty from registry databases confirmed by use of patients’ medical records with gold-standard definitions
| TSA (N = 2,588) | |
|---|---|
Potential periprosthetic infections identified  from registry databases from registry databases | 46 |
 No records No records | 2 |
 Superficial infection or hematoma Superficial infection or hematoma | 7 |
 No documentation of infection No documentation of infection | 5 |
| Confirmed total deep periprosthetic infections | 32 |
 Staphylococcus coagulase negative Staphylococcus coagulase negative | 5 |
 S aureus
*
S aureus
*
| 10 |
 Methicillin-resistant S aureus Methicillin-resistant S aureus | 0 |
 P acnes
P acnes
| 6 |
 Streptococcus (Streptococcus pneumoniae or Streptococcus (Streptococcus pneumoniae or  β-hemolytic Streptococcus) β-hemolytic Streptococcus) | 2 |
 Serratia
Serratia
| 1 |
 Clostridium species Clostridium species | 0 |
 Pseudomonas
Pseudomonas
| 0 |
 Bacillus species Bacillus species | 0 |
 Enterococcus species Enterococcus species | 0 |
 Clinical septic arthritis (with or without Clinical septic arthritis (with or without  pus or serosanguineous fluid or necrotic pus or serosanguineous fluid or necrotic  joint tissue or positive blood culture but joint tissue or positive blood culture but  no organism on joint fluid/tissue culture no organism on joint fluid/tissue culture  or culture not done)† or culture not done)† | 8 |
Superficial infection was defined as infection in skin and subcutaneous tissue without any extension beyond the fascial planes and without any extension into joint or pericapsular tissue. In all cases, these were treated with oral antibiotics, mostly without any incision and drainage.
Staphylococcus aureus was the most common organism (31%), followed by Propionibacterium (19%). The underlying organism in other cases is shown in Table II. Seven patients were diagnosed clinically and treated as having septic arthritis in the absence of positive cultures. There were some differences in the spectrum of organisms in different time periods from 1976-2008 (Table III). Infections in the earlier periods, 1976-1990, with identifiable microorganisms were exclusively Staphylococcus infections (S aureus or Staphylococcus coagulase negative), and the majority of those in the period 1991-2000 were Staphylococcus infections; however, in the most recent period of 2001-2008, Propionibacterium acnes caused infection in a large number of the patients.
Table III
Time trend in type of microbial infection
| Time period | No. at risk | Organism | No. |
|---|---|---|---|
| 1976-1990 (n = 4) | 660 patients/764 shoulders | S aureus | 2 |
| Clinically septic,* no organism | 1 | ||
| Necrosis, no organism | 1 | ||
| 1991-2000 (n = 14) | 650 patients/793 shoulders | S aureus | 3 |
| Staphylococcus coagulase negative | 3 | ||
| P acnes | 1 | ||
| Serratia | 1 | ||
| Clinically septic, no organism | 6 | ||
| 2001-2008 (n = 14) | 864 patients/1,031 shoulders | S aureus | 5 |
| Staphylococcus coagulase negative | 2 | ||
| Streptococcus | 2 | ||
| P acnes | 5 |
Overall infection-free survival
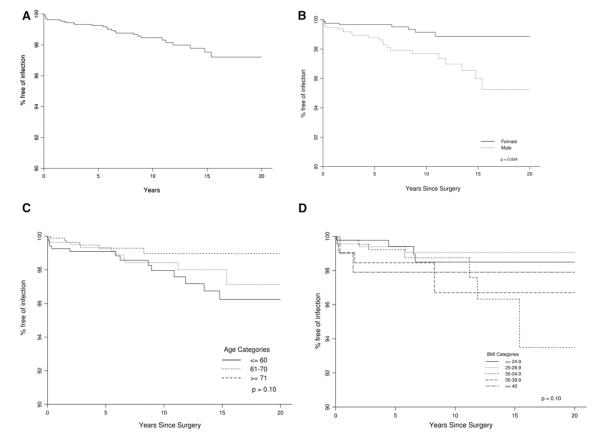
Estimates of survival free of deep periprosthetic infection at 5-, 10-, and 20-year follow-up were 99.3% (95% CI, 98.9%-99.6%), 98.5% (95% CI, 97.8%-99.1%), and 97.2% (95% CI, 96.0%-98.4%), respectively. The number of TSAs available for observation at 5 years was 1,470; at 10 years, it was 837; and at 20 years, it was 64. Infection-free survival rates by age, gender, and BMI are depicted in Figure. 1.
Significant correlates of deep periprosthetic infections
A female patient undergoing TSA had a significantly lower risk of a deep periprosthetic infection, with a hazard ratio of 0.29 (Table IV). Older age was associated with a lower risk of infection, with a hazard ratio of 0.78 per 10-year increase in age (Table IV). BMI, ASA class, Deyo-Charlson index, implant fixation, and underlying diagnosis were not significantly associated with risk of deep periprosthetic infection. We also examined individual Deyo-Charlson comorbidities, and none were significantly associated with the risk of deep periprosthetic infection.
Table IV
Association of clinical and demographic factors with periprosthetic infection in TSA on univariate analyses (n = 32)
| Variable | No. of shoulders | No. of deep infections | Kaplan-Meier estimates (95% CI) | Cox proportional hazards | ||||
|---|---|---|---|---|---|---|---|---|
| 5 y | 10 y | 20 y | Hazard ratio (95% CI) | P value | Overall P value | |||
| Male | 1,236 | 24 | 98.8 (98.1-99.5) | 97.7 (96.6-98.8) | 95.3 (92.8-97.7) | 1.0 (reference) | .006 | |
| Female | 1,352 | 8 | 99.7 (99.3-100) | 99.1 (98.5-99.8) | 98.9 (98.1-99.7) | 0.29 (0.12-0.71) | ||
| Age at surgery, per 10 y | 0.78 (0.61-0.98) | .04 | ||||||
| Deyo-Charlson index | 0.98 (0.69-1.40) | .93 | ||||||
| BMI* | .41 | |||||||
 <24.9 kg/m2 <24.9 kg/m2 | 475 | 4 | 99.4 (98.6-100) | 98.5 (97.0-100) | 98.5 (97.0-100) | 1.0 (reference) | ||
 25-29.9 kg/m2 25-29.9 kg/m2 | 744 | 5 | 99.4 (98.8-100) | 99.1 (98.2-99.9) | 99.1 (98.2-99.9) | 0.82 (0.22-3.02) | .76 | |
 30-34.9 kg/m2 30-34.9 kg/m2 | 521 | 7 | 99.2 (98.3-100) | 98.7 (97.5-100) | 93.5 (87.3-100) | 1.67 (0.47-5.95) | .43 | |
 35-39.9 kg/m2 35-39.9 kg/m2 | 235 | 4 | 98.5 (96.7-100) | 96.7 (93.0-100) | 96.7 (93.0-100) | 2.48 (0.63-9.83) | .19 | |
 ≥40 kg/m2 ≥40 kg/m2 | 126 | 2 | 97.9 (95.1-100) | 97.9 (95.1-100) | 97.9 (95.1-100) | 2.46 (0.45-13.34) | .29 | |
| ASA class† | ||||||||
 ASA (1/2) ASA (1/2) | 1,236 | 11 | 99.2 (98.6-99.7) | 98.9 (98.2-99.6) | 97.0 (93.1-100) | 1.0 (reference) | ||
 ASA (3/4) ASA (3/4) | 780 | 9 | 99.1 (98.2-99.9) | 98.2 (96.8-99.7) | 95.9 (92.5-99.5) | 1.41 (0.55-3.66) | .47 | |
| Implant fixation | ||||||||
 No cement No cement | 103 | 1 | 98.9 (96.6-100) | 98.9 (96.6-100) | 98.9 (96.6-100) | 1.0 (reference) | ||
 Cement Cement | 2,485 | 31 | 99.3 (98.9-99.6) | 98.4 (97.8-99.1) | 97.1 (95.8-98.4) | 1.95 (0.26-14.76) | .52 | |
| Diagnosis | ||||||||
 No osteoarthritis No osteoarthritis | 948 | 15 | 98.9 (98.1-99.6) | 98.1 (97.0-99.1) | 97.4 (96.0-98.8) | 1.0 (reference) | ||
 Osteoarthritis Osteoarthritis | 1,640 | 17 | 99.5 (99.1-99.9) | 98.7 (98.0-99.5) | 96.8 (94.8-98.9) | 0.82 (0.37-1.80) | .62 | |
On multivariable analysis (n = 32), older age and female gender were significantly associated with lower risk of infection. Female gender was significantly associated with lower risk of deep periprosthetic infection, with a hazard ratio of 0.29 (95% CI, 0.12-0.71; P =.007). Older age had a borderline significant association with a lower risk of deep periprosthetic infection, with a hazard ratio of 0.77 per 10 years of age (95% CI, 0.61-1.00; P =.05).
Sensitivity analysis was performed by limiting the multivariable analyses to 24 confirmed deep periprosthetic infections, excluding the 8 patients with clinical septic arthritis without positive joint fluid cultures (as recommended by the reviewers). The association of female gender with risk of deep periprosthetic infection persisted, with a hazard ratio of 0.23 (95% CI, 0.08-0.63; P = .004). Age was no longer significantly associated, with a hazard ratio of 0.85 (95% CI, 0.62-1.16; P =.30), but higher BMI was now associated, with a hazard ratio of 1.01 per 1-kg/m2 increase (95% CI, 1.003-1.02; P = .011), where it was previously not significant. No other changes were noted.
We compared the OR time and receipt of transfusion (yes/no) between those without infection and those without infection. We found longer OR time in those with deep periprosthetic infection (mean, 223 minutes; SD, 60 minutes) compared with those without infection (mean, 194 minutes; SD, 65 minutes), as expected. The hazard ratio for infection was 1.08 per 15-minute increase in the OR time (95% CI, 1.01-1.16; P = .03). There was no association between transfusion (yes vs no) and the hazard of infection, with a hazard ratio of 1.67 (95% CI, 0.63-4.41; P = .30).
Discussion
In our study the rate of deep periprosthetic infection risk was low in TSA patients with a high infection-free survival rate of 97% at 20-year follow-up. Men were significantly more likely and older patients were significantly less likely to have deep periprosthetic infections after TSA. We noted some change in etiopathogenesis of deep periprosthetic infection over a 33-year period, with Propionibacterium recognized more commonly as the causative organism for periprosthetic infections in the most recent time period (ie, 2001-2008).
We reviewed data from a 33-year period from a large institutional registry and found rates of deep periprosthetic infections in TSA similar to those reported in a few studies previously. In a systematic review of 40 studies of the Neer type II prosthesis, the rate of periprosthetic infections was 0.9% for TSA in 3,584 patients at a mean follow-up of 59 months.16 Similarly, in a previous 23-year study, 1.1% of primary shoulder arthroplasties (25 of 2,279) (3.6% of revision cases [7 of 194]) were diagnosed with deep periprosthetic infections,13 with S aureus and Staphylococcus coagulase negative as the most common organisms. To put this in perspective, prosthetic infection was reported in 1.6% of patients within 2 years and 0.6% between 2 and 10 years in those who had undergone total hip arthroplasty.12
To our knowledge, there are rare published systematic studies examining the factors associated with shoulder periprosthetic infections. In a previous study, reoperation for hematoma formation was a risk factor for deep periprosthetic infection after primary or revision shoulder arthroplasty (2 of 12 patients with hematoma later underwent resection arthroplasty for deep infection).4 A previous review suggested underlying diagnosis of rheumatoid arthritis, presence of diabetes, use of systemic corticosteroid or immunosuppressive medications, previous shoulder surgery, or repeated intra-articular corticosteroid injections as risk factors for periprosthetic infections, based on small series.17
In our study we examined both underlying diagnoses and medical comorbidity, but we did not find any significant association with risk of deep periprosthetic infections. Instead, in this study we found that male gender and younger age increased the risk, which was confirmed on multivariable-adjusted analyses. These findings are novel and add to the literature. The strength of our study is the follow-up of a large number of patients and confirmation of each case with data from the medical records. Our study finding of higher infection risk in men is similar to a finding of higher risk of periprosthetic infection in men after elective total hip arthroplasty in a Medicare sample.12 We do not understand why younger patients and men might be more susceptible to the development of a periprosthetic infection. Younger patients are much more likely to have had previous trauma or rheumatoid arthritis, and younger men are more likely to have had significant trauma. Perhaps the poorer tissue quality in patients with previous trauma or the systemic effects and medical treatment of the patients with rheumatoid arthritis played a role, despite diagnostic considerations not being significant when directly tested. When analyses were limited to 24 joint culture–proven deep periprosthetic infections, men and patients with higher BMI were at higher risk (but not younger patients). A higher risk of periprosthetic infection in more obese patients is similar to that reported in patients with primary hip arthroplasty8 and can be postulated to result from several hypothesized alterations in host immune system with obesity. However, we did not find an association of comorbidity with risk of infection, as was found in this total hip arthroplasty cohort.12 This may be because of the small number of infections in our study making it underpowered to detect such a difference or a difference in etiology of infection by the type of joint (shoulder vs hip). Our study identifies risk factors for periprosthetic infections, but this should not be interpreted as a recommendation of screening of only men and younger patients with TSA for infection. The screening for periprosthetic infections after TSA should be based on the clinical judgment of expert orthopaedic surgeons, taking these findings into consideration.
Another interesting finding is the possible changing microbiology of shoulder periprosthetic infection that we noted in our study. Although Staphylococcus was the offending organism in the majority of patients with identifiable organisms in the earlier periods, P acnes was almost as common as Staphylococcus in the most recent time period, 2001-2008. This may indicate a changing pattern in offending organism for shoulder periprosthetic infection. It is also possible that recent recognition of P acnes as an underlying organism may have led to increased surveillance for this organism; in addition, changes in culture incubation periods and laboratory assays over the study period may have also influenced the rate of detection of P acnes. Most laboratories now test for P acnes, although this was not the case 2 decades ago. It is interesting that whereas 2 patients had culture-negative septic arthritis in 1976-1990 and 6 patients during 1991-2000, none in the most recent period has culture-negative septic arthritis. Though speculative, it is possible that several of the culture-negative septic arthritis cases in the patients before 1990 could have had P acnes. It is unlikely that any change in pattern or type of antibiotics in the perioperative period has contributed to this, because patients typically have received 24 hours of perioperative antibiotic coverage, typically with cefazolin intravenously, with minimal changes in the types of antibiotics used over the years. However, contribution from minor changes cannot be ruled out. Future studies should examine whether the prevalence of this organism in periprosthetic infections is truly increasing. There was also some indication that the low total number of infections in the first period 1976-1990 (n = 4) may be increasing over the subsequent periods of observation. This may denote a higher index of suspicion, availability of better microbiologic techniques to diagnose uncommon and rare organisms, inclusion of patients with higher comorbidity in more recent time periods, or a simple increase in the rate of infections. If these findings are confirmed by other medical centers, they may have potential implications on the choice of perioperative antibiotics for patients undergoing shoulder arthroplasty in the future. In exploratory analyses, we analyzed whether age or gender is associated with the risk of P acnes and did not find any evidence in this small study.
We confirm the high infection-free survival rate noted in a previous study13 with our study. We found that the 20-year infection-free survival rate was high, at 97%. This needs to be confirmed with long-term follow-up studies from other institutions and/or national registries.
Our study has several limitations. Our observations are based on patients seen in a large medical center that provides both orthopaedic care for the local population and referral specialty care. Generalizability to other cohorts remains to be seen; reproducibility of these findings with studies from other centers/registries will support generalizability. Even a larger cohort of patients needs to be followed up to study risk factors for periprosthetic infections, because we found only 32 periprosthetic infections over a 33-year period of observation. With only 32 cases, this study was likely underpowered to look at risk factors for infections. For example, we could not examine individual comorbidities as predictors of infections, to avoid overfitting the model and to avoid multiple comparisons, when the overall outcome occurred in 32 cases only. Similarly, very few patients had uncemented implants, which also likely did not allow assessment of this variable as a predictor of infections. Finally, no consensus exists for definitions for periprosthetic infections; to overcome this limitation, we developed a working definition of periprosthetic infections a priori, based on the consensus of 3 clinicians, 2 of whom are experienced orthopaedic surgeons, with more than 50 years of cumulative experience in performing shoulder arthroplasty. Readers may appropriately debate whether inclusion of a definition using “clinically suspected septic arthritis plus either purulent or serosanguineous joint fluid or necrotic joint tissue (culture negative or culture not performed)” was specific enough for periprosthetic infections. The inclusion of this algorithm was based on clinical observation that if the orthopaedic surgeon clinically suspected septic arthritis (based on history and physical examination and documented in their notes) and had these additional findings during the surgery, most patients would be treated as having septic arthritis. However, we provide main analyses limited to the 24 patients with joint fluid culture–proven infection. This definition is subject to further discussion and may need explicit endorsement by infectious experts as well, because several patients can present as culture negative early in their disease.
Conclusion
We found a low risk of deep periprosthetic infection after TSA in a follow-up of up to 20 years. Staphylococcus and Propionibacterium were the most common organisms associated with deep periprosthetic infections after TSA. Two patient factors, male gender and younger age, were risk factors for deep periprosthetic infections. Further studies are needed to investigate the underlying reasons for these differences by studying these and additional factors, such as bone characteristics, implant characteristics, and specific comorbidities, in even larger series of patients than presented here.
Acknowledgments
This material is the result of work supported by National Institutes of Health Clinical Translational Science Award 1 KL2 RR024151-01 (Mayo Clinic Center for Clinical and Translational Research) and the resources and the use of facilities at the Birmingham VA Medical Center, Birmingham, Alabama, USA.
Footnotes
Disclaimer There are no financial conflicts related to this work. J.A.S. has received speaker honoraria from Abbott; research and travel grants from Allergan, Takeda, Savient, Wyeth, and Amgen; and consultant fees from Savient, URL Pharmaceuticals, and Novartis. J.W.S. has received royalties from Aircast and Biomet and consultant fees from Tornier and owns stock in Tornier. R.H.C. has received royalties from Smith & Nephew.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
This study was approved by the Mayo Clinic’s Institutional Review Board, and all investigations were conducted in conformity with ethical principles of research.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jse.2012.01.006
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3586318?pdf=render
Citations & impact
Impact metrics
Article citations
Isolation and Antibiofilm Activity of Bacteriophages against <i>Cutibacterium acnes</i> from Patients with Periprosthetic Joint Infection.
Viruses, 16(10):1592, 10 Oct 2024
Cited by: 0 articles | PMID: 39459925 | PMCID: PMC11512206
Positive Cutibacterium acnes Intervertebral Discs Are Not Associated with Subsidence Following Anterior Cervical Discectomy and Fusion at 3 or 6 Months.
J Clin Med, 13(18):5619, 22 Sep 2024
Cited by: 0 articles | PMID: 39337106 | PMCID: PMC11432799
Impact of postoperative skin disinfection with chlorhexidine on bacterial colonisation following shoulder arthroplasty surgery: a controlled randomised study.
Infect Prev Pract, 6(2):100365, 26 Apr 2024
Cited by: 0 articles | PMID: 38765917 | PMCID: PMC11098957
Antimicrobial effects of blue light therapy against cutibacterium acnes: optimal dosing and impact of serial treatments.
JSES Int, 8(2):328-334, 18 Dec 2023
Cited by: 0 articles | PMID: 38464448 | PMCID: PMC10920142
Next-generation Sequencing Results Require Higher Inoculum for Cutibacterium acnes Detection Than Conventional Anaerobic Culture.
Clin Orthop Relat Res, 481(12):2484-2491, 21 Jun 2023
Cited by: 2 articles | PMID: 37341498
Go to all (116) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Periprosthetic infections after shoulder hemiarthroplasty.
J Shoulder Elbow Surg, 21(10):1304-1309, 11 Dec 2011
Cited by: 45 articles | PMID: 22154310 | PMCID: PMC3310339
Periprosthetic fractures associated with primary total shoulder arthroplasty and primary humeral head replacement: a thirty-three-year study.
J Bone Joint Surg Am, 94(19):1777-1785, 01 Oct 2012
Cited by: 42 articles | PMID: 23032588 | PMCID: PMC3448303
Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty.
Clin Orthop Relat Res, 472(9):2809-2815, 07 Jun 2014
Cited by: 80 articles | PMID: 24906812 | PMCID: PMC4117904
Detritic synovitis can mimic a Propionibacterium periprosthetic infection.
Int Orthop, 40(1):95-98, 10 Nov 2015
Cited by: 3 articles | PMID: 26555186
Review
Funding
Funders who supported this work.
NCRR NIH HHS (3)
Grant ID: KL2 RR024151-04
Grant ID: 1 KL2 RR024151-01
Grant ID: KL2 RR024151