Abstract
Free full text

Phosphate Import in Plants: Focus on the PHT1 Transporters
Abstract
The main source of phosphorus for plants is inorganic phosphate (Pi), which is characterized by its poor availability and low mobility. Uptake of this element from the soil relies heavily upon the PHT1 transporters, a specific family of plant plasma membrane proteins that were identified by homology with the yeast PHO84 Pi transporter. Since the discovery of PHT1 transporters in 1996, various studies have revealed that their function is controlled by a highly complex network of regulation. This review will summarize the current state of research on plant PHT1 multigenic families, including physiological, biochemical, molecular, cellular, and genetics studies.
Introduction
Phosphorus is an essential macro-element for life. It plays a key role in many crucial processes such as heredity (DNA, RNA), cellular compartmentalization (membrane lipids), energy metabolism (ATP), and phosphorylation-based signaling mechanisms (Poirier and Bucher, 2002; Vance et al., 2003; Misson et al., 2005; Jouhet et al., 2007). Despite its abundance in the environment (ranked as the 11th most abundant element), phosphorus is neither easily accessible nor evenly distributed in most soils. For example, 20–80% of phosphorus in soil can be present as organic pools (mostly composed of plant and microorganism residues), which cannot be absorbed directly by the plants (Richardson, 1994). In plants, phosphorus represents 0.1–0.5% of the dry weight, and this element is acquired in the form of phosphate [inorganic phosphate (Pi)], an inorganic form of P. Depending on the pH, several anions can exist ( m2
m2 s−1). As a consequence, Pi uptake by the plant creates a depleted area around the roots (Ullrich-Eberius et al., 1984; Furihata et al., 1992; Schachtman et al., 1998). Therefore, Pi can easily be considered as one of the least available plant macronutrients (Raghothama, 1999).
s−1). As a consequence, Pi uptake by the plant creates a depleted area around the roots (Ullrich-Eberius et al., 1984; Furihata et al., 1992; Schachtman et al., 1998). Therefore, Pi can easily be considered as one of the least available plant macronutrients (Raghothama, 1999).
Before the modern intensification of agriculture, farmers relied mostly on phosphorus naturally present in soils or from manure. However, as a growing world population has put high demands on food production (with adverse effects on soil degradation), agriculture has become dependent on external sources of phosphate fertilizers (such as guano, ground bones, and phosphate rock) to increase crop yields (Cordell et al., 2009). Phosphate rock is an abundant and cheap source of phosphorus, and has quickly become the main source of Pi for fertilizer production. Despite this abundance, phosphate rock is still a non-renewable resource, which required 10–15 million years for its production (Cordell et al., 2009). As of 2009, the total mineable phosphate rock reserves were estimated at 15–16 billion tons (containing approximately 30% Pi), according to the US Geological Survey (an organization that collects data worldwide to estimate levels of our natural resources). The gravity of the situation is apparent when it is taken into consideration that 170 million tons of phosphate rock were extracted that same year for fertilizer production. The demand for phosphate rock will only steadily increase, with the mounting concerns to feed a growing global population, and the development of plant culture dedicated to biofuel production. These facts clearly strengthen the estimate of remaining Pi rock reserves. According to these data, our Pi rock reserves should be exhausted in only 50–100 years (Gilbert, 2009; Gross, 2010). A 700% increase in the price of Pi was observed in 2008, foreshadowing the impact that Pi scarcity can have on the economy (Cordell et al., 2009). This underscores an urgent need for an alternative to the use of phosphate rock reserves, which are largely devoted (82%) to fertilizer production. Moreover, this production process demands the exploitation of high quality Pi reserves in order to avoid contamination by toxic heavy metals such as cadmium or isotopes of the natural decay series of uranium or thorium (Othman and Al-Masri, 2007; Casacuberta et al., 2011; Da Conceicao et al., 2011). Improving our knowledge of Pi acquisition and use by plants will undoubtedly have a positive effect on reducing the dependency on fertilizer supply for crop production.
years (Gilbert, 2009; Gross, 2010). A 700% increase in the price of Pi was observed in 2008, foreshadowing the impact that Pi scarcity can have on the economy (Cordell et al., 2009). This underscores an urgent need for an alternative to the use of phosphate rock reserves, which are largely devoted (82%) to fertilizer production. Moreover, this production process demands the exploitation of high quality Pi reserves in order to avoid contamination by toxic heavy metals such as cadmium or isotopes of the natural decay series of uranium or thorium (Othman and Al-Masri, 2007; Casacuberta et al., 2011; Da Conceicao et al., 2011). Improving our knowledge of Pi acquisition and use by plants will undoubtedly have a positive effect on reducing the dependency on fertilizer supply for crop production.
Inorganic phosphate concentration in plant tissues has been measured at 5–20 mM (Raghothama, 1999), whereas the level available in soils is typically less than 10
mM (Raghothama, 1999), whereas the level available in soils is typically less than 10 μM (Bieleski, 1973). This sharp concentration gradient between the plant and the soil illustrates the crucial role of Pi transporters. The study of the PHT1 family of phosphate transporters is particularly appropriate, due to their presence in the plant plasma membrane (at the interface between the cell and the external medium). Several reviews have focused on this family of transporters (Raghothama, 1999; Smith et al., 2000; Rausch and Bucher, 2002; Smith and Barker, 2002; Bucher, 2007), since the identification of phosphate transporters in Arabidopsis thaliana in 1996 (Muchhal et al., 1996). More recent advances have revealed novel processes that control this family of transporters. The present article will synthesize this literature with special attention to PHT1 regulation and the plant model A. thaliana.
μM (Bieleski, 1973). This sharp concentration gradient between the plant and the soil illustrates the crucial role of Pi transporters. The study of the PHT1 family of phosphate transporters is particularly appropriate, due to their presence in the plant plasma membrane (at the interface between the cell and the external medium). Several reviews have focused on this family of transporters (Raghothama, 1999; Smith et al., 2000; Rausch and Bucher, 2002; Smith and Barker, 2002; Bucher, 2007), since the identification of phosphate transporters in Arabidopsis thaliana in 1996 (Muchhal et al., 1996). More recent advances have revealed novel processes that control this family of transporters. The present article will synthesize this literature with special attention to PHT1 regulation and the plant model A. thaliana.
Identification of PHT1 Phosphate Transporters
Beginning in the early 1990s, several high affinity phosphate transporters were cloned in various fungi. The first one, PHO84, was identified in the yeast Saccharomyces cerevisiae (Bun-Ya et al., 1991). Several years later, transporter homologs were found in Neurospora crassa (Versaw, 1995; Versaw and Metzenberg, 1995) and in the mycorrhizal fungus Glomus versiforme (Harrison and Van Buuren, 1995). Their discovery enabled the identification of phosphate transporters in plants. Two separate approaches were used for their identification in Arabidopsis, either by the heterologous complementation of the yeast pho84 mutant by plant cDNA (Muchhal et al., 1996) or by the identification of Arabidopsis ESTs closely related to these proteins (Mitsukawa et al., 1997; Smith et al., 1997). Later, their function as phosphate transporters was confirmed in planta by the analysis of Arabidopsis mutants such as pht1;1 and pht1;4 and the corresponding double mutant (Misson et al., 2004; Shin et al., 2004). The nearly simultaneous identification of these genes by several teams introduced some initial confusion to the literature, as different nomenclatures were proposed (PT or PHT) by these authors. Adding to this confusion, as the genes turned out to belong to a wide multigenic family, several authors published descriptions of different homologs under the same name (Muchhal et al., 1996; Smith et al., 1997; Okumura et al., 1998). Not until the complete sequencing of the A. thaliana genome in 2000 was it revealed that the PHT1 family is comprised of nine members (PHT1;1 through PHT1;9; Figure Figure1).1). Their names have now been unified according to the rule of the Commission on Plant Gene Nomenclature (Rausch and Bucher, 2002).

Sequence alignment of several PHT1 transporters from Medicago truncatula (Mt), Arabidopsis thaliana (At), Hordeum vulgare (Hv), and Oryza sativa (Os). High or low affinity characterized PHT1 are indicated by orange or green colors respectively. Stars indicate the 7 amino acids with non-conservative changes between the three low affinity (MtPT1, Mt PT2, MtPT3) and the high affinity transporters (MtPT5) identified by Liu et al. (2008). The serine identified in AtPHT1.1 (Bayle et al., 2011) that regulates exit from the ER is boxed and the putative ER exit site is underlined.
Homologs of PHT1 transporters have been characterized in a wide range of species, since their initial identification in Arabidopsis. This non-exclusive list includes: potato (Leggewie et al., 1997), Lupinus albus (Liu et al., 2001), tomato (Daram et al., 1998; Liu et al., 1998a), Catharanthus roseus (Kai et al., 1997), Medicago truncatula (Liu et al., 1998b; Xiao et al., 2006), barley (Smith et al., 1999), tobacco (Baek et al., 2001), lotus (Nakamori et al., 2002), rice (Paszkowski et al., 2002; Ming et al., 2005), maize (Nagy et al., 2006), wheat (Tittarelli et al., 2007), Populus trichocarpa (Loth-Pereda et al., 2011) as well as more distant organisms such as Chlamydomonas (Chang et al., 2005).
PHT1 Structure
Sequence analysis of different PHT1 transporters highlights an amino acid sequence conserved from fungi to plants. Hydrophobicity analysis predicts that these transporters share a common topology with 12 membrane-spanning domains, which are separated into two groups of six domains by a charged hydrophilic loop (Raghothama, 1999). Both C- and N-termini are expected to be oriented inside the cell, with the protein inserted in the plasma membrane (Raghothama, 1999). The PHT1 family members encode closely related proteins, as indicated by a greater than 76% identity in their protein sequences between various plant species (A. thaliana, tomato, M. truncatula, potato, and C. roseus; Raghothama, 1999; cf. Figure Figure11 for examples). Strong homologies for this protein family are also observed between plants and yeast: Arabidopsis PHT1 and yeast PHO84 proteins share 34% identity and around 50% similarity.
In Arabidopsis, these transporters range in size from 520 to 550 amino acids, with an estimated molecular weight around 58 kDa. As previously reported in Medicago (Chiou et al., 2001) or in Arabidopsis (Figure (Figure2)2) this weight is lower than expected. Moreover, these western blots exhibit also higher weight molecular bands that are compatible with the presence of multimeric proteins (Figure (Figure2;2; Chiou et al., 2001). This hypothesis will require further experiments for validation, such as those performed for NRT2.1 (Wirth et al., 2007). In these experiments multimers were also identified on SDS PAGE gel before validation by crosslinking. Nevertheless, this view is also supported by the semi-dominant character of the pht1;1–3 mutation in Arabidopsis (Catarecha et al., 2007), a trait generally associated with multimeric transporters (Ludewig et al., 2003; Loque et al., 2007) in which incorporating the inactive subunits into the multimeric pore affects its global structure.
kDa. As previously reported in Medicago (Chiou et al., 2001) or in Arabidopsis (Figure (Figure2)2) this weight is lower than expected. Moreover, these western blots exhibit also higher weight molecular bands that are compatible with the presence of multimeric proteins (Figure (Figure2;2; Chiou et al., 2001). This hypothesis will require further experiments for validation, such as those performed for NRT2.1 (Wirth et al., 2007). In these experiments multimers were also identified on SDS PAGE gel before validation by crosslinking. Nevertheless, this view is also supported by the semi-dominant character of the pht1;1–3 mutation in Arabidopsis (Catarecha et al., 2007), a trait generally associated with multimeric transporters (Ludewig et al., 2003; Loque et al., 2007) in which incorporating the inactive subunits into the multimeric pore affects its global structure.
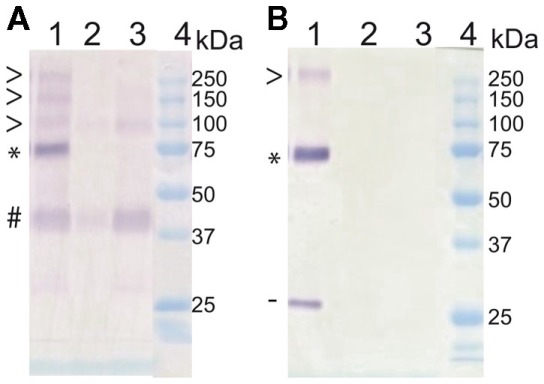
Western blot analysis of PHT1;1, PHT1;2, and PHT1;3 on root membrane protein extracts reveal the presence of potential multimers. Plants were grown in vitro on medium supplemented with high (500 μM) or low (10
μM) or low (10 μM) Pi content (+P or −P respectively). Five micrograms of membrane protein were loaded per lane, on 10% acrylamide SDS PAGE gels before protein transfer onto nitrocellulose membranes. (A) The primary antibody was designed against two peptides common to PHT1;1, PHT1;2, and PHT1;3. In parallel, an anti GFP antibody (Roche) was used for control western blots at the same dilution (B). Lanes were loaded as follows: (1) Transgenic line expressing 35S:PHT1;1:GFP construct; and wild type control on +P (2) or −P (3) and Bio-Rad Precision Plus Protein Standards (4). GFP tagged (*) and untagged (#) PHT1 monomeric form, putative multimers (>) and cleaved GFP (−).
μM) Pi content (+P or −P respectively). Five micrograms of membrane protein were loaded per lane, on 10% acrylamide SDS PAGE gels before protein transfer onto nitrocellulose membranes. (A) The primary antibody was designed against two peptides common to PHT1;1, PHT1;2, and PHT1;3. In parallel, an anti GFP antibody (Roche) was used for control western blots at the same dilution (B). Lanes were loaded as follows: (1) Transgenic line expressing 35S:PHT1;1:GFP construct; and wild type control on +P (2) or −P (3) and Bio-Rad Precision Plus Protein Standards (4). GFP tagged (*) and untagged (#) PHT1 monomeric form, putative multimers (>) and cleaved GFP (−).
PHT1 are Pi:H+ Co-Transporters
The study of Pi uptake kinetics in planta using radioactive Pi isotopes (P33 or P32) identified two distinct phases, which were interpreted as the co-existence of a high and a low affinity system (Cogliati and Clarkson, 1983; Drew and Saker, 1984; Drew et al., 1984). Pi uptake kinetics have been measured for several species (Lemna gibba, Brassica nigra, C. roseus, tobacco, Arabidopsis), with Km values for the high affinity system in the range of 2.5–12.3 μM, whereas Km values for the low affinity system were usually observed between 50 and 100
μM, whereas Km values for the low affinity system were usually observed between 50 and 100 μM (Ullrich-Eberius et al., 1984; Lefebvre et al., 1990; Furihata et al., 1992; Shimogawara and Usuda, 1995; Dunlop et al., 1997). In rare cases, only high affinity phosphate transporters could be detected (Drew and Saker, 1984), and it remains to be determined if this is linked to the plant samples or the experimental design.
μM (Ullrich-Eberius et al., 1984; Lefebvre et al., 1990; Furihata et al., 1992; Shimogawara and Usuda, 1995; Dunlop et al., 1997). In rare cases, only high affinity phosphate transporters could be detected (Drew and Saker, 1984), and it remains to be determined if this is linked to the plant samples or the experimental design.
Inorganic phosphate uptake is an energy-mediated process that promotes the alkalinization of the medium, which points toward a Pi/H+ co-transport (Ullrich and Novacky, 1990). The measurements of phosphate absorption and proton flux revealed a stoichiometry of two to four protons for each phosphate ion transported across the plasma membrane (Ullrich-Eberius et al., 1981, 1984; Sakano, 1990).
It should be noted that phosphate transporters (in particular PHT1 proteins) can also transport Pi analogs such as phosphite (Ticconi et al., 2001; Varadarajan et al., 2002) and arsenate (Lee et al., 2003; Catarecha et al., 2007; Wu et al., 2011). This last compound is highly toxic and can be found in the soil or water of certain areas. Interestingly, both arsenate and phosphite, like Pi, down-regulate PHT1 transcription (Ticconi et al., 2001; Varadarajan et al., 2002; Catarecha et al., 2007).
A Range of Affinities
Several approaches have been used to investigate the Pi transport properties of the various PHT1 family members. One approach relies on the complementation by plant phosphate transporters of the yeast pho84 mutant or pho84/pho89 double mutant (PHO84 and PHO89 are the two major high affinity Pi transporters present in yeast). The Km values measured for several PHT1 family members were: 280 and 130 μM, for two of the potato PHT1 transporters (Muchhal et al., 1996; Leggewie et al., 1997; Liu et al., 1998b); 97
μM, for two of the potato PHT1 transporters (Muchhal et al., 1996; Leggewie et al., 1997; Liu et al., 1998b); 97 μM for the rice OsPT6 (Ai et al., 2008) or 23
μM for the rice OsPT6 (Ai et al., 2008) or 23 μM for OsPT8 (Jia et al., 2011); and 192
μM for OsPT8 (Jia et al., 2011); and 192 μM for Medicago MtPT1 (Liu et al., 1998b).
μM for Medicago MtPT1 (Liu et al., 1998b).
Although a range of affinities can be determined from yeast complementation assays, in most cases these measurements greatly exceed the expected Km calculated from in planta radioactive Pi uptake experiments (from 2.5 to 100 μM as mentioned in a previous section). This suggests that yeast complementation can only partially reflect the behavior of PHT1 proteins in plants, and it is important to keep this information in mind when predicting the affinities of PHT1 proteins in the plant.
μM as mentioned in a previous section). This suggests that yeast complementation can only partially reflect the behavior of PHT1 proteins in plants, and it is important to keep this information in mind when predicting the affinities of PHT1 proteins in the plant.
Because of the discrepancies between the in planta affinities and the values obtained in yeast, alternative methods may be needed for precise Pi uptake measurements. One such technique is the heterologous transformation of Xenopus oocytes with PHT1 transporters, although measured affinities based on this method can also largely differ from the expected values in planta. For instance, the rice Pi transporter OsPT2 exhibits low affinity characteristics in the mM scale, when expressed in oocytes (Ai et al., 2008). By contrast, using the same method reveals a Km value of 19 μM for the barley high affinity Pi transporter HvPHT1 (Preuss et al., 2011). One revealing potential study could be to compare values obtained for both systems (yeast vs. oocytes) for several transporters.
μM for the barley high affinity Pi transporter HvPHT1 (Preuss et al., 2011). One revealing potential study could be to compare values obtained for both systems (yeast vs. oocytes) for several transporters.
To avoid using a heterologous system, the kinetic parameters can be investigated by gene over-expression in plant cell culture. This technique was used to estimate Km values for AtPHT1;1 (3.1 μM, Mitsukawa et al., 1997), and for barley HvPHT1;1 (9.06
μM, Mitsukawa et al., 1997), and for barley HvPHT1;1 (9.06 μM) and HvPHT1;6 (385
μM) and HvPHT1;6 (385 μM, Rae et al., 2003). These data are much closer to the affinities expected for Pi uptake measurements in planta, although the high Km determined for HvPHT1;6 reinforces the idea that PHT1 members could encode both high and low affinity transporters.
μM, Rae et al., 2003). These data are much closer to the affinities expected for Pi uptake measurements in planta, although the high Km determined for HvPHT1;6 reinforces the idea that PHT1 members could encode both high and low affinity transporters.
One study has attempted to identify which amino acid alterations are responsible for the variation observed in transporter affinity, in M. truncatula. The results indicate that slight variations in the protein sequence of the PHT1 proteins could influence their affinity for Pi transport (Liu et al., 2008): when several M. truncatula PHT1 transporters with an 84% amino acid identity were expressed in yeast, their Km ranged broadly from 13 to 858 μM. Analysis of the transporter sequences revealed that only seven amino acids showed non-conservative changes between the three low affinity (MtPT1, Mt PT2, MtPT3) and the high affinity transporters (MtPT5). These amino acid positions are located all along the protein sequence (Figure (Figure1).1). The predicted structural model hypothesizes that they are clustered in two regions of the protein: one on the extracellular surface, and one within the membrane (Liu et al., 2008). The authors posit that these particular extracellular membrane areas could be directly responsible in controlling the PHT1 affinities. Nevertheless, sequence comparisons with other PHT1 transporters that exhibit distinct affinities for Pi could not reveal a correlation with these results (Figure (Figure1).1). This suggests either independent evolution in separate species, or that the amino acids identified from the M. truncatula data cannot account for affinity changes in other species.
μM. Analysis of the transporter sequences revealed that only seven amino acids showed non-conservative changes between the three low affinity (MtPT1, Mt PT2, MtPT3) and the high affinity transporters (MtPT5). These amino acid positions are located all along the protein sequence (Figure (Figure1).1). The predicted structural model hypothesizes that they are clustered in two regions of the protein: one on the extracellular surface, and one within the membrane (Liu et al., 2008). The authors posit that these particular extracellular membrane areas could be directly responsible in controlling the PHT1 affinities. Nevertheless, sequence comparisons with other PHT1 transporters that exhibit distinct affinities for Pi could not reveal a correlation with these results (Figure (Figure1).1). This suggests either independent evolution in separate species, or that the amino acids identified from the M. truncatula data cannot account for affinity changes in other species.
Spatial Distribution
Most members of the PHT1 family exhibit strong expression in roots, and this property is shared between monocotyledonous and dicotyledonous species (Muchhal et al., 1996; Leggewie et al., 1997; Smith et al., 1997; Liu et al., 1998a,b; Schunmann et al., 2004a; Koyama et al., 2005; Nagy et al., 2006; Xiao et al., 2006; Tittarelli et al., 2007; Jia et al., 2011; Loth-Pereda et al., 2011). However, PHT1 proteins are also detected in aerial organs such as leaves or flowers (Figure (Figure33).
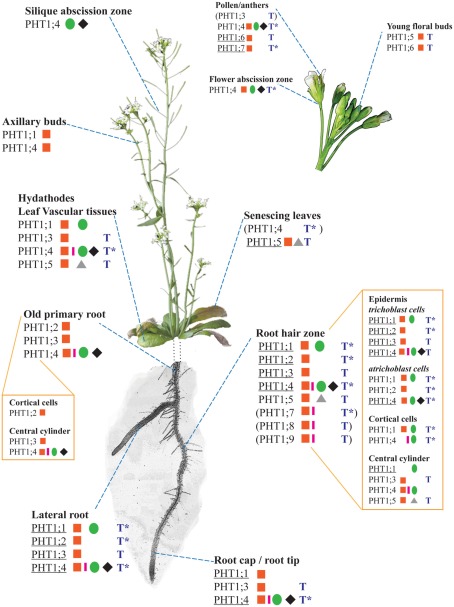
Expression patterns of the nine PHT1 transporters from Arabidopsis thaliana, based on a compilation of histological and transcriptomics data (combining information for both +P and −P conditions). The different plant parts are not presented to scale; in particular, the root system has been simplified so that only a single primary root and lateral root are represented. References corresponding to histological studies (promoter::GFP/GUS fusions or in situ hybridization) are identified on the figure by the following symbols: red square (Mudge et al., 2002), green circle (Karthikeyan et al., 2002), pink rectangle (Misson et al., 2004), black rhombus (Karthikeyan et al., 2009), and gray triangle (Nagarajan et al., 2011). Results based on transcriptomics studies are indicated by a blue T, and they correspond to a summary of several data sets presented on the eFP server (Winter et al., 2007). *Due to the design of most transcriptomic chips, it is not possible to distinguish the expression patterns of PHT1;1/PHT1;2 as well as PHT1;4/PHT1;7. When a PHT1 transporter is predominantly (but not exclusively) expressed in one or several tissues, its name is underlined. The subset of expression data indicated between brackets () are based on RT-PCR and/or transcriptomics only (i.e., not validated by promoter::reporter studies).
More precisely, in situ hybridization performed in tomato revealed the presence of LePT1 transcript in the root cap and in the external layers of the root (Daram et al., 1998; Liu et al., 1998a). Similar experiments in M. truncatula and A. thaliana localized MtPT1 and AtPHT1;4 in the root epidermis and root hairs (Chiou et al., 2001; Misson et al., 2004). Immunoblot analyses of tomato LePT1 (Muchhal and Raghothama, 1999) and potato StPT2 (Gordon-Weeks et al., 2003), or use of translational GFP fusions with either AtPHT1;1, AtPHT1;2, or AtPHT1;4 (Gonzalez et al., 2005; Bayle et al., 2011) have all provided similar results, localizing these transporters mainly in epidermis and root hairs. Additionally, translational fusion experiments between PHT1 and GFP reporter genes confirmed that these phosphate transporters are targeted to the plasma membrane in various species (Gonzalez et al., 2005; Bayle et al., 2011; Jia et al., 2011). Transcriptional fusions between PHT1 promoters and the GUS or GFP reporter genes have also facilitated the study of the expression patterns of PHT1 members from different species, along with the identification of promoter elements involved in the control of these expression patterns (Karthikeyan et al., 2002; Mudge et al., 2002; Schunmann et al., 2004a,b; Misson et al., 2005; Jia et al., 2011). In the specific case of the plant model A. thaliana, the full genome sequence permitted extensive analyses of all Arabidopsis PHT1 family members, using either RT-PCR (Mudge et al., 2002; Misson et al., 2005) or promoter fusions with GUS or GFP reporter genes (Karthikeyan et al., 2002; Mudge et al., 2002). A landmark contribution to this field was provided by Mudge et al. (2002), who performed a systematic study of the nine PHT1 transporters. Subsequently, the expression patterns were confirmed or analyzed more precisely by several other laboratories (Karthikeyan et al., 2002, 2009; Misson et al., 2004; Nagarajan et al., 2011). In addition, the publication of multiple transcriptomics studies allows us to take into account data sets that were not initially developed for the study of phosphate transport. Powerful tools such as the eFP browser provide an efficient way of analyzing this vast amount of data (Winter et al., 2007). Figure Figure33 summarizes the expression patterns for the nine PHT1 members of A. thaliana, and compares their published expression patterns to values contained within transcriptomics data sets.
It appears that the vast majority of the transporters (eight of the nine Arabidopsis PHT1 members) are expressed at least in roots, in line with their major role in Pi uptake from the soil. Within the roots, the expression of phosphate transporters is mostly concentrated in root epidermis and central cylinder, particularly in the root hair zone (Karthikeyan et al., 2002; Mudge et al., 2002; Misson et al., 2004; Koyama et al., 2005; Xiao et al., 2006; Hirsch et al., 2011).
Most PHT1 transporters are not present in a single cell type, but are found in diverse tissues from various organs, often in overlapping patterns with other PHT1 members, suggesting a greater complexity in their roles (Karthikeyan et al., 2002; Mudge et al., 2002; Figure Figure3).3). However, each PHT1 member taken individually is defined by a preferential expression in a given tissue, which may indicate the location of its predominant role. It should be noted that this location is often strongly influenced by environmental or developmental factors. For instance, strong AtPHT1;6 expression in pollen could reveal a role for PHT1 transporters during flower development (Karthikeyan et al., 2002; Mudge et al., 2002). Functional confirmation of these expression patterns can be performed by reverse genetic analyses: the role of PHT1 protein in soil Pi uptake was demonstrated with Arabidopsis pht1;4 and pht1;1 single and double mutants, which are dramatically attenuated in their phosphate uptake capacities (Misson et al., 2004; Shin et al., 2004). A similar strategy was recently applied to understand the importance of the strong expression of PHT1;5 in senescing leaves (Nagarajan et al., 2011; Figure Figure2):2): analysis of the pht1;5 mutant confirmed a role in senescing leaves, and revealed its role in source-to-sink Pi mobilization. Altogether, this literature suggests a major role for PHT1 transporters in Pi uptake from soil, and additional roles in Pi re-mobilization from other organs.
One category of PHT1 transporters which cannot be studied in Arabidopsis are the PHT1 transporters that are induced by mycorrhizal symbiosis (endo and ectomycorhizal associations). Arabidopsis is among the minority of plants which is incapable of establishing any symbiosis with mycorrhizal fungi. This symbiosis is however widespread among the plant kingdom (80% of vascular plants), and its impact on the capacity of plants to acquire Pi from the soil has been well established (Smith and Read, 1997). This adaptation of plants has probably evolved to benefit from the ability of the fungus to explore a greater part of the soil (exploring up to 100-fold more soil volume) and to have access to mineralized organic phosphorus (Bucher, 2007).
The induction of specific plant PHT1 transporters in response to mycorrhization is a widely reported feature of these symbioses (Javot et al., 2007b; Hata et al., 2010; Loth-Pereda et al., 2011). In the case of the endomycorrhizal symbiosis, the fungus penetrates into the root system and develops hyphal structures (referred to as arbuscules) in the cortex. These arbuscules are composed of a trunk and arbuscular branches, which are enveloped by the plasma membrane of the cortical cell. The expression of a subset of PHT1 members is induced in cells containing arbuscules, which are believed to be the site of nutrient exchange between the plant and the fungus (Smith and Read, 1997; Javot et al., 2007a; Loth-Pereda et al., 2011). The symbiosis-specific PHT1 phosphate transporter MtPT4 from the plant M. truncatula is only detected in cells containing arbuscules, and it has exclusively been located in the plasma membrane that surrounds arbuscular branches (Pumplin and Harrison, 2009). The fine regulation of arbuscule development and associated stimulation of PHT1 expression was recently illustrated by the study of the dynamics of the rice transporter OsPT11 fused to GFP (Kobae and Hata, 2010).
Promising techniques using uptake of radioactive Pi isotopes in planta can be applied to study specific PHT1 transporters. It is now possible to visualize and analyze the precise dynamics of Pi distribution in planta. This technique, using X-ray films or imaging plates, has revealed that the main location for phosphate uptake in Arabidopsis is found close to the root tips, and that PHT1 proteins are involved in this process (Misson et al., 2004). A recent improvement in visualizing Pi flux dynamics is the use of a CsI scintillator to convert β-rays into visible light (Kanno et al., 2007). As illustrated in Figure Figure4,4, it is now possible to compare precisely and in a non-destructive way the entry of Pi into various genotypes of plants at the same time (in particular for mutations affecting PHT1 transporters).
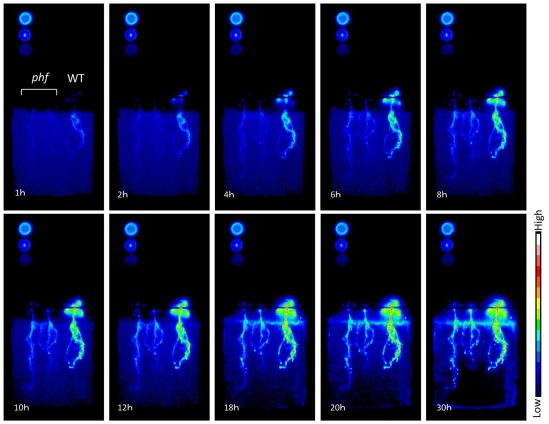
Time course for phosphate absorption based on 32P detection in Arabidopsis. Plants were supplied with 10 μM Pi with 32P (H3PO4, 12.5
μM Pi with 32P (H3PO4, 12.5 kBq/ml). Images of radioactivity were obtained every 3
kBq/ml). Images of radioactivity were obtained every 3 min; 10 images are presented here. For each panel, two phf mutants (left) and one wild type (Col-0; right) plantlet are shown. Circles on the left are 32P standards:descending from the upper left corner, they represent 2.5, 1, and 0.5k
min; 10 images are presented here. For each panel, two phf mutants (left) and one wild type (Col-0; right) plantlet are shown. Circles on the left are 32P standards:descending from the upper left corner, they represent 2.5, 1, and 0.5k Bq/μl. The plants shown are 11
Bq/μl. The plants shown are 11 days old, grown in vitro using 1/10 MS medium containing 500
days old, grown in vitro using 1/10 MS medium containing 500 μM Pi and subsequently transferred to 10
μM Pi and subsequently transferred to 10 μM Pi for 3
μM Pi for 3 days before analysis.
days before analysis.
Transcriptional Regulation of PHT1 Transporters
The tissue specificity of PHT1 described above appears to be mostly controlled at the transcriptional level. It is likely that the spatial distribution of PHT1 proteins requires regulatory elements located in the promoters, which remain poorly characterized (Schunmann et al., 2004a,b; Karthikeyan et al., 2009). Nevertheless, they are probably well conserved between monocots and dicots, since fusion of the AtPHT1;1 promoter with the GUS reporter gene has given fairly similar expression patterns in both rice and Arabidopsis transformants (Koyama et al., 2005).
The expression of specific plant PHT1 transporters that are induced in roots in response to mycorrhizal symbiosis is also under transcriptional control in species as diverse as: rice (Paszkowski et al., 2002), Medicago (Harrison et al., 2002; Javot et al., 2007a), potato (Nagy et al., 2005), maize (Nagy et al., 2006), various cereals (Glassop et al., 2005), tomato (Nagy et al., 2005; Balestrini et al., 2007; Xu et al., 2007), and trees such as poplar (Loth-Pereda et al., 2011).
The internal Pi concentration of cells is a major factor in the control of PHT1 expression. This role has been clearly illustrated by various transcriptomics experiments (Hammond et al., 2003; Wu et al., 2003; Misson et al., 2005; Morcuende et al., 2007; Winter et al., 2007; Calderon-Vazquez et al., 2008; Bustos et al., 2010; Thibaud et al., 2010), or with the assistance of reporter genes (Karthikeyan et al., 2002; Mudge et al., 2002; Misson et al., 2004.). Interestingly, plants can adjust PHT1 transcription when challenged with a broad spectrum of Pi concentrations (from 0 to 1250 μM) that are outside of the expected range in soils, typically observed below 10
μM) that are outside of the expected range in soils, typically observed below 10 μM (Muchhal and Raghothama, 1999; Karthikeyan et al., 2002; Misson et al., 2004). Low basal levels of gene expression are usually observed when the Pi concentration of the medium exceeds 250
μM (Muchhal and Raghothama, 1999; Karthikeyan et al., 2002; Misson et al., 2004). Low basal levels of gene expression are usually observed when the Pi concentration of the medium exceeds 250 μM (Muchhal and Raghothama, 1999; Karthikeyan et al., 2002; Misson et al., 2004).
μM (Muchhal and Raghothama, 1999; Karthikeyan et al., 2002; Misson et al., 2004).
Variation of Pi concentration in the medium promotes a rapid modulation of PHT1 transcripts. In Arabidopsis, the detection of Pi deficiency can occur within 12–24 h after removal of Pi from the medium (Muchhal and Raghothama, 1999; Misson et al., 2004, 2005). Not surprisingly, re-supply of Pi triggered a faster response (Karthikeyan et al., 2002), presumably since Pi accumulation occurs more rapidly than its depletion from the vacuole (Stefanovic et al., 2011). This phenomenon can be greatly affected by the protocol for cultivating plants (hydroponics vs. solid medium), as gelling agents are often contaminated with traces of Pi, which can impact the responses of the plant (Jain et al., 2009). The analysis of promoter sequences from barley, wheat, and Arabidopsis
PHT1 transporters prompted the identification of regulatory boxes involved in the response to Pi starvation (Schunmann et al., 2004b; Tittarelli et al., 2007; Karthikeyan et al., 2009; Thibaud et al., 2010). These studies revealed the presence of the P1BS motif, which is the binding site for the R2R3 Myb transcription factor PHR1 (PHOSPHATE STARVATION RESPONSE 1, Rubio et al., 2001; Nilsson et al., 2007) in Arabidopsis. This protein is a homolog of Chlamydomonas reinhardtii protein PSR1 (PHOSPHORUS STARVATION RESPONSE 1, Wykoff et al., 1999). PHR1 belongs to a wide multigenic family that includes PHL1 (Bustos et al., 2010). Both PHR1 and PHL1 bind to the P1BS DNA motif (GNATATNC; Rubio et al., 2001), and play a crucial role in the Pi responsiveness of PHT1 transporters as demonstrated with both phr1 and phr1/phl1 mutants (Rubio et al., 2001; Bustos et al., 2010). This DNA binding motif appears to be conserved between monocots and dicots (Zhou et al., 2008). In addition, the P1BS motif participates in the systemically regulated PHT1 response to internal Pi concentration, as revealed by the full genome analysis in split root experiments (Thibaud et al., 2010).
h after removal of Pi from the medium (Muchhal and Raghothama, 1999; Misson et al., 2004, 2005). Not surprisingly, re-supply of Pi triggered a faster response (Karthikeyan et al., 2002), presumably since Pi accumulation occurs more rapidly than its depletion from the vacuole (Stefanovic et al., 2011). This phenomenon can be greatly affected by the protocol for cultivating plants (hydroponics vs. solid medium), as gelling agents are often contaminated with traces of Pi, which can impact the responses of the plant (Jain et al., 2009). The analysis of promoter sequences from barley, wheat, and Arabidopsis
PHT1 transporters prompted the identification of regulatory boxes involved in the response to Pi starvation (Schunmann et al., 2004b; Tittarelli et al., 2007; Karthikeyan et al., 2009; Thibaud et al., 2010). These studies revealed the presence of the P1BS motif, which is the binding site for the R2R3 Myb transcription factor PHR1 (PHOSPHATE STARVATION RESPONSE 1, Rubio et al., 2001; Nilsson et al., 2007) in Arabidopsis. This protein is a homolog of Chlamydomonas reinhardtii protein PSR1 (PHOSPHORUS STARVATION RESPONSE 1, Wykoff et al., 1999). PHR1 belongs to a wide multigenic family that includes PHL1 (Bustos et al., 2010). Both PHR1 and PHL1 bind to the P1BS DNA motif (GNATATNC; Rubio et al., 2001), and play a crucial role in the Pi responsiveness of PHT1 transporters as demonstrated with both phr1 and phr1/phl1 mutants (Rubio et al., 2001; Bustos et al., 2010). This DNA binding motif appears to be conserved between monocots and dicots (Zhou et al., 2008). In addition, the P1BS motif participates in the systemically regulated PHT1 response to internal Pi concentration, as revealed by the full genome analysis in split root experiments (Thibaud et al., 2010).
Additional regulatory sequences have been proposed from in silico analyses (including HLH, and NIT2 motifs highly present in the promoters of the PHT1 genes), although no mechanism has been characterized yet (Mukatira et al., 2001). The genes PHO1 and PHO2 have also been identified as critical to the control of Pi homeostasis (Hamburger et al., 2002; Aung et al., 2006; Bari et al., 2006). The corresponding pho1 and pho2 mutants accumulate Pi in either roots or leaves, respectively (Poirier et al., 1991; Delhaize and Randall, 1995), and affect the transcription of PHT1;8 and PHT1;9 (Bari et al., 2006; Rouached et al., 2011). It is likely that PHO1 (membrane protein of unknown function) and PHO2 (E2 conjugase protein) act along with additional elements, since neither of these two proteins are transcription factors.
Several transcription factors have been found in addition to PHR1 that regulate PHT1 expression. For example, genetic dissection of the AtPHT1;4 promoter identified putative binding sequences for AtMyb2 and AtMyc2 (two positive regulators responding to water deprivation and abscisic acid stimuli) and also WRKY proteins (Karthikeyan et al., 2009), which are involved in biotic and abiotic stress responses. Reducing WRKY75 expression through RNAi silencing (Devaiah et al., 2007a) or the over-expression of MYB62 (Devaiah et al., 2009) produces plants that exhibit a decreased level of several PHT1 transporters, although there is no evidence for a direct interaction between these transcription factors and PHT1 promoters.
Further regulatory components were identified based on genetic evidence and transcriptomic analysis of Pi starvation. These include the zinc finger transcription factor ZAT6 (Devaiah et al., 2007b), the regulatory protein SPX3 (Duan et al., 2008), the nuclear actin-related protein ARP6, and the histone H2A.Z (Smith et al., 2010). These various proteins are reported to modulate PHT1 genes (see Chiou and Lin, 2011 for a recent review of the Pi regulatory pathway), although a mechanism of action remains to be proposed.
Factors other than Pi concentration also influence PHT1 expression, including active photosynthesis or sugars (Jain et al., 2007; Karthikeyan et al., 2007; Hammond and White, 2008, 2011; Lei et al., 2011). Cytokinins have also been observed to prevent PHT1 induction during Pi deficiency; this action relies on the cytokinin receptors CRE1/AHK3 and AHK4 (Franco-Zorrilla et al., 2002, 2005). Many genes responding to Pi deficiency are stimulated by sugars or are enhanced in the cre1/ahk4 mutant (regardless of cytokinin applications), suggesting cross-talk between Pi deficiency, sugars, and cytokinin (Franco-Zorrilla et al., 2005).
Post-Transcriptional Regulation of PHT1
Beyond transcriptional control, PHT1 targeting and accumulation in the plasma membrane are also modulated by multiple steps of post-translational regulations. A mutant allele of the PHF1 locus (PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1) has been identified, which displays a severe decrease in Pi influx (Gonzalez et al., 2005). In accordance with its status as an endoplasmic reticulum (ER) resident protein, the loss of PHF1 results in the abnormal accumulation of PHT1;1, PHT1;2, and PHT1;4 in the ER, suggesting that PHF1 could be involved in intracellular trafficking of several PHT1 family proteins (Gonzalez et al., 2005; Bayle et al., 2011). These observations can explain how the phf1-1 mutant shows a strong reduction (80%) of Pi uptake capacity compared to WT when grown in low Pi (Gonzalez et al., 2005; Figure Figure4).4). Recently, similar results were found in rice, where a mutant of OsPHF1 was shown to affect several members of PHT1 family, including the low affinity Pi transporter OsPT2 and the high affinity Pi transporter OsPT8 (Chen et al., 2011). A similar targeting defect was described for axr4, an auxin-resistant mutant that is affected in the expression of an ER accessory protein; this defect promotes the retention of the auxin influx facilitator AUX1 in the ER (Dharmasiri et al., 2006). These results suggest that several membrane proteins may require assistance in transiting through the ER compartment. PHF1 exhibits structural homologies with yeast SEC12 protein, an ER resident component involved in the formation of COPII vesicles (Gonzalez et al., 2005). These vesicles are involved in the export of newly synthesized cargo proteins, including PHT1, from the ER to the Golgi apparatus in eukaryotic cells. Although PHF1 shares structural homologies with SEC12, multiple amino acids that have been conserved between all SEC12 proteins are lost in the plant PHF1 homologs. This suggests a functional specialization that has diverged from that of SEC12 (Gonzalez et al., 2005). Indeed, cell biology experiments in Arabidopsis revealed the absence of co-localization between COPII markers and PHF1, demonstrating the independence of PHF1’s role from COPII formation (Bayle et al., 2011).
Phospho-proteomic studies have determined that phosphorylation events occur at the C-end of PHT1 proteins when Pi is abundant in the environment (Nuhse et al., 2004; Hem et al., 2007). A phosphorylation-mimicking mutagenesis of PHT1;1 (at Ser 514, Figure Figure1)1) resulted in its accumulation in the ER (Bayle et al., 2011), implicating phosphorylation as an additional way for plant cells to modulate PHT1 exit from the ER. Phosphorylation events on serine residues located at the C-end of the PHT1;1 protein could provide additional negative charges in the vicinity of the putative ER export site (D/E-X-D/E), thereby altering the recognition of this motif and resulting in the accumulation of PHT1;1 in the ER (Figure (Figure1).1). This mechanism is likely conserved between the different PHT1 transporters, since phosphorylation of other Arabidopsis members of this family (PHT1;4, PHT1;5; PHT1;7, PHT1;9) at the C-end of the protein has been reported (Bayle et al., 2011). This regulation recalls the previously identified phosphorylation of the C-terminal serine residue during subcellular trafficking of PIP2;1 toward the plasma membrane (Prak et al., 2008). However, phosphorylation has distinct effects on these proteins. Whereas phosphorylation of PIP2;1 is required for its proper localization, in the case of PHT1;1, phosphorylation was found to prevent the protein from reaching its final destination.
Finally, a new type of post-transcriptional regulation has recently been identified, which specifically degrades plasma membrane PHT1 in the presence of phosphate (Bayle et al., 2011). This mechanism is dependent upon endocytosis and subsequent degradation of the protein in the vacuole. Similar regulatory mechanisms have been identified for many types of plasma membrane proteins in yeast, plants, and animals (Lagerstedt et al., 2002; Persson et al., 2003; Takano et al., 2005). As for the broader family of Pi transporters, this regulation has already been reported for the yeast PHO84 transporter (Lagerstedt et al., 2002; Persson et al., 2003). An important distinction here is that the regulatory mechanism identified in yeast requires the phosphorylation of an amino acid to observe this degradation phenomenon, whereas this particular amino acid is not conserved in plant PHT1 (Lundh et al., 2009). Thus, despite the high homologies between PHO84 and PHT1 proteins, similar regulatory mechanisms could rely on distinct types of machineries. This probably reflects adaptive steps used by plants to cope with a complex environment and a multicellular organization.
Conclusion
Great strides have been made in the characterization of the PHT1 family of Pi transporters since their identification in 1996. The fact that these transporters belong to broad multigenic family often exhibiting overlapping expression patterns limits the analysis of their role in planta. Another obstacle to the characterization of PHT1 proteins is their hydrophobic nature, which has impeded the study of their biochemistry and structural biology. This issue will eventually be surmounted as improved techniques in genetics, cell and molecular biology become more readily available. In view of the ever-expanding array of tools available to researchers, rapid advances are anticipated in our understanding of the finely regulated, sophisticated mechanisms of the PHT1 family.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Brandon Loveall (from the “Improvence” editing company) for English proof reading of the manuscript.
References
- Ai P., Sun S., Zhao J., Fan X., Xin W., Guo Q., Yu L., Shen Q., Wu P., Miller A. J., Xu G. (2008). Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 57, 798–80910.1111/j.1365-313X.2008.03726.x [Abstract] [CrossRef] [Google Scholar]
- Aung K., Lin S. I., Wu C. C., Huang Y. T., Su C. L., Chiou T. J. (2006). pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 141, 1000–101110.1104/pp.106.078063 [Abstract] [CrossRef] [Google Scholar]
- Baek S. H., Chung I. M., Yun S. J. (2001). Molecular cloning and characterization of a tobacco leaf cDNA encoding a phosphate transporter. Mol. Cells 11, 1–6 [Abstract] [Google Scholar]
- Balestrini R., Gomez-Ariza J., Lanfranco L., Bonfante P. (2007). Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol. Plant Microbe Interact. 20, 1055–106210.1094/MPMI-20-9-1055 [Abstract] [CrossRef] [Google Scholar]
- Bari R., Datt Pant B., Stitt M., Scheible W. R. (2006). PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 141, 988–99910.1104/pp.106.079707 [Abstract] [CrossRef] [Google Scholar]
- Bayle V., Arrighi J. F., Creff A., Nespoulous C., Vialaret J., Rossignol M., Gonzalez E., Paz-Ares J., Nussaume L. (2011). Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23, 1523–153510.1105/tpc.110.081067 [Abstract] [CrossRef] [Google Scholar]
- Bieleski R. L. (1973). Phosphate pools, phosphate transport, and phosphate availability. Annu. Rev. Plant Physiol. 24, 225–25210.1146/annurev.pp.24.060173.001301 [CrossRef] [Google Scholar]
- Bucher M. (2007). Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 173, 11–2610.1111/j.1469-8137.2006.01935.x [Abstract] [CrossRef] [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y. (1991). The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 11, 3229–3238 [Europe PMC free article] [Abstract] [Google Scholar]
- Bustos R., Castrillo G., Linhares F., Puga M. I., Rubio V., Perez-Perez J., Solano R., Leyva A., Paz-Ares J. (2010). A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 6, e1001102.10.1371/journal.pgen.1001102 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Calderon-Vazquez C., Ibarra-Laclette E., Caballero-Perez J., Herrera-Estrella L. (2008). Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant- and species-specific levels. J. Exp. Bot. 59, 2479–249710.1093/jxb/ern115 [Abstract] [CrossRef] [Google Scholar]
- Casacuberta N., Masque P., Garcia-Orellana J. (2011). Fluxes of (238)U decay series radionuclides in a dicalcium phosphate industrial plant. J. Hazard. Mater. 190, 245–25210.1016/j.jhazmat.2011.03.035 [Abstract] [CrossRef] [Google Scholar]
- Catarecha P., Segura M. D., Franco-Zorrilla J. M., Garcia-Ponce B., Lanza M., Solano R., Paz-Ares J., Leyva A. (2007). A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell 19, 1123–113310.1105/tpc.106.041871 [Abstract] [CrossRef] [Google Scholar]
- Chang C. W., Moseley J. L., Wykoff D., Grossman A. R. (2005). The LPB1 gene is important for acclimation of Chlamydomonas reinhardtii to phosphorus and sulfur deprivation. Plant Physiol. 138, 319–32910.1104/pp.105.059550 [Abstract] [CrossRef] [Google Scholar]
- Chen J. Y., Liu Y., Ni J., Wang Y. F., Bai Y. H., Shi J., Gan J., Wu Z. C., Wu P. (2011). OsPHF1 regulates the plasma membrane localization of low- and high-affinity inorganic phosphate transporters and determines inorganic phosphate uptake and translocation in rice. Plant Physiol. 157, 269–27810.1104/pp.111.181693 [Abstract] [CrossRef] [Google Scholar]
- Chiou T. J., Lin S. I. (2011). Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 62, 185–20610.1146/annurev-arplant-042110-103849 [Abstract] [CrossRef] [Google Scholar]
- Chiou T. J., Liu H., Harrison M. J. (2001). The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. Plant J. 25, 281–29310.1046/j.1365-313x.2001.00963.x [Abstract] [CrossRef] [Google Scholar]
- Cogliati D. H., Clarkson D. T. (1983). Physiological changes in, and phosphate uptake by potato plants during development of and recovery from phosphate deficiency. Physiol. Plant 58, 287–29410.1111/j.1399-3054.1983.tb04183.x [CrossRef] [Google Scholar]
- Cordell D., Drangert J.-O., White S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Change 19, 292–30510.1016/j.gloenvcha.2008.10.009 [CrossRef] [Google Scholar]
- Da Conceicao F. T., Antunes M. L., Durrant S. F. (2011). Radionuclide concentrations in raw and purified phosphoric acids from Brazil and their processing wastes: implications for radiation exposures. Environ. Geochem. Health.10.1007/s10653-011-9394-2 [Abstract] [CrossRef] [Google Scholar]
- Daram P., Brunner S., Persson B. L., Amrhein N., Bucher M. (1998). Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta 206, 225–23310.1007/s004250050394 [Abstract] [CrossRef] [Google Scholar]
- Delhaize E., Randall P. J. (1995). Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol. 107, 207–213 [Abstract] [Google Scholar]
- Devaiah B. N., Karthikeyan A. S., Raghothama K. G. (2007a). WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 143, 1789–180110.1104/pp.106.093971 [Abstract] [CrossRef] [Google Scholar]
- Devaiah B. N., Nagarajan V. K., Raghothama K. G. (2007b). Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol. 145, 147–15910.1104/pp.107.101691 [Abstract] [CrossRef] [Google Scholar]
- Devaiah B. N., Madhuvanthi R., Karthikeyan A. S., Raghothama K. G. (2009). Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant 2, 43–5810.1093/mp/ssn081 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dharmasiri S., Swarup R., Mockaitis K., Dharmasiri N., Singh S. K., Kowalchyk M., Marchant A., Mills S., Sandberg G., Bennett M. J., Estelle M. (2006). AXR4 is required for localization of the auxin influx facilitator AUX1. Science 312, 1218–122010.1126/science.1122847 [Abstract] [CrossRef] [Google Scholar]
- Drew M. C., Saker L. R. (1984). Uptake and long-distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley – evidence of non-allosteric regulation. Planta 160, 500–50710.1007/BF00411136 [Abstract] [CrossRef] [Google Scholar]
- Drew M. C., Saker L. R., Barber S. A., Jenkins W. (1984). Changes in the kinetics of phosphate and potassium absorption in nutrient-deficient barley roots measured by a solution-depletion technique. Planta 160, 490–49910.1007/BF00411136 [Abstract] [CrossRef] [Google Scholar]
- Duan K., Yi K., Dang L., Huang H., Wu W., Wu P. (2008). Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J. 54 965–97510.1111/j.1365-313X.2008.03460.x [Abstract] [CrossRef] [Google Scholar]
- Dunlop J., Phung H. T., Meeking R., White D. W. R. (1997). The kinetics associated with phosphate absorption by Arabidopsis and its regulation by phosphorus status. Aust. J. Plant Physiol. 24, 623–62910.1071/PP96137 [CrossRef] [Google Scholar]
- Franco-Zorrilla J. M., Martin A. C., Leyva A., Paz-Ares J. (2005). Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol. 138, 847–85710.1104/pp.105.060517 [Abstract] [CrossRef] [Google Scholar]
- Franco-Zorrilla J. M., Martin A. C., Solano R., Rubio V., Leyva A., Paz-Ares J. (2002). Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J. 32, 353–36010.1046/j.1365-313X.2002.01431.x [Abstract] [CrossRef] [Google Scholar]
- Furihata T., Suzuki M., Sakurai H. (1992). Kinetic characterization of two phosphate uptake systems with different affinities in suspension-cultured Catharanthus roseus protoplants. Plant Cell Physiol. 33, 1151–1157 [Google Scholar]
- Gilbert N. (2009). Environment: the disappearing nutrient. Nature 461, 716–71810.1038/461716a [Abstract] [CrossRef] [Google Scholar]
- Glassop D., Smith S. E., Smith F. W. (2005). Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 222, 688–69810.1007/s00425-005-0015-0 [Abstract] [CrossRef] [Google Scholar]
- Gonzalez E., Solano R., Rubio V., Leyva A., Paz-Ares J. (2005). Phosphate transporter traffic facilitator1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17, 3500–351210.1105/tpc.105.036640 [Abstract] [CrossRef] [Google Scholar]
- Gordon-Weeks R., Tong Y., Davies T. G., Leggewie G. (2003). Restricted spatial expression of a high-affinity phosphate transporter in potato roots. J. Cell. Sci. 116, 3135–314410.1242/jcs.00615 [Abstract] [CrossRef] [Google Scholar]
- Gross M. (2010). Fears over phosphorus supplies. Curr. Biol. 20, R386–R38710.1016/j.cub.2010.06.042 [Abstract] [CrossRef] [Google Scholar]
- Hamburger D., Rezzonico E., Macdonald-Comber Petetot J., Somerville C., Poirier Y. (2002). Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14, 889–90210.1105/tpc.000745 [Abstract] [CrossRef] [Google Scholar]
- Hammond J. P., Bennett M. J., Bowen H. C., Broadley M. R., Eastwood D. C., May S. T., Rahn C., Swarup R., Woolaway K. E., White P. J. (2003). Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 132, 578–59610.1104/pp.103.020941 [Abstract] [CrossRef] [Google Scholar]
- Hammond J. P., White P. J. (2008). Sucrose transport in the phloem: integrating root responses to phosphorus starvation. J. Exp. Bot. 59, 93–10910.1093/jxb/erm221 [Abstract] [CrossRef] [Google Scholar]
- Hammond J. P., White P. J. (2011). Sugar signalling in root responses to low P availability. Plant Physiol. 156, 1033–104010.1104/pp.111.175612 [Abstract] [CrossRef] [Google Scholar]
- Harrison M. J., Dewbre G. R., Liu J. (2002). A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14, 2413–242910.1105/tpc.004861 [Abstract] [CrossRef] [Google Scholar]
- Harrison M. J., Van Buuren M. L. (1995). A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378, 626–62910.1038/378626a0 [Abstract] [CrossRef] [Google Scholar]
- Hata S., Kobae Y., Banba M. (2010). Interactions between plants and arbuscular mycorrhizal fungi. Int. Rev. Cell Mol. Biol. 281, 1–4810.1016/S1937-6448(10)81001-9 [Abstract] [CrossRef] [Google Scholar]
- Hem S., Rofidal V., Sommerer N., Rossignol M. (2007). Novel subsets of the Arabidopsis plasmalemma phosphoproteome identify phosphorylation sites in secondary active transporters. Biochem. Biophys. Res. Commun. 363, 375–38010.1016/j.bbrc.2007.08.177 [Abstract] [CrossRef] [Google Scholar]
- Hirsch J., Marin E., Floriani M., Chiarenza S., Richaud P., Nussaume L., Thibaud M. C. (2006). Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 88, 1767–177110.1016/j.biochi.2006.05.007 [Abstract] [CrossRef] [Google Scholar]
- Hirsch J., Misson J., Crisp P. A., David P., Bayle V., Estavillo G. M., Javot H., Chiarenza S., Mallory A. C., Maizel A., Declerck M., Pogson B. J., Vaucheret H., Crespi M., Desnos T., Thibaud M. C., Nussaume L., Marin E. (2011). A novel fry1 allele reveals the existence of a mutant phenotype unrelated to 5’→3’ exoribonuclease (XRN) activities in Arabidopsis thaliana roots. PLoS ONE 6, e16724.10.1371/journal.pone.0016724 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jain A., Poling M. D., Karthikeyan A. S., Blakeslee J. J., Peer W. A., Titapiwatanakun B., Murphy A. S., Raghothama K. G. (2007). Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol. 144, 232–24710.1104/pp.106.092130 [Abstract] [CrossRef] [Google Scholar]
- Jain A., Poling M. D., Smith A. P., Nagarajan V. K., Lahner B., Meagher R. B., Raghothama K. G. (2009). Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol. 150, 1033–104910.1104/pp.109.136184 [Abstract] [CrossRef] [Google Scholar]
- Javot H., Penmetsa R. V., Terzaghi N., Cook D. R., Harrison M. J. (2007a). A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 104, 1720–172510.1073/pnas.0608136104 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Javot H., Pumplin N., Harrison M. J. (2007b). Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ. 30, 310–32210.1111/j.1365-3040.2006.01617.x [Abstract] [CrossRef] [Google Scholar]
- Jia H., Ren H., Gu M., Zhao J., Sun S., Zhang X., Chen J., Wu P., Xu G. (2011). The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 156, 1164–117510.1104/pp.111.175240 [Abstract] [CrossRef] [Google Scholar]
- Jouhet J., Marechal E., Block M. A. (2007). Glycerolipid transfer for the building of membranes in plant cells. Prog. Lipid Res. 46, 37–5510.1016/j.plipres.2006.06.002 [Abstract] [CrossRef] [Google Scholar]
- Kai M., Masuda Y., Kikuchi Y., Osaki M., Tadano T. (1997). Isolation and characterization of a cDNA from Catharanthus roseus which is highly homologous with phosphate transporter. Soil Sci. Plant Nutr. 43, 227–235 [Google Scholar]
- Kanno S., Rai H., Ohya T., Hayashi Y., Tanoi K., Nakanishi T. M. (2007). Real-time imaging of radioisotope labeled compounds in a living plant. J. Radioanal. Nucl. Chem. 272, 565–57010.1007/s10967-007-0625-z [CrossRef] [Google Scholar]
- Karthikeyan A. S., Ballachanda D. N., Raghothama K. G. (2009). Promoter deletion analysis elucidates the role of cis elements and 5’UTR intron in spatiotemporal regulation of AtPht1;4 expression in Arabidopsis. Physiol. Plant 136, 10–1810.1111/j.1399-3054.2009.01207.x [Abstract] [CrossRef] [Google Scholar]
- Karthikeyan A. S., Varadarajan D. K., Jain A., Held M. A., Carpita N. C., Raghothama K. G. (2007). Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225, 907–91810.1007/s00425-006-0408-8 [Abstract] [CrossRef] [Google Scholar]
- Karthikeyan A. S., Varadarajan D. K., Mukatira U. T., D’Urzo M. P., Damsz B., Raghothama K. G. (2002). Regulated expression of Arabidopsis phosphate transporters. Plant Physiol. 130, 221–23310.1104/pp.020007 [Abstract] [CrossRef] [Google Scholar]
- Kobae Y., Hata S. (2010). Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol. 51, 341–35310.1093/pcp/pcq013 [Abstract] [CrossRef] [Google Scholar]
- Koyama T., Ono T., Shimizu M., Jinbo T., Mizuno R., Tomita K., Mitsukawa N., Kawazu T., Kimura T., Ohmiya K., Sakka K. (2005). Promoter of Arabidopsis thaliana phosphate transporter gene drives root-specific expression of transgene in rice. J. Biosci. Bioeng. 99, 38–4210.1263/jbb.99.38 [Abstract] [CrossRef] [Google Scholar]
- Lagerstedt J. O., Zvyagilskaya R., Pratt J. R., Pattison-Granberg J., Kruckeberg A. L., Berden J. A., Persson B. L. (2002). Mutagenic and functional analysis of the C-terminus of Saccharomyces cerevisiae Pho84 phosphate transporter. FEBS Lett. 526, 31–3710.1016/S0014-5793(02)03109-5 [Abstract] [CrossRef] [Google Scholar]
- Lee D. A., Chen A., Schroeder J. I. (2003). Ars1, an Arabidopsis mutant exhibiting increased tolerance to arsenate and increased phosphate uptake. Plant J. 35, 637–64610.1046/j.1365-313X.2003.01835.x [Abstract] [CrossRef] [Google Scholar]
- Lefebvre D. D., Duff S. M., Fife C. A., Julien-Inalsingh C., Plaxton W. C. (1990). Response to phosphate deprivation in Brassica nigra suspension cells: enhancement of intracellular, cell surface, and secreted phosphatase activities compared to increases in pi-absorption rate. Plant Physiol. 93, 504–51110.1104/pp.93.2.522 [Abstract] [CrossRef] [Google Scholar]
- Leggewie G., Willmitzer L., Riesmeier J. W. (1997). Two cDNAs from potato are able to complement a phosphate uptake-deficient yeast mutant: identification of phosphate transporters from higher plants. Plant Cell 9, 381–39210.1105/tpc.9.3.381 [Abstract] [CrossRef] [Google Scholar]
- Lei M., Liu Y., Zhang B., Zhao Y., Wang X., Zhou Y., Raghothama K. G., Liu D. (2011). Genetic and genomic evidence that sucrose is a global regulator of plant response to phosphate starvation in Arabidopsis. Plant Physiol. 156, 1116–113010.1104/pp.110.171736 [Abstract] [CrossRef] [Google Scholar]
- Liu C., Muchhal U. S., Uthappa M., Kononowicz A. K., Raghothama K. G. (1998a). Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol. 116, 91–9910.1104/pp.116.1.91 [Abstract] [CrossRef] [Google Scholar]
- Liu H., Trieu A. T., Blaylock L. A., Harrison M. J. (1998b). Cloning and characterization of two phosphate transporters from Medicago truncatula roots: regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi. Mol. Plant Microbe Interact. 11, 14–2210.1094/MPMI.1998.11.1.14 [Abstract] [CrossRef] [Google Scholar]
- Liu J., Uhde-Stone C., Li A., Vance C., Allan D. (2001). A phosphate transporter with enhanced expression on proteoid roots of white lupin (Lupinus albus L.). Plant Soil 237, 257–26610.1023/A:1013396825577 [CrossRef] [Google Scholar]
- Liu J., Versaw W. K., Pumplin N., Gomez S. K., Blaylock L. A., Harrison M. J. (2008). Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. J. Biol. Chem. 283, 24673–2468110.1074/jbc.M806338200 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Loque D., Lalonde S., Looger L. L., von Wiren N., Frommer W. B. (2007). A cytosolic trans-activation domain essential for ammonium uptake. Nature 446, 195–19810.1038/nature05579 [Abstract] [CrossRef] [Google Scholar]
- Loth-Pereda V., Orsini E., Courty P. E., Lota F., Kohler A., Diss L., Blaudez D., Chalot M., Nehls U., Bucher M., Martin F. (2011). Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa. Plant Physiol. 156, 2141–215410.1104/pp.111.180646 [Abstract] [CrossRef] [Google Scholar]
- Ludewig U., Wilken S., Wu B., Jost W., Obrdlik P., El Bakkoury M., Marini A. M., Andre B., Hamacher T., Boles E., von Wiren N., Frommer W. B. (2003). Homo- and hetero-oligomerization of ammonium transporter-1 NH4 uniporters. J. Biol. Chem. 278, 45603–4561010.1074/jbc.M307424200 [Abstract] [CrossRef] [Google Scholar]
- Lundh F., Mouillon J. M., Samyn D., Stadler K., Popova Y., Lagerstedt J. O., Thevelein J. M., Persson B. L. (2009). Molecular mechanisms controlling phosphate-induced downregulation of the yeast Pho84 phosphate transporter. Biochemistry 48, 4497–450510.1021/bi9001198 [Abstract] [CrossRef] [Google Scholar]
- Ming F., Mi G. H., Lu Q., Yin S., Zhang S. S., Guo B., Shen D. L. (2005). Cloning and characterization of cDNA for the Oryza sativa phosphate transporter. Cell. Mol. Biol. Lett. 10, 401–411 [Abstract] [Google Scholar]
- Misson J., Raghothama K. G., Jain A., Jouhet J., Block M. A., Bligny R., Ortet P., Creff A., Somerville S., Rolland N., Doumas P., Nacry P., Herrerra-Estrella L., Nussaume L., Thibaud M. C. (2005). A genome-wide transcriptional analysis using Arabidopsis thaliana affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. U.S.A. 102, 11934–1193910.1073/pnas.0505266102 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Misson J., Thibaud M. C., Bechtold N., Raghothama K., Nussaume L. (2004). Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol. Biol. 55, 727–74110.1007/s11103-004-1965-5 [Abstract] [CrossRef] [Google Scholar]
- Mitsukawa N., Okumura S., Shirano Y., Sato S., Kato T., Harashima S., Shibata D. (1997). Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc. Natl. Acad. Sci. U.S.A. 94, 7098–710210.1073/pnas.94.13.7098 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Morcuende R., Bari R., Gibon Y., Zheng W., Pant B. D., Blasing O., Usadel B., Czechowski T., Udvardi M. K., Stitt M., Scheible W. R. (2007). Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 30, 85–11210.1111/j.1365-3040.2006.01608.x [Abstract] [CrossRef] [Google Scholar]
- Muchhal U. S., Pardo J. M., Raghothama K. G. (1996). Phosphate transporters from the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 93, 10519–1052310.1073/pnas.93.19.10519 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Muchhal U. S., Raghothama K. G. (1999). Transcriptional regulation of plant phosphate transporters. Proc. Natl. Acad. Sci. U.S.A. 96, 5868–587210.1073/pnas.96.10.5868 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mudge S. R., Rae A. L., Diatloff E., Smith F. W. (2002). Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 31, 341–35310.1046/j.1365-313X.2002.01356.x [Abstract] [CrossRef] [Google Scholar]
- Mukatira U. T., Liu C., Varadarajan D. K., Raghothama K. G. (2001). Negative regulation of phosphate starvation-induced genes. Plant Physiol. 127, 1854–186210.1104/pp.010876 [Abstract] [CrossRef] [Google Scholar]
- Nagarajan V. K., Jain A., Poling M. D., Lewis A. J., Raghothama K. G., Smith A. P. (2011). Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs, and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 156, 1149–116310.1104/pp.111.174805 [Abstract] [CrossRef] [Google Scholar]
- Nagy R., Karandashov V., Chague V., Kalinkevich K., Tamasloukht M., Xu G., Jakobsen I., Levy A. A., Amrhein N., Bucher M. (2005). The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 42, 236–25010.1111/j.1365-313X.2005.02364.x [Abstract] [CrossRef] [Google Scholar]
- Nagy R., Vasconcelos M. J., Zhao S., Mcelver J., Bruce W., Amrhein N., Raghothama K. G., Bucher M. (2006). Differential regulation of five Pht1 phosphate transporters from maize (Zea mays L.). Plant Biol. (Stuttg.) 8, 186–19710.1055/s-2005-873052 [Abstract] [CrossRef] [Google Scholar]
- Nakamori K., Takabatake R., Umehara Y., Kouchi H., Izui K., Hata S. (2002). Cloning, functional expression, and mutational analysis of a cDNA for Lotus japonicus mitochondrial phosphate transporter. Plant Cell Physiol. 43, 1250–125310.1093/pcp/pcf141 [Abstract] [CrossRef] [Google Scholar]
- Nilsson L., Muller R., Nielsen T. H. (2007). Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ. 30, 1499–151210.1111/j.1365-3040.2007.01734.x [Abstract] [CrossRef] [Google Scholar]
- Nuhse T. S., Stensballe A., Jensen O. N., Peck S. C. (2004). Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell 16, 2394–240510.1105/tpc.104.023150 [Abstract] [CrossRef] [Google Scholar]
- Okumura S., Mitsukawa N., Shirano Y., Shibata D. (1998). Phosphate transporter gene family of Arabidopsis thaliana. DNA Res. 5, 261–26910.1093/dnares/5.5.261 [Abstract] [CrossRef] [Google Scholar]
- Othman I., Al-Masri M. S. (2007). Impact of phosphate industry on the environment: a case study. Appl. Radiat. Isot. 65, 131–14110.1016/j.apradiso.2006.06.014 [Abstract] [CrossRef] [Google Scholar]
- Paszkowski U., Kroken S., Roux C., Briggs S. P. (2002). Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 99, 13324–1332910.1073/pnas.202474599 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Persson B. L., Lagerstedt J. O., Pratt J. R., Pattison-Granberg J., Lundh K., Shokrollahzadeh S., Lundh F. (2003). Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 43, 225–24410.1007/s00294-003-0400-9 [Abstract] [CrossRef] [Google Scholar]
- Poirier Y., Bucher M. (2002). “Phosphate transport and homeostasis in Arabidopsis,” in The Arabidopsis Book, eds Somerville C. R., Meyerowitz E. M., editors. (Rockville, MD: American Society of Plant Biologists; ), 1–35 [Europe PMC free article] [Abstract] [Google Scholar]
- Poirier Y., Thoma S., Somerville C., Schiefelbein J. (1991). Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 97, 1087–109310.1104/pp.97.3.1087 [Abstract] [CrossRef] [Google Scholar]
- Prak S., Hem S., Boudet J., Viennois G., Sommerer N., Rossignol M., Maurel C., Santoni V. (2008). Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol. Cell. Proteomics 7, 1019–103010.1074/mcp.M700566-MCP200 [Abstract] [CrossRef] [Google Scholar]
- Preuss C. P., Huang C. Y., Tyerman S. D. (2011). Proton-coupled high-affinity phosphate transport revealed from heterologous characterization in Xenopus of barley-root plasma membrane transporter, HvPHT1;1. Plant Cell Environ. 34, 681–68910.1111/j.1365-3040.2010.02272.x [Abstract] [CrossRef] [Google Scholar]
- Pumplin N., Harrison M. J. (2009). Live-cell imaging reveals periarbuscular membrane domains and organelle location in Medicago truncatula roots during arbuscular mycorrhizal symbiosis. Plant Physiol. 151, 809–81910.1104/pp.109.141879 [Abstract] [CrossRef] [Google Scholar]
- Rae A. L., Cybinski D. H., Jarmey J. M., Smith F. W. (2003). Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol. Biol. 53, 27–3610.1023/B:PLAN.0000009259.75314.15 [Abstract] [CrossRef] [Google Scholar]
- Raghothama K. G. (1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 665–69310.1146/annurev.arplant.50.1.665 [Abstract] [CrossRef] [Google Scholar]
- Rausch C., Bucher M. (2002). Molecular mechanisms of phosphate transport in plants. Planta 216, 23–3710.1007/s00425-002-0921-3 [Abstract] [CrossRef] [Google Scholar]
- Richardson A. E. (1994). “Soil microorganisms and phosphorus availability,” in Soil Biota: Management in Sustainable Farming Systems, eds Pankhurst C. E., Doube B. M., Gupta V. V. S. R., Grace P. R., editors. 50–62 [Google Scholar]
- Rouached H., Stefanovic A., Secco D., BulakArpat A., Gout E., Bligny R., Poirier Y. (2011). Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis. Plant J. 65, 557–57010.1111/j.1365-313X.2010.04442.x [Abstract] [CrossRef] [Google Scholar]
- Rubio V., Linhares F., Solano R., Martin A. C., Iglesias J., Leyva A., Paz-Ares J. (2001). A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 15, 2122–213310.1101/gad.204401 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sakano K. (1990). Proton/phosphate stoichiometry in uptake of inorganic phosphate by cultured cells of Catharanthus roseus (L.) G. Don. Plant Physiol. 93, 479–48310.1104/pp.93.2.479 [Abstract] [CrossRef] [Google Scholar]
- Schachtman D. P., Reid R. J., Ayling S. M. (1998). Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116, 447–45310.1104/pp.116.2.447 [Abstract] [CrossRef] [Google Scholar]
- Schunmann P. H., Richardson A. E., Smith F. W., Delhaize E. (2004a). Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.). J. Exp. Bot. 55, 855–86510.1093/jxb/erh103 [Abstract] [CrossRef] [Google Scholar]
- Schunmann P. H., Richardson A. E., Vickers C. E., Delhaize E. (2004b). Promoter analysis of the barley Pht1;1 phosphate transporter gene identifies regions controlling root expression and responsiveness to phosphate deprivation. Plant Physiol. 136, 4205–421410.1104/pp.104.045823 [Abstract] [CrossRef] [Google Scholar]
- Shimogawara K., Usuda H. (1995). Uptake of inorganic phosphate by suspension-cultured tobacco cells: kinetics and regulation by Pi starvation. Plant Cell Physiol. 36, 341–351 [Google Scholar]
- Shin H., Shin H. S., Dewbre G. R., Harrison M. J. (2004). Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 39, 629–64210.1111/j.1365-313X.2004.02161.x [Abstract] [CrossRef] [Google Scholar]
- Smith A. P., Jain A., Deal R. B., Nagarajan V. K., Poling M. D., Raghothama K. G., Meagher R. B. (2010). Histone H2A.Z regulates the expression of several classes of phosphate starvation response genes but not as a transcriptional activator. Plant Physiol. 152, 217–22510.1104/pp.109.145532 [Abstract] [CrossRef] [Google Scholar]
- Smith F. W., Cybinski D. H., Rae A. L., editors. (eds). (1999). Regulations of Expression of Genes Encoding Phosphate Transporters in Barley Roots. Dordrecht: Kluwer Academic Publishers [Google Scholar]
- Smith F. W., Ealing P. M., Dong B., Delhaize E. (1997). The cloning of two Arabidopsis genes belonging to a phosphate transporter family. Plant J. 11, 83–9210.1046/j.1365-313X.1997.11010083.x [Abstract] [CrossRef] [Google Scholar]
- Smith F. W., Rae A. L., Hawkesford M. J. (2000). Molecular mechanisms of phosphate and sulphate transport in plants. Biochim. Biophys. Acta 1465, 236–24510.1016/S0005-2736(00)00141-3 [Abstract] [CrossRef] [Google Scholar]
- Smith S. E., Barker S. J. (2002). Plant phosphate transporter genes help harness the nutritional benefits of arbuscular mycorrhizal symbiosis. Trends Plant Sci. 7, 189–19010.1016/S1360-1385(02)02260-4 [Abstract] [CrossRef] [Google Scholar]
- Smith S. E., Read D. J. (1997). Mycorrhizal Symbiosis, 2nd Edn. San Diego, CA: Academic Press [Google Scholar]
- Stefanovic A., Arpat A. B., Bligny R., Gout E., Vidoudez C., Bensimon M., Poirier Y. (2011). Over-expression of PHO1 in Arabidopsis leaves reveals its role in mediating phosphate efflux. Plant J. 66, 689–69910.1111/j.1365-313X.2011.04532.x [Abstract] [CrossRef] [Google Scholar]
- Takano J., Miwa K., Yuan L. X., Von Wiren N., Fujiwara T. (2005). Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. U.S.A. 102, 12276–1228110.1073/pnas.0502060102 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Thibaud M. C., Arrighi J. F., Bayle V., Chiarenza S., Creff A., Bustos R., Paz-Ares J., Poirier Y., Nussaume L. (2010). Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J. 64, 775–78910.1111/j.1365-313X.2010.04375.x [Abstract] [CrossRef] [Google Scholar]
- Ticconi C. A., Delatorre C. A., Abel S. (2001). Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiol. 127, 963–97210.1104/pp.010396 [Abstract] [CrossRef] [Google Scholar]
- Tittarelli A., Milla L., Vargas F., Morales A., Neupert C., Meisel L. A., Salvo G. H., Penaloza E., Munoz G., Corcuera L. J., Silva H. (2007). Isolation and comparative analysis of the wheat TaPT2 promoter: identification in silico of new putative regulatory motifs conserved between monocots and dicots. J. Exp. Bot. 58, 2573–258210.1093/jxb/erm123 [Abstract] [CrossRef] [Google Scholar]
- Ullrich C. I., Novacky A. J. (1990). Extra- and intracellular pH and membrane potential changes induced by K, Cl, H(2)PO(4), and NO(3) uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiol. 94, 1561–156710.1104/pp.94.4.1561 [Abstract] [CrossRef] [Google Scholar]
- Ullrich-Eberius C. I., Novacky A., Fischer E., Luttge U. (1981). Relationship between energy-dependent phosphate uptake and the electrical membrane potential in Lemna gibba G1. Plant Physiol. 67, 797–80110.1104/pp.67.4.797 [Abstract] [CrossRef] [Google Scholar]
- Ullrich-Eberius C. I., Novacky A., Van Bel A. J. E. (1984). Phosphate uptake in Lemna gibba G1: energetics and kinetics. Planta 161, 46–5210.1007/BF00951459 [Abstract] [CrossRef] [Google Scholar]
- Vance C. P., Uhde-Stone C., Allan D. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157, 423–44710.1046/j.1469-8137.2003.00695.x [Abstract] [CrossRef] [Google Scholar]
- Varadarajan D. K., Karthikeyan A. S., Matilda P. D., Raghothama K. G. (2002). Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 129, 1232–124010.1104/pp.010835 [Abstract] [CrossRef] [Google Scholar]
- Versaw W. K. (1995). A phosphate-repressible, high-affinity phosphate permease is encoded by the pho-5+ gene of Neurospora crassa. Gene 153, 135–13910.1016/0378-1119(94)00814-9 [Abstract] [CrossRef] [Google Scholar]
- Versaw W. K., Metzenberg R. L. (1995). Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. U.S.A. 92, 3884–388710.1073/pnas.92.9.3884 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Von Vexhull H. R., Mutert E. (1998). “Global extent, development and economic impact of acid soils,” in Plant-Soil Interactions at Low pH: Principles and Management, eds Date R. A., Grundon N. J., Rayment G. E., Probert M. E., editors. (Dordrecht: Kluwer; ), 5–19 [Google Scholar]
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G. V., Provart N. J. (2007). An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2, e718.10.1371/journal.pone.0000718 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wirth J., Chopin F., Santoni V., Viennois G., Tillard P., Krapp A., Lejay L., Daniel-Vedele F., Gojon A. (2007). Regulation of root nitrate uptake at the NRT2.1 protein level in Arabidopsis thaliana. J. Biol. Chem. 32, 23541–2355210.1074/jbc.M700901200 [Abstract] [CrossRef] [Google Scholar]
- Wu P., Ma L., Hou X., Wang M., Wu Y., Liu F., Deng X. W. (2003). Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 132, 1260–127110.1104/pp.103.021022 [Abstract] [CrossRef] [Google Scholar]
- Wu Z., Ren H., McGrath S. P., Wu P., Zhao F.-J. (2011). Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol. 157, 498–50810.1104/pp.111.181065 [Abstract] [CrossRef] [Google Scholar]
- Wykoff D. D., Grossman A. R., Weeks D. P., Usuda H., Shimogawara K. (1999). Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc. Natl. Acad. Sci. U.S.A. 96, 15336–1534110.1073/pnas.96.26.15336 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xiao K., Liu J., Dewbre G., Harrison M., Wang Z. Y. (2006). Isolation and characterization of root-specific phosphate transporter promoters from Medicago truncatula. Plant Biol. (Stuttg.) 8, 439–44910.1055/s-2005-873053 [Abstract] [CrossRef] [Google Scholar]
- Xu G. H., Chague V., Melamed-Bessudo C., Kapulnik Y., Jain A., Raghothama K. G., Levy A. A., Silber A. (2007). Functional characterization of LePT4: a phosphate transporter in tomato with mycorrhiza-enhanced expression. J. Exp. Bot. 58, 2491–250110.1093/jxb/erm096 [Abstract] [CrossRef] [Google Scholar]
- Zhou J., Jiao F., Wu Z., Li Y., Wang X., He X., Zhong W., Wu P. (2008). OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol. 146, 1673–168610.1104/pp.107.111443 [Abstract] [CrossRef] [Google Scholar]
Articles from Frontiers in Plant Science are provided here courtesy of Frontiers Media SA
Full text links
Read article at publisher's site: https://doi.org/10.3389/fpls.2011.00083
Read article for free, from open access legal sources, via Unpaywall:
https://www.frontiersin.org/articles/10.3389/fpls.2011.00083/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3389/fpls.2011.00083
Article citations
Candida albicans' inorganic phosphate transport and evolutionary adaptation to phosphate scarcity.
PLoS Genet, 20(8):e1011156, 13 Aug 2024
Cited by: 1 article | PMID: 39137212 | PMCID: PMC11343460
SlDELLA interacts with SlPIF4 to regulate arbuscular mycorrhizal symbiosis and phosphate uptake in tomato.
Hortic Res, 11(9):uhae195, 21 Jul 2024
Cited by: 0 articles | PMID: 39257536 | PMCID: PMC11384114
Short-term artificial adaptation of Rhizoglomus irregulare to high phosphate levels and its implications for fungal-plant interactions: phenotypic and transcriptomic insights.
Front Plant Sci, 15:1385245, 23 Apr 2024
Cited by: 0 articles | PMID: 38716338 | PMCID: PMC11074448
Evolution of the SPX gene family and its role in the response mechanism to low phosphorus stress in self-rooted apple stock.
BMC Genomics, 25(1):488, 16 May 2024
Cited by: 0 articles | PMID: 38755552 | PMCID: PMC11108120
Modulation of plant immunity and biotic interactions under phosphate deficiency.
J Plant Res, 137(3):343-357, 02 May 2024
Cited by: 0 articles | PMID: 38693461
Review
Go to all (187) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The conservation of phosphate-binding residues among PHT1 transporters suggests that distinct transport affinities are unlikely to result from differences in the phosphate-binding site.
Biochem Soc Trans, 44(5):1541-1548, 01 Oct 2016
Cited by: 9 articles | PMID: 27911737
Review
The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation.
New Phytol, 195(2):356-371, 11 May 2012
Cited by: 98 articles | PMID: 22578268
Environmental Control of Phosphorus Acquisition: A Piece of the Molecular Framework Underlying Nutritional Homeostasis.
Plant Cell Physiol, 62(4):573-581, 01 Sep 2021
Cited by: 5 articles | PMID: 33508134
Review
Plant phosphorus acquisition in a common mycorrhizal network: regulation of phosphate transporter genes of the Pht1 family in sorghum and flax.
New Phytol, 205(4):1632-1645, 23 Jan 2015
Cited by: 54 articles | PMID: 25615409





