Abstract
Background
The Thai phase 3 HIV vaccine trial RV 144 showed modest efficacy of a vaccine against HIV acquisition. Baseline variables of age, sex, marital status, and risk did not modify vaccine efficacy. We did a post-hoc analysis of the trial's data to investigate behavioural risk and efficacy every 6 months after vaccination.Methods
RV 144 was a randomised, multicentre, double-blind, placebo-controlled efficacy trial testing the combination of the HIV vaccines ALVAC-HIV (vCP1521) and AIDSVAX B/E to prevent HIV infection or reduce setpoint viral load. Male and female volunteers aged 18-30 years were recruited from the community. In this post-hoc analysis of the modified intention-to-treat population (16,395 participants), HIV risk behaviour was assessed with a self-administered questionnaire at the time of initial vaccination in the trial and every 6 months thereafter for 3 years. We classified participants' behaviour as low, medium, or high risk. Both the acquisition endpoint and the early viral-load endpoint were examined for interactions with risk status over time and temporal effects after vaccination. Multiple proportional hazards regression models with treatment and time-varying risk covariates were analysed.Findings
Risk of acquisition of HIV was low in each risk group, but 9187 (58·2%) participants reported higher-risk behaviour at least once during the study. Participants classified as high or increasing risk at least once during follow-up were compared with those who maintained low-risk or medium-risk behaviour as a time-varying covariate, and the interaction of risk status and acquisition efficacy was significant (p=0·01), with greater benefit in low-risk individuals. Vaccine efficacy seemed to peak early--cumulative vaccine efficacy was estimated to be 60·5% (95% CI 22-80) through the 12 months after initial vaccination--and declined quickly. Vaccination did not seem to affect viral load in either early or late infections.Interpretation
Future HIV vaccine trials should recognise potential interactions between challenge intensity and risk heterogeneity in both population and treatment effects. The regimen tested in the RV 144 phase 3 trial might benefit from extended immunisation schedules.Funding
US Army Medical Research and Materiel Command and Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health.Free full text

Ad hoc analysis of behavior and time as co-variates of the Thai phase III efficacy trial: RV 144
Abstract
Background
The Thai phase III HIV vaccine trial's modest efficacy (VE 31.2% 95% CI 1.1, 51.2) represents the first demonstration that a vaccine can protect against HIV acquisition. Baseline variables of age, gender, marital status, and risk did not modify vaccine efficacy (VE). Here we explore behavioral risk and efficacy at 6 monthly intervals following vaccination.
Methods
Behavioral risk was assessed with a self-administered questionnaire every 6 months during trial participation. Both the acquisition endpoint and the early viral load endpoint are examined for interactions with risk status over time and temporal effects following vaccination.
Finding
Risk for HIV acquisition is low in each risk group, but the majority of participants reported higher-risk behavior at least once during the study (N= 9187, 58%). In post-hoc analyses, comparing those participants categorized as high or rising risk at least once during study follow-up versus those who maintained low or medium risk behavior as a time-varying covariate, the interaction of risk status and acquisition efficacy is significant (P = 0.010) with greater benefit in the lower risk individuals. VE appears to peak early with an estimate of cumulative VE = 60% through 12 months after initial vaccination (95% CI 22 –80%), and declines quickly. Vaccination did not appear to affect viral load in either early or late infections.
Interpretation
Future HIV vaccine trials must recognize potential interactions between challenge intensity and risk heterogeneity in the population and treatment effects. The regimen tested in the Thai phase III trial may benefit from extended immunization schedules.
INTRODUCTION
The results of the Thai HIV vaccine trial, RV144, provide new hope that a vaccine to prevent acquisition of HIV infection is possible.1 While the observed efficacy was modest and insufficient to warrant licensure, RV 144 provides both insights and opportunities. The Thai trial had two salient, hypothesis-generating observations: 1) that efficacy appeared greater in participants at lower risk for HIV infection and 2) efficacy appeared maximal early and decreased with time. We have undertaken a detailed, post-hoc statistical exploration to determine whether we can gain further insight into these observations.
In contrast to prior HIV efficacy trials, RV 144 enrolled predominantly heterosexuals residing from a population with low prevalence of HIV.1 Most sexual encounters in RV 144 were unlikely to be associated with HIV transmission risk. Participants in well-defined high risk groups including sex workers, homosexual/bisexual men or injecting drug users represented a minority of the study population and accrued a minority of endpoint infections. The study was not designed to evaluate risk-stratified efficacy rates, and no statistically significant interaction between baseline risk and efficacy was observed1 although estimated VE was >40% in the lower risk groups at baseline and under 5% in the high risk participants. In other disease settings, sufficient challenge doses may overwhelm a vaccine induced protective immune response.2 Possibly, the modest success observed in RV 144 may be a reflection of generally lower levels of challenge encountered in the study population.
In general, non-human primate challenge studies with high dose, intravenous SIV and pathogenic SHIV have suggested that protection from infection was not feasible but a favorable modification of early viral burden and clinical outcome was realistically achievable.1 A notable outcome in RV 144 was the absence of an effect on viral load among vaccine recipients.1 Most of these non-human primate studies employed intravenous or non-physiologic, high-dose mucosal challenge doses of virus. Recent non-human primate challenge studies utilizing a repeat, low-dose, mucosal challenge with SIV after vaccination have shown protection from acquisition with no or variable effect on viral load or clinical outcome among animals with breakthrough infection.3, 4 These observations are consistent with the notion that currently available vaccines afford a reduction in acquisition risk that are observed in settings that more closely model human trans-mucosal risk. Taken together, these findings also suggest that the immune responses associated with protection from infection are largely distinct from those needed for reduction of viremia and improved clinical outcome as suggested in a summary of data from human trials of ALVAC-protein boost breakthrough infections and an SIV NHP challenge study.5–7
The RV 144 study was designed to acquire endpoints over 3.5 years after initial vaccination in over 16,000 volunteers with 90% statistical power to address the acquisition objective of 50% efficacy. This population size and extended follow-up was needed in view of the ten-fold reduction in HIV incidence as a consequence of the vigorous public health campaign for HIV/AIDS prevention in Thailand.8, 9 The trial was not designed to define time-dependent effects. Nevertheless, inspection of the data suggests that efficacy waned during the extended observation period although the interaction was not statistically significant.1
Here we further explore, in a ad-hoc analysis, the interaction of risk behavior and efficacy over the full course of the study and examine time dependent estimates of efficacy to guide the design of future VE trials for HIV.
METHODS
Study Population
The main study methods and results including the screening, enrollment and retention data by arm have been published previously.1 In brief, the trial was a randomized, multicenter, double-blind, placebo-controlled efficacy trial testing the combination of ALVAC-HIV® (vCP1521) and AIDSVAX B/E® to prevent HIV infection or reduce set-point viral load. Male and female volunteers aged 18–30 years were recruited from the community without regard to HIV risk through a separate screening protocol. Volunteers received a trial information briefing and written informed consent was obtained for participation in the screening protocol.HIV testing was performed, and a follow-up visit was scheduled 2–3 weeks later at one of the eight clinical research sites.
Study Procedures
Volunteers returned for follow-up after the screening visit, were informed of their HIV test results and if seronegative, the Phase III trial written informed consent was obtained and vaccinations initiated. The protocol was reviewed and approved by the Ethical Committees of the Ministry of Public Health, the Royal Thai Army, Mahidol University, and the Human Subjects Research Review Board of the U.S. Army Medical Research and Materiel Command.
Vaccinations occurred over a 24-week period (0, 4, 12, 24 weeks). The ALVAC-HIV® (vCP1521) or placebo prime was given in the left arm at weeks 0, 4, 12, and 24. Boosting with AIDSVAX B/E or placebo was delivered in the right arm at weeks 12 and 24.
The volunteers were followed with HIV testing (with appropriate pre- and post-test counseling) every 6 months for 3 years. Plasma for HIV-1 diagnostics was collected at 0 and 24 weeks, and every 6 months during the follow-up phase. Education on risk behavior reduction was provided during each vaccination and post-test counseling visit. The scheme for clinical trial conduct from screening to treatment and analysis allocation is published elsewhere.1
Study Objectives and Endpoints
Assessment of HIV risk behavior within the preceding 6 months occurred at baseline, week 24 and at each 6-month follow-up visit via a self-administered questionnaire. The volunteer was asked to categorize whether their everyday behavior placed them at risk for HIV infection. The questionnaire then inventoried specific risk behaviors for HIV acquisition. At each visit, a participant was categorized as “high risk” if in the last 6 months: (1) reported that their behavior placed them at risk for HIV, (2) shared needles when injecting drugs, (3) 2 or more sex partners; (4) HIV positive partner; (5) no condom use during the last sexual contact (≤ 6 months) with a commercial sex worker (CSW), casual partner, same gender partner, drug injecting partner or partner with multiple partners; (6) recent STD symptoms; (7) drug use while incarcerated; or (8) baseline employment as CSWs or in the entertainment industry. Volunteers were considered low risk if in the prior 6 months they: (1) perceived their behavior did not place them at risk for HIV; (2) reported 0/1 sex partners and no sex with CSWs, casual partners, same gender partner, HIV infected partner, drug-injecting partner or a partner with many partners; (3) or reported no STD symptoms or incarceration. The moderate risk category contained persons with neither low nor high risk. For example, an individual with a single partner described as CSW, IDU, same gender or casual partner but who used condoms at the last sexual encounter is neither high nor low risk and therefore categorized as medium risk. Also, individuals who did not answer a risk question item but were otherwise consistent with high risk were classified as moderate risk. The risk score categories were constructed from the baseline responses using the combined arm infection results in a blinded fashion. The terms high, moderate and low are relative within this population and do not equate to typical definitions in high-risk cohorts.
Statistical Considerations
Volunteers with prevalent infection at baseline were excluded (N=7) and the modified intent-to-treat population was used for the analyses (N= 16395). To accommodate withdrawals, the percentages of individuals with identified risk characteristics over the study course were estimated by the product-limit survival method. Multiple proportional hazards regression models with treatment and time-varying risk covariates were analyzed. Because of its simplicity and similar VE results seen in subjects with initial low or medium risk, results for the time-varying covariate model with risk categorized as high or ever increasing (versus other) along with its interaction with treatment are presented. VE was also evaluated for maximum level of risk reported during the study and risk level reported in the study interval prior to seroconversion. VE estimates from the Kaplan-Meier infection estimates were computed at semiannual time points. A nonparametric estimate of the relative hazard function was computed along with its confidence intervals.10 Descriptive statistics were computed and pointwise Wilcoxon tests of viral load were performed. Two-tailed p-values are reported.
Role of funding sources
The study was funded by the US Army Medical Materiel Command and the Division of AIDS, National Institute of Allergy and Infectious Diseases. ALVAC-HIV (vCP1521) and ALVAC placebo was supplied by the manufacturer, Sanofi-Pasteur. AIDSVAX and AIDSVAX placebo (VaxGen) was purchased by DAIDS/NIAID for the purpose of this trial.
RESULTS
Interaction of risk and efficacy
We previously reported that baseline behavioral risk characteristics were balanced by treatment arm and associated with different placebo arm transmission rates ranging from 0.227 per 100 PY in the low risk group to 0.364 in the high risk group (p=0.005 adjusted for treatment).1 However, estimates of VE were not significantly different when compared by the baseline behavioral risk co-variate or any other parameter assessed including sex, age, and baseline partnership status.1 Behavioral characteristics were evaluated at each subsequent visit and for the study overall; the percent of total participants categorized as low risk increased, and the medium and high risk categories declined through the first 52 weeks of study and remained stable thereafter (figure 1). The distribution of risk for both overall category and individual risk items (data not shown) between treatment arms remained balanced. Condom use was stable over the course of the study for all partner types. Overall risk behavior category was similar between men and women (data not shown).
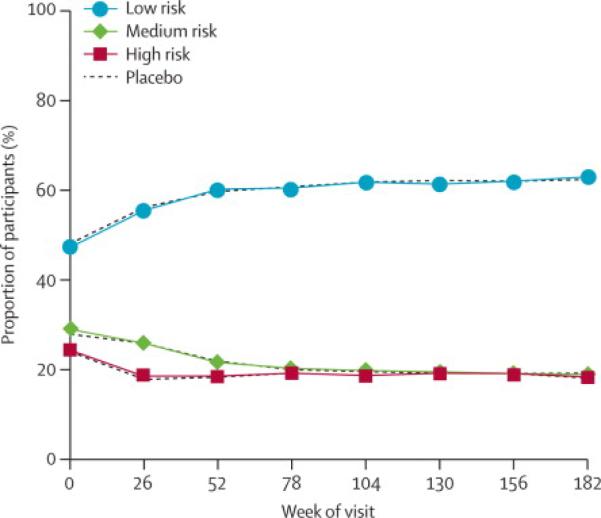
Risk category over time. Percent of participants categorized as low, medium or high risk based upon questionnaires administered at entry and every six months. Vaccine and placebo groups are shown separately for each category.
Participants were able to skip a question or report, “don't know/not sure”, and these response rates declined as the study progressed. For example, “declined to answer” for injecting drug use with needle sharing was 7.4 % (N=1218) of respondents at study entry and decreased to 2.0 % (N=303) by trial end with a small corresponding increase in reported rates both affirming and denying this behavior over time. Thirty five percent (N=105) of the participants who declined to answer at trial end also declined to answer this item at baseline. Although the rates for individual and overall risk category did not systematically increase over time, the number of individuals who reported a high risk factor at least once increased (table 1). No treatment group differences were identified in the risk categories at baseline or through time. At baseline, 7 of the 15 risk characteristics had higher numbers in the placebo group with a maximum excess risk disparity of 18 subjects. Many participants (N=5613 36%) reported a self-assessment of high-risk behavior at least once during the study. Generally, all other specific risk items were far less common. Taken together the percent of participants self categorized or assigned to the high risk group based on specific responses rose from 24.1% (N=3495) at entry to58.2% (N=9187) when considering all time points available in the study. The number of HIV infections in baseline high risk (N=45) and low risk participants (N=46) was similar despite different transmission rates because low risk participants were more common at baseline. However, over time the majority of infections were identified in participants who reported high-risk behavior at baseline or at least one subsequent visit. An additional 39 infections occurred in participants who initially reported low risk behavior (n=28, 14 placebo and 14 vaccine) or medium risk behavior (n=11, 6 placebo and 5 vaccine) and later reported at least one interval with a higher risk behavior category. When considering two categories of risk for the entire study data-set as those reporting high or increased risk behavior at least once versus those reporting medium or lower or constant low risk throughout the study on a time-varying basis, the interaction of risk with VE is significant (p = 0.010). The VE estimate for participants who maintained their low at entry or medium at entry risk throughout the entire study is 68% (p=0.002 95% CI (34%, 84%)). In this analysis, little vaccine effect is seen for the higher risk group (VE=5% 95% CI(−46%, 38%)).
Table 1
Behavioral risk indicators at baseline and ever reported during the study.
| Baseline | Baseline | Ever | Ever | |
|---|---|---|---|---|
| Risk Characteristic | N | percent | N | percent |
| Everyday behavior puts at risk | 1620 | 9.9% | 5613 | 36.1% |
| Needle sharing | 133 | 0.8% | 1250 | 8.2% |
| 2–4 sex partners | 1034 | 6.3% | 2745 | 17.5% |
| > 4 sex partners | 205 | 1.3% | 502 | 3.2% |
| No condom with casual partner | 936 | 5.7% | 2490 | 15.9% |
| No condom/sex worker partner | 62 | 0.4% | 291 | 1.9% |
| No condom/same gender partner | 169 | 1% | 429 | 2.7% |
| Condom / HIV infected partner | 227 | 1.45 | 597 | 3.8% |
| No condom / HIV infected partner | 29 | 0.2% | 143 | 0.9% |
| No condom / IDU partner | 18 | 0.1% | 97 | 0.6% |
| No condom / many sex partners | 258 | 1.6% | 753 | 4.8% |
| STD symptoms | 479 | 2.9% | 1613 | 10.4% |
| Jailed with drug injection | 38 | 0.2% | 181 | 1.2% |
| Occupation as sex worker | 86 | 0.5% | NA | |
| Occupation in entertainment | 470 | 2.9% | NA | |
| High Risk Category Group total | 3945 | 24.1% | 9187 | 58.2% |
Although this post-hoc analysis identifies an interaction with risk, the most important risk behaviors for HIV transmission globally contributed little to the observations within RV 144. For example, though injecting drug use with needle sharing was commonly reported (N=1250 8.2% overall) only 5 HIV infections were observed (2 vaccine and 3 placebo). Among men reporting sex with men, only 16 HIV infections were observed (8 vaccine and 8 placebo).
Efficacy over time
Over the course of the study, as shown in table 2, observed VE declined at each interval with endpoint ascertainment after 12 months but the interaction of time from first immunization and outcome was not significant (p=0.36).1 Nevertheless, the early time point efficacy estimates observed after completion of the vaccine series are substantially greater than at study conclusion with a VE of 54% and 60% at the 6 and 12 month intervals. The transmission rate for the placebo group varied modestly from 0.38 per 100PY in year one to 0.26 per 100PY in the final year of observation. In proportional hazards models that specified the log hazard ratio (vaccine/placebo) as various smooth functions of time (linear, log-linear, quadratic, piece-wise cubic polynomials in 3 or 4 segments), the results were generally consistent with efficacy most clearly evident early and declining from the second year of observation through study end (data not shown). A nonparametric analysis gives a similar result with early instantaneous hazards efficacy waning to zero by 18 months (figure 2).
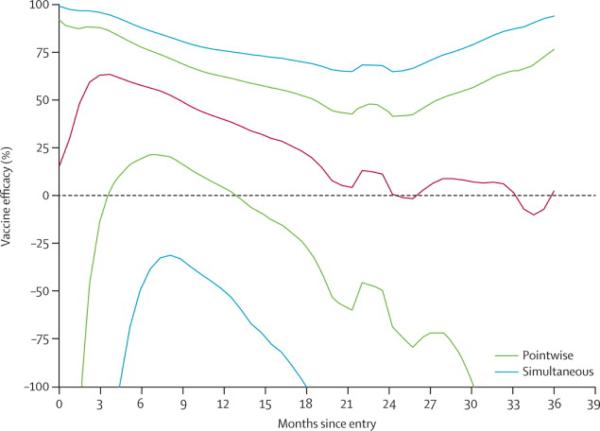
Vaccine efficacy point estimates over time. Vaccine efficacy rates are given over time (black line) with 95% confidence interval determined by pairwise comparison (dotted line) and simulated data sets (solid purple line).
Table 2
Cumulative VE at 6 month intervals for the mITT population (i.e. 100*(1-vaccine infection %/placebo infection %) for the mITT population determined from Kaplan-Meier infection rates at 6 month inverals
| Vaccine | Placebo | ||||||
|---|---|---|---|---|---|---|---|
| Time (Months) | Events | Infection (%) | Standard Error (%) | Events | Infection (%) | Standard Error (%) | Efficacy (%) |
| 6 | 5 | 0.06 | 0.28 | 11 | 0.14 | 0.042 | 54.5 |
| 12 | 12 | 0.15 | 0.044 | 30 | 0.38 | 0.069 | 59.9 |
| 18 | 24 | 0.31 | 0.063 | 43 | 0.55 | 0.083 | 44.0 |
| 24 | 32 | 0.41 | 0.072 | 50 | 0.64 | 0.09 | 35.7 |
| 30 | 37 | 0.48 | 0.078 | 58 | 0.74 | 0.097 | 36.0 |
| 36 | 45 | 0.58 | 0.086 | 65 | 0.84 | 0.103 | 30.4 |
| 42 | 51 | 0.68 | 0.096 | 74 | 0.96 | 0.111 | 29.2 |
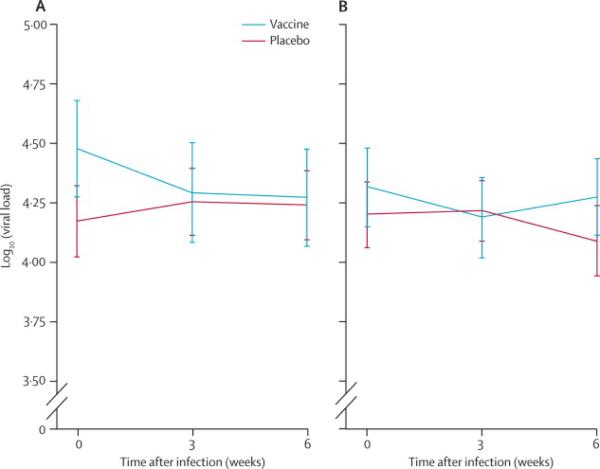
In view of these observations, an additional post-hoc analysis considered a timing of infection and viral load. The viral load endpoint was determined as the average of three samples acquired over the first 6 weeks after serodiagnosis of HIV. This viral load endpoint was evaluated for acquisition events arising within 600 days of initial vaccination, a period with higher efficacy rate estimates, separately from those observed after this period where efficacy declined. No difference in viral load at any time point following infection, or mean early viral load (the co-primary endpoint), was detected for vaccine and placebo comparisons when segregating analysis by proximity to vaccination (figure 3A and 3B). Vaccine impact on viral load did not differ either by baseline risk group or when categorized as high or increased over baseline risk versus others.
DISCUSSION
The Thai phase III HIV vaccine trial showed modest benefit which was unrelated to baseline variables including risk assessment. The risk assessment variable is significant in respect to outcome when considered over the course of the study. Further, the efficacy estimate was highest in the first 6 months after completion of vaccination and waned rapidly.
Risk of infection is a complex, compound estimate of multiple effects but may be simply formulated as follows (adapted from Fisher et al.)11
Aggregate risk of infection is a function of donor “challenge” (risk that source is HIV positive, viral load, presence of co-factors, ie STD, bleeding and others), recipient susceptibility (genetically defined host characteristics, route and presence of cofactors, ie STD, bleeding and others) and frequency of exposure.
For example, HIV infection among individuals with deletions of CCR5 are uncommon, but given sufficient exposures, the cumulative probability of encountering a donor with X4 virus mitigates the protective benefit of this element of genetic resistance.12 Further, data from human vaccine and challenge experiments provide convincing evidence that induction of protective immunity can be overcome with a single sufficiently large challenge.2 In the case of non-human primate studies, it has long been considered likely that reproducible and efficient infectious challenge doses and routes may require a magnitude of immune response that is currently unachievable for the current generation of vaccines. Similarly in Vax003, a trial that utilized AIDSVAX B/E in Thai injecting drug users, the lack of observed efficacy may have been due to the stringency of intravenous challenge, when compared to intravaginal and intrarectal routes. This may be due to the circumvention of mucosal barriers and associated genetic bottlenecks or the avoidance of vaccine associated immune responses associated with mucosal sites. Several lines of evidence suggest that the challenge experienced by IDU is higher in magnitude and in genetic diversity.13–15 It is possible that the human vaccines tested in efficacy trials thus far provided only modest time-sensitive reduction in host susceptibility that was undone by the aggregate transmission challenge intensity, i.e. the product of the number and quality of host risk behaviors and susceptibility factors and the frequency and magnitude of exposures.
It is reasonable to assume that vaccine induced immune responses are independent of volunteer risk status. The absence of efficacy in higher risk participants within RV 144 may reflect either the higher challenge per exposure or more frequent exposure or both, with infection when the threshold exposure occurred at a time when immune responses were inadequate to the task of containment (figure 4). In contrast, the population maintaining lower risk throughout the study, in whom efficacy is observed, are likely to have less frequent exposures and possibly less challenging exposures. This concept is illustrated in figure 4. Frequency and route of exposure, and co-factors such as STDs, constitute the basis for considering different levels of challenge intensity16, 17 and correspondingly, different magnitudes of vaccine induced immunity required to achieve protection. Further, it can be deduced from figure 4, that vaccine-induced responses must be both higher in magnitude and better sustained to achieve similar efficacy in high intensity and low intensity challenge populations. The model depicted in figure 4 conforms to the observations of a modest, time-limited protective immune response as observed in RV 144 and suggests an additional boost or other augmentation of immune response would improve efficacy for all risk categories.
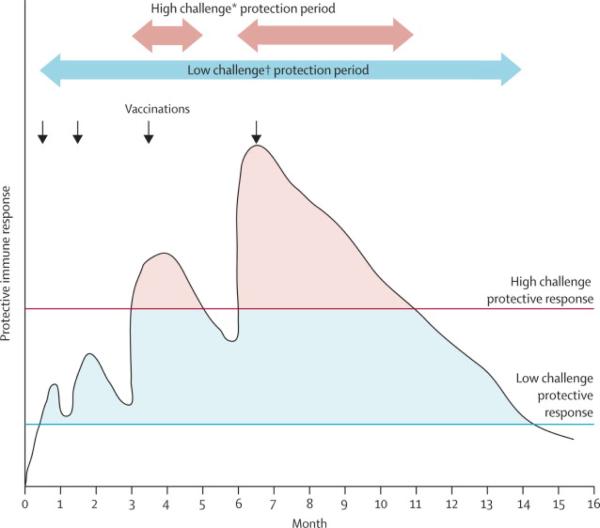
Schematic representation of the interaction of challenge intensity and VE. The black line represents magnitude of protective vaccine induced immunity as it rises after each immunization and wanes subsequently over time.
A second possibility assumes a more complex model with different immune responses that protect against higher and lower risk challenges (for instance, these might be manifest in different mucosal compartments); the differential decay of these protective responses could also explain the early protective effect seen in RV144. Correlation of immune responses induced by the ALVAC-HIV + AIDSVAX B/E regimen to the temporal pattern of protection will be critical in guiding future vaccine development.
The commonly held view has been that most vaccines under development for HIV had no hope of preventing acquisition of HIV infection due to the absence of neutralizing antibody.18, 19 It has been thought that a more realistic goal for vaccine platforms inducing T cell immunity was to reduce early viral load and slow disease progression as has been observed in high dose NHP challenge studies20–22 and inferred from studies of elite controllers and long-term non-progressors with HIV (reviewed in Virgin, et al.23). Despite the apparent absence of neutralizing antibody against primary HIV isolates or broadly neutralizing activity the ALVAC prime/AIDSVAX boost reduced acquisition by 31.2% at 42 months. This regimen failed to provide any early viral load benefit even when examined in the period shortly after vaccination, where acquisition efficacy appears highest. This observation is consistent with the notion that efficacy for acquisition requires a different set of immune effectors than achieving a reduction in viral load and altered prognosis. Interestingly, Haynes et al. reported that IgG against a conformational gp120 V1V2 epitope was inversely correlated with infection in RV144.24 These data prompted Barouch et al. to look for anti-V2 responses in non-human primates vaccinated with adenovirus type 26 and modified vacciniaankara vectored SIV inserts, and anti-(SIV)V2 responses in that study were also inversely correlated with infection risk.7 These findings raise additional hypotheses related to the potential decay of immune responses in the plasma or mucosal compartments.
One may reasonably question whether a vaccine affording modest protection in low challenge intensity settings is valuable as a public health intervention; others argue that such a vaccine might be cost effective in the Thai setting.25, 26 Nevertheless, the value of identifying the potential to protect from human transmission by vaccination cannot be underestimated. Further elucidating the nature of protection afforded in more permissive settings may allow optimization of vaccine strategies to achieve qualitatively and quantitatively superior products with expanded efficacy potential. The data presented here should be carefully considered in terms of the inherent risks of a post-hoc analysis and are intended to identify areas deserving further consideration in future efficacy trials. It is likely not the primary findings of RV 144 that are most important to future HIV vaccine development but the questions these data engender. Among these, the temporal nature of protection and the interaction with risk deserve more detailed consideration in HIV vaccine trials.
SYSTEMATIC REVIEW
A systematic search of the literature for HIV vaccine efficacy trials was accomplished using PubMed to identify all randomized controlled trials with behavioral data and a positive outcome published in English. No prior studies of HIV vaccines showing any degree of efficacy were identified.
INTERPRETATION
The efficacy observed in RV 144 which enrolled a low incidence population and benefit waned quickly after 12 months rapidly and accrued mainly to those who did not report or perceive traditional HIV risk behaviors. Future studies will need to employ designs to account for participant risk and temporal effects and this pox-protein prime boost regimen may show improved efficacy with extended boosts.
AKNOWLEDGEMENTS
The study was funded by the US National Institutes of Health, Division of AIDS and by the US Army Medical Research and Material Command. The authors recognize the critical support and commitment of the volunteers, research staff and communities of Chonburi and Rayong where the study was conducted.
Funding: Funding was provided by the US Army, Research and Material Command, and the Division of AIDS, National Institutes of Allergy and Infectious Disease, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS The manuscript was written by Merlin Robb, Jerome Kim, Don Stablein and Nelson Michael who also contributed to study design, management, and data analysis. Peter Gilbert contributed to data analysis and manuscript preparation. SupachaiRerks-Ngarm, SorachaiNitayaphan, PunneePitisuttithum, JaranitKaewkungwal, PrayuraKunasol, ChirasakKhamboonruang, PrasertThongcharoen, Patricia Morgan, Michael Benenson, Robert Paris, Joseph Chiu, and Elizabeth Adamscontributed to the design, execution, collection and analysis of primary trial data and risk information. Donald Francis, Sanjay Gurunathan, Jim Tartagliaare representatives of the manufacturers of the study test products and were involved in design, oversight, data analysis and interpretation.
The opinions in this paper are those of the authors and are not to be construed as official and do not reflect the views of the Department of Army or the Department of Defense.
CONFLICT OF INTEREST STATEMENT Sanjay Gurunathan and Jim Tartaglia are employees of Sanofi-Pasteur, the commercial manufacturer of ALVAC. The remaining authors have no conflict of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/s1473-3099(12)70088-9
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3530398?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/s1473-3099(12)70088-9
Article citations
A single dose of an ALVAC vector-based RABV virus-like particle candidate vaccine induces a potent immune response in mice, cats and dogs.
Emerg Microbes Infect, 13(1):2406280, 30 Sep 2024
Cited by: 0 articles | PMID: 39295522 | PMCID: PMC11443554
Immune correlates analysis of the Imbokodo (HVTN 705/HPX2008) efficacy trial of a mosaic HIV-1 vaccine regimen evaluated in Southern African people assigned female sex at birth: a two-phase case-control study.
EBioMedicine, 108:105320, 04 Sep 2024
Cited by: 0 articles | PMID: 39236556 | PMCID: PMC11404224
Impact of Recombinant VSV-HIV Prime, DNA-Boost Vaccine Candidates on Immunogenicity and Viremia on SHIV-Infected Rhesus Macaques.
Vaccines (Basel), 12(4):369, 29 Mar 2024
Cited by: 0 articles | PMID: 38675751 | PMCID: PMC11053682
Safety and immunogenicity of a subtype C ALVAC-HIV (vCP2438) vaccine prime plus bivalent subtype C gp120 vaccine boost adjuvanted with MF59 or alum in healthy adults without HIV (HVTN 107): A phase 1/2a randomized trial.
PLoS Med, 21(3):e1004360, 19 Mar 2024
Cited by: 1 article | PMID: 38502656 | PMCID: PMC10986991
A remarkable genetic shift in a transmitted/founder virus broadens antibody responses against HIV-1.
Elife, 13:RP92379, 15 Apr 2024
Cited by: 0 articles | PMID: 38619110 | PMCID: PMC11018346
Go to all (162) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial.
Lancet HIV, 7(4):e238-e248, 06 Feb 2020
Cited by: 31 articles | PMID: 32035516 | PMCID: PMC7247755
Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand.
N Engl J Med, 361(23):2209-2220, 20 Oct 2009
Cited by: 2072 articles | PMID: 19843557
HIV gp120 vaccine - VaxGen: AIDSVAX, AIDSVAX B/B, AIDSVAX B/E, HIV gp120 vaccine - Genentech, HIV gp120 vaccine AIDSVAX - VaxGen, HIV vaccine AIDSVAX - VaxGen.
Drugs R D, 4(4):249-253, 01 Jan 2003
Cited by: 11 articles | PMID: 12848591
Human immunodeficiency virus vaccine trials.
Cold Spring Harb Perspect Med, 2(12):a007351, 01 Dec 2012
Cited by: 38 articles | PMID: 23209178 | PMCID: PMC3543076
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R37 AI054165
Grant ID: Y01 AI002642-12