Abstract
Free full text

Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice
Abstract
Inhibition of leukocyte adhesion to the vascular endothelium represents a novel and important approach for decreasing sickle cell disease (SCD) vaso-occlusion. Using a humanized SCD–mouse-model of tumor necrosis factor-α–induced acute vaso-occlusion, we herein present data demonstrating that short-term administration of either hydroxyurea or the phosphodiesterase 9 (PDE9) inhibitor, BAY73-6691, significantly altered leukocyte recruitment to the microvasculature. Notably, the administration of both agents led to marked improvements in leukocyte rolling and adhesion and decreased heterotypic red blood cell-leukocyte interactions, coupled with prolonged animal survival. Mechanistically, these rheologic benefits were associated with decreased endothelial adhesion molecule expression, as well as diminished leukocyte Mac-1-integrin activation and cyclic guanosine monophosphate (cGMP)–signaling, leading to reduced leukocyte recruitment. Our findings indicate that hydroxyurea has immediate beneficial effects on the microvasculature in acute sickle-cell crises that are independent of the drug's fetal hemoglobin-elevating properties and probably involve the formation of intravascular nitric oxide. In addition, inhibition of PDE9, an enzyme highly expressed in hematopoietic cells, amplified the cGMP-elevating effects of hydroxyurea and may represent a promising and more tissue-specific adjuvant therapy for this disease.
Introduction
Sickle cell disease (SCD) is a genetic disorder caused by a point mutation in the HBB gene, resulting in the production of abnormal sickle hemoglobin (HbS).1,2 HbS polymerizes at low oxygen levels, making the red blood cell (RBC) more rigid and, eventually, irreversibly sickled. This causes the complex pathophysiology of SCD that includes hemolysis, chronic inflammation, elevated cell adhesion, leukocytosis, increased oxidative stress, and endothelial activation/dysfunction, which can culminate in the acute vaso-occlusive processes that are responsible for much of the morbidity observed in patients.1,2
Vaso-occlusion comprises multistep and multicellular processes that appear to be initiated by the adhesion of red cells and leukocytes to activated endothelium via a mechanism in which inflammation, hypoxic events, oxidative stress, and reduced nitric oxide availability probably play roles.3–7 Data from in vivo studies using SCD mice5,8,9 and in vitro studies10 indicate that the recruitment of large, less deformable leukocytes to the vessel wall, and their subsequent interactions with circulating RBCs, may initiate vaso-occlusion. As such, drugs that inhibit the adhesion of leukocytes to vascular endothelium may represent an important approach for decreasing, or even preventing, vaso-occlusion.11
Research over recent years indicates that reduced nitric oxide (NO) bioavailability may contribute to manifestations of SCD, such as pulmonary hypertension and cutaneous leg ulceration.12,13 Whether reduced NO signaling has a direct role in the vaso-occlusive process is currently unknown; however, several studies indicate that nitric oxide-based therapies may be beneficial for increasing regional blood flow,14 reducing pain,15 and treating stroke16 in SCD. Furthermore, studies demonstrate that elevation of NO, or supplementation of its substrate, arginine, can reduce SCD neutrophil adhesive properties in vitro, and can improve microvascular functions,17 increase survival, and prevent lung injury during hypoxia in SCD mice.18,19
Hydroxyurea (HU), a drug approved by the United States Food and Drug Administration for use in adults with SCD, is currently the only drug proven to modify the disease process by improving hematologic parameters and hospitalization.20,21 HU is thought to act principally by increasing fetal hemoglobin (HbF) production in erythrocytes, thereby inhibiting HbS polymerization (see scheme, Figure 1). Although HU is known to inhibit DNA synthesis via inactivation of ribonucleotide reductase, it is also suggested to act as a donor of NO in vitro.22,23 HU may also induce HBG (encoding γ-globin) expression in erythroid progenitor cells in vitro via a cyclic guanosine monophosphate (cGMP)–dependent pathway.24 Although numerous studies indicate that HU might have benefits in SCD that could be independent of its HbF-inducing properties, including reductions in leukocyte counts and increased erythrocyte cation transport,2,21 to date no immediate short-term benefits have, to our knowledge, been reported after its administration in SCD patients or in mouse models.
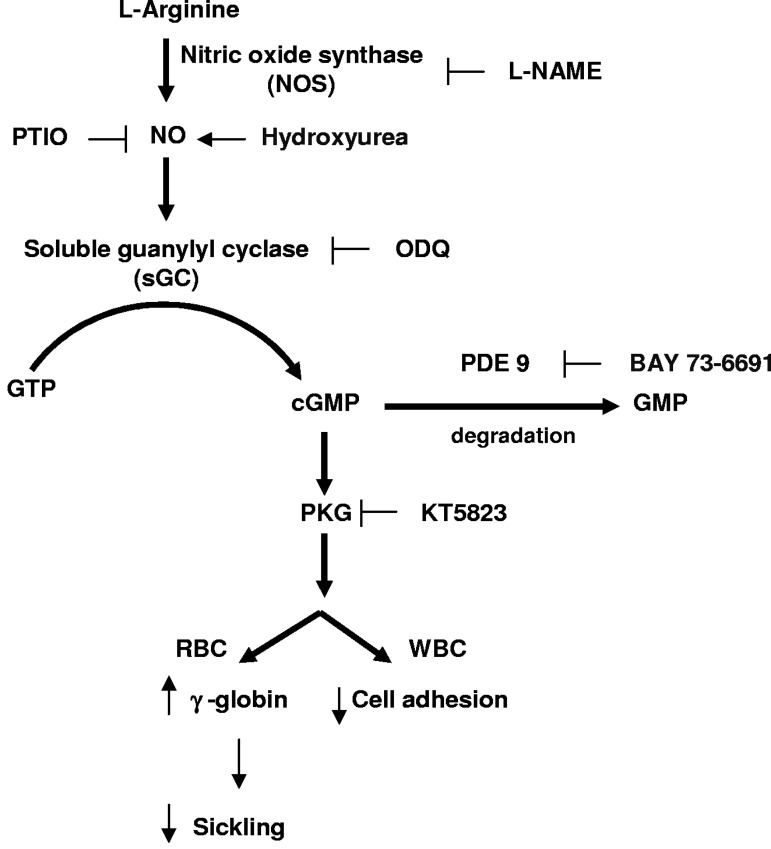
The NO-cGMP pathway. HU acts as a NO donor in vivo and/or directly activates intracellular sGC. NO stimulates intracellular sGC to produce cGMP from guanosine-5′-triphosphate. Stimulation of cGMP-dependent protein kinase (PKG) by cGMP in erythroid lineage cells elevates HBG and fetal hemoglobin production and decreases leukocyte (WBC) adhesive mechanisms. PDE9 degrades intracellular cGMP to GMP and is highly expressed in hematopoietic cells. BAY73-6691 is a selective inhibitor of PDE9, and therefore elevates intracellular cGMP in cells that express the enzyme. PTIO is a radical scavenger for nitric oxide. L-NAME is an arginine analog that inhibits nitric oxide synthase activity. ODQ (1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one) is a selective inhibitor of sGC, whereas KT5823 selectively inhibits PKG.
Modulation of intracellular levels of the NO second messenger, cGMP, may represent an effective and cell-specific approach for amplifying intracellular NO-dependent signaling.25 In addition to the induction of HBG production in erythroid lineage cells,26 activation of this pathway also reduces the adhesive properties of leukocytes, in vitro.27 Recent data demonstrate that the cGMP-degrading enzyme, phosphodiesterase 9 (PDE9), is highly expressed in hematopoietic cells, possibly providing a more tissue-specific drug target (see scheme, Figure 1).28 The specific PDE9 inhibitor, BAY73-6691, has been reported to increase HBG γ-globin expression in K562 erythroleukemic cells, and also to reduce SCD neutrophil adhesive properties in vitro.28,29
The aim of this study was to investigate the effects of the acute administration of HU alone, and in combination with the PDE9-inhibiting agent, BAY73-6691, in an in vivo model of inflammation-induced vaso-occlusion in SCD mice, and to further determine whether these effects are mediated through a cGMP-dependent pathway.
Methods
Animals
C57BL/6 mice were commercially obtained from the NCI Breeding Laboratories. Chimeric male SCD mice were generated by transplantation of nucleated bone marrow cells harvested from Berkeley SCD mice (Tg[Hu-miniLCRα1GγAγδβS] Hba−/−Hbb−/−) into lethally irradiated C57BL/6 mice, as previously described.5,11 Only chimeric SCD mice expressing > 97% human globin were used in intravital microscopy experiments at 3 to 5 months after transplantation; these mice are hereafter referred to as SCD mice. Nontransplanted, native SCD mice (aged 3-6 months; hereafter referred to as native SCD mice) were used in selected experiments.30 Animals were fed on a 20% protein diet (Rodent Diet 20 [5053]; Picolab) without additional arginine supplementation. All experimental procedures were approved by the Animal Care and Use Committees of the Mount Sinai School of Medicine, and the Albert Einstein College of Medicine, New York.
Intravital microscopy
Mice were anesthetized with a mixture of 2% α-chloralose and 10% urethane in phosphate-buffered saline (PBS; 6 mL/kg) and a polyethylene tube was inserted into the trachea to facilitate spontaneous respiration. The cremaster muscle was surgically exteriorized and continuously superfused with bicarbonate-buffered saline (37°C, pH 7.4), equilibrated with a mixture of 95% N2 and 5% CO2. One hundred and eighty minutes before surgery, inflammation was induced in mice by the injection of tumor necrosis factor (TNF)–α (0.5 μg intraperitoneally) and mice were concomitantly treated with hydroxyurea (100 mg/kg intravenously), BAY73-6691 (3 mg/kg intravenously), a combination of the 2 drugs or vehicle (2% dimethylsulfoxide in PBS). Drugs were administered (either individually or in combination) in a single injection of 150 μL. The doses of HU and BAY73-6691 used were chosen based on dose-response curves (50-250 mg/kg HU and 1-6 mg/kg BAY73-6691) conducted in nontransplanted C57BL/6 mice (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In some experiments, hydroxyurea was also administered orally (250 mg/kg orally; dose also chosen based on dose-response curve, data not shown), and inflammation was induced with TNF-α. Some protocols used nontransplanted C57BL/6 wild-type (WT) mice, where mice were pretreated (60 minutes before TNF-α) with Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME; 300 mg/kg, intravenously), an arginine analog that inhibits NO production by inhibiting nitric oxide synthase activity (NOS), or (30 minutes before TNF-α) with PTIO (2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; 1 mg/kg, intravenously), a scavenger of NO that does not affect NOS; or the guanylate cyclase inhibitor, 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ; 15 mg/kg, intraperitoneally), or the protein kinase G (PKG) inhibitor, KT5823 (1 mg/kg, intraperitoneally; Figure 1). For all protocols, microvessels (8-15 for each mouse) were visualized immediately after surgery using a custom-designed intravital microscope (Nikon-Morrell; 60× magnification) and videotaped continuously for 1 to 2 minutes. Images were recorded using a charge-coupled video camera device (Hamamatsu) and a Sony SVHS SVO-9500 video recorder. Leukocyte (WBC) rolling, adhesion, and extravasation were monitored and analyzed for 30 minutes after surgery. The definitions of rolling, adhesion, extravasation and interactions between RBCs and WBCs, which were analyzed by videotape playback, were previously described.5,11 All SCD mice were monitored until death or up to 660 minutes, at which time they were killed. Blood cell counts were determined using a Beckman Coulter automatic cell counter (AcT). All reagents and drugs were obtained from Sigma-Aldrich.
Fluorescence intravital microscopy and Mac-1 activity assay
Mice were prepared for intravital microscopy, as described in the previous section. Inflammation was induced with TNF-α (0.5 μg intraperitoneally) and mice were treated intravenously with a combination of BAY73-6691 and HU or vehicle. Five minutes before analysis, mice were injected intravenously with 109 bovine serum albumin–coated yellow-green fluorescent microspheres (FluoSpheres-Invitrogen), as previously described,9 and binding of spheres to adherent leukocytes was quantified using Slidebook Version 5.0 software (Intelligent Imaging Innovations) on a Zeiss Examiner D1 microscope (20× magnification) equipped for epifluorescence imaging. Images were recorded using a Hamamatsu coolsnap HQ2 camera.
Quantification of endothelial cell adhesion molecule expression by confocal microscopy
Cremaster muscle tissues were harvested at the end of intravital microscopy experiments, embedded in OCT (optimal cutting temperature; Sakura), snap frozen in liquid nitrogen and cryosectioned on a Leica cryostat. Sections were fixed with methanol, incubated with fluorescently coupled antibodies and imaged on a Zeiss Examiner D1 microscope equipped with a CSU-1X spinning disk confocal scanhead (Yokogawa) and 4-stack laser system (405 nm, 488 nm, 561 nm, and 642 nm wavelengths). Images were obtained as 3-dimensional stacks spanning the whole thickness of the tissue. For investigations of expression levels, P/E-selectin and ICAM-1 antibodies were conjugated with phycoerythrin (PE) and all quantifications were performed using mask analysis (Slidebook Version 5.0 software; Intelligent Imaging Innovations) based on PECAM-1 (antibody conjugated with allophycocyanin - APC) expression and quantifying expression of other fluorescent channels within the mask containing PECAM+ pixels, delineating vascular structures.
Quantification of plasma cGMP
Blood was collected from mice at the time of death. Plasma was separated from the blood by centrifuging at 2500g for 10 minutes. Samples were then stored at −80°C. Plasma cGMP was quantified using commercially available enzyme-linked immunosorbent assay (ELISA) kits (GE Healthcare).
Statistical analyses
Values are expressed as mean ± SEM. Parametric comparisons were performed using analysis of variance with Newman-Keuls posttest or t test when only 2 variants were available. RBC-WBC interactions and microsphere binding assays were analyzed using nonparametric comparisons (Dunn posttest or Mann-Whitney test). Statistical significance for the Kaplan-Meier survival curve was assessed using a log-rank (Mantel-Cox) test. Differences among groups were considered significant at P ≤ .05.
Results
Rapid cGMP modulation with hydroxyurea and BAY73-6691 inhibits leukocyte recruitment in TNF-α–inflamed SCD mice
Intraperitoneal administration of TNF-α in mice induces a well-described inflammation in the cremasteric microcirculation,5,9,31 associated with increased leukocyte adhesion to the vessel wall. In SCD mice, ensuing heterotypic interactions between RBCs and adhered WBCs may reduce blood flow and result in previously described vaso-occlusive type phenomena in the microvasculature.5,8 Modulation of cGMP-dependent intercellular signaling may represent a novel therapeutic approach for reducing leukocyte-endothelial interactions.27 As HU has been suggested to increase cGMP signaling24 and has clear beneficial effects in SCD,1,2 we treated a group of SCD mice with a single dose of HU (intravenously), concomitantly with TNF-α, and quantified leukocyte responses by intravital microscopy at 3 hours after treatment. We found that, although treatment of SCD mice with HU alone did not alter leukocyte rolling (Figure 2A), unexpectedly, significant reductions in leukocyte adhesion and extravasation (~ 40% inhibition) were observed (Figure 2C-D,F). To determine whether HU exerted similar effects when administered orally, HU was also given orally (250 mg/kg) to TNF-α–inflamed SCD mice; similar alterations in leukocyte adhesion and extravasation were observed at 3 hours after TNF-α, and notably, leukocyte rolling and WBC-RBC interactions ameliorated significantly (Figure 3A-D).
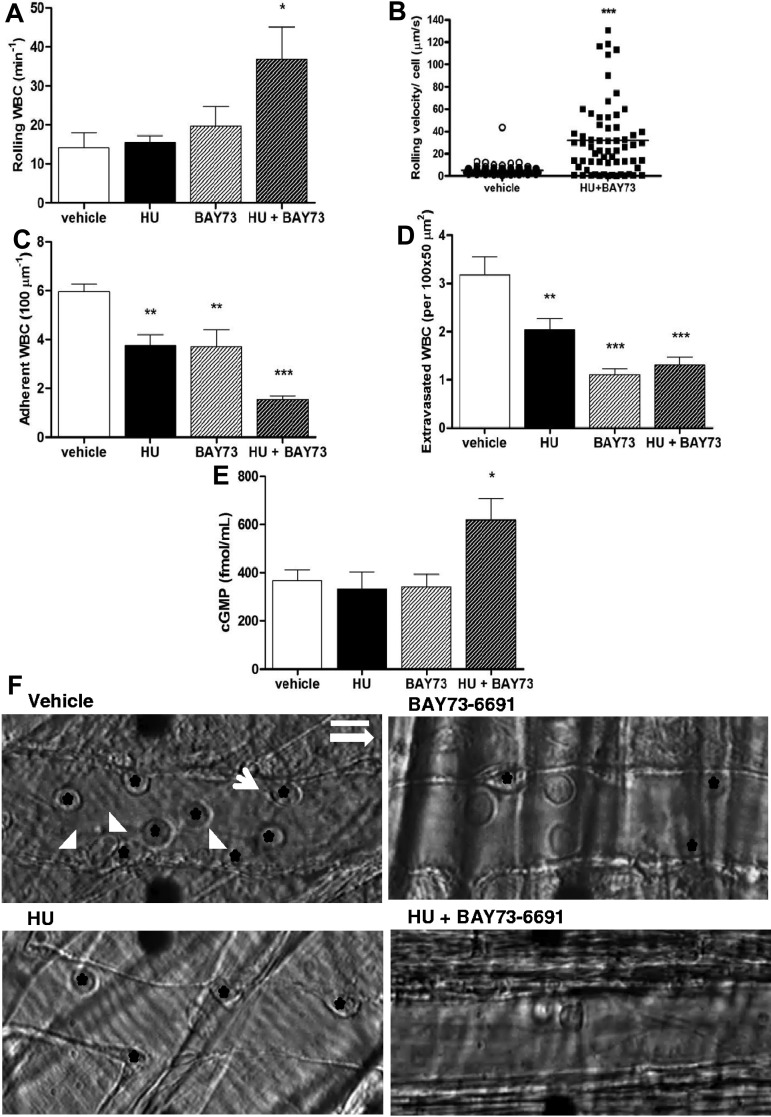
Effects of coadministration of HU and BAY73-6691 on leukocyte recruitment in TNF-α–treated SCD mice. (A) Leukocyte rolling; (B) leukocyte rolling velocity (velocity of each leukocyte observed; n = 64-82 venules from 3-4 mice per group); (C) leukocyte adhesion, and (D) leukocyte extravasation (n = 5-7 mice per group for A-C-D). (E) Plasma cGMP levels at the time of death/sacrifice as determined by ELISA (n = 5-6 mice per group; *P < .05, **P < .01, ***P < .001). (F) Representative images of SCD mice venules at 180 minutes after TNF-α stimulation. Black stars indicate adherent leukocytes; white arrowheads, RBCs; and white arrow, RBC-WBC interaction. The longer white arrow indicates the direction of blood flow. Scale bar: 10 μm.
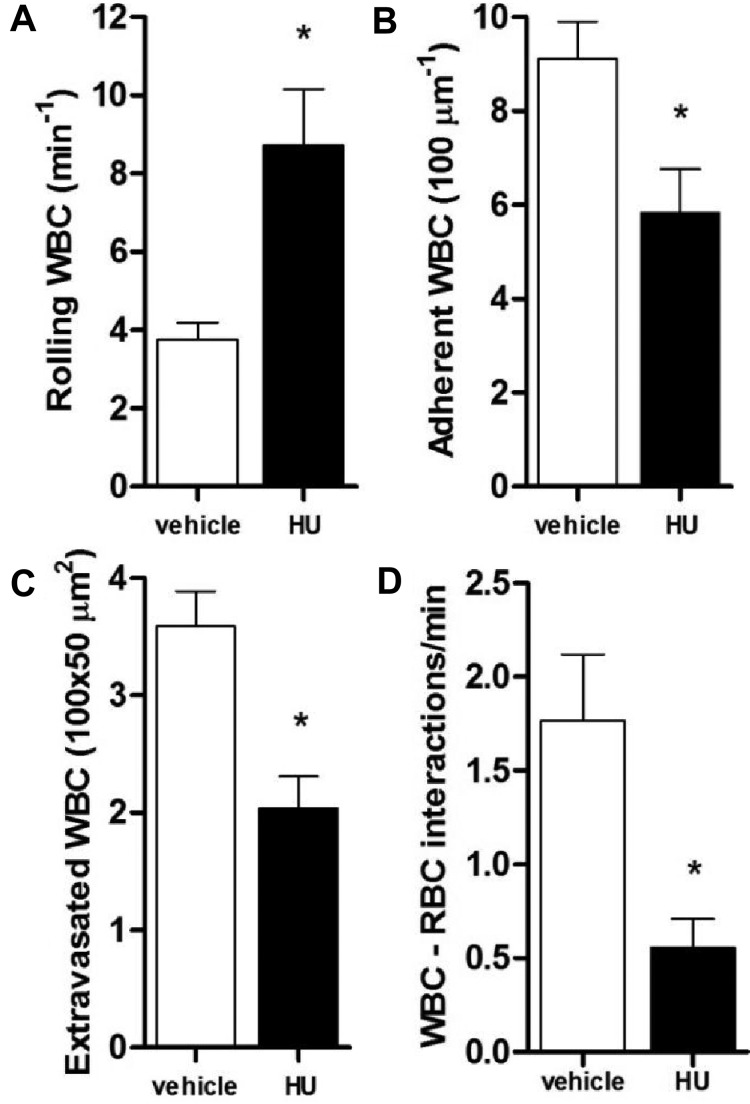
Effects of oral administration of hydroxyurea on leukocyte recruitment in TNF-α–treated SCD mice. HU (250 mg/kg) was given orally to SCD mice at the time of TNF-α administration. (A) Leukocyte rolling; (B) adhesion; (C) extravasation; and (D) quantification of RBC-WBC interactions were determined at 180 minutes after TNF-α (*P < .05 compared with vehicle; n = 3-5 mice per group).
To evaluate the effects of a direct modulator of intracellular cGMP on leukocyte recruitment, we administered (intravenously) BAY73-6691, a specific inhibitor of the cGMP-degrading enzyme PDE9 (see scheme, Figure 1), to TNF-α–stimulated SCD mice. BAY73-6691 administration caused significant reductions in both leukocyte adhesion (~ 40% inhibition) and extravasation (~ 70% inhibition; Figure 2C-D,F). Interestingly, coadministration of both HU and BAY73-6691 led to an even more pronounced anti-inflammatory effect than with either drug alone, increasing the number of rolling leukocytes, as well as their rolling velocities, and further reducing leukocyte adhesion and extravasation (Figure 2A-D,F). As bone marrow transplantation involves minor MHC (major histocompatibility complex) mismatch, which could potentially affect the endothelial cell phenotype and interactions with leukocytes, we confirmed the immediate effects of HU and BAY73-6691 administration in native SCD mice that had not been submitted to transplantation. Figure 4A through C confirms that coadministration of these 2 drugs to TNF-α–stimulated native SCD mice inhibits leukocyte adhesion and extravasation in a manner similar to that observed in chimeric SCD animals; the number of rolling leukocytes was also increased, but not significantly.
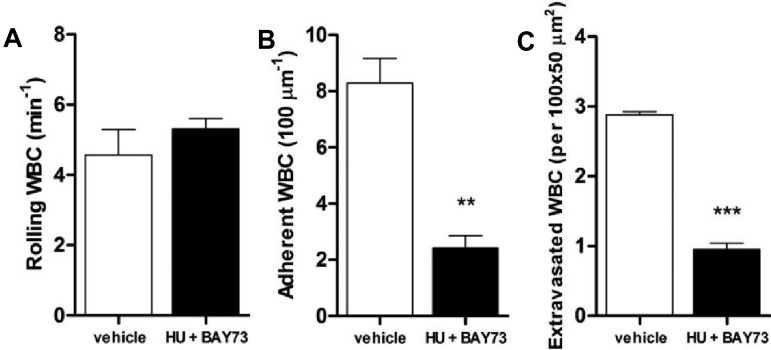
Effects of coadministration of HU and BAY73-6691 on leukocyte recruitment in TNF-α–treated native SCD mice. HU (100 mg/Kg, intravenously) and BAY73-6691 (3 mg/Kg, intravenously) were administered to native SCD mice at the time of TNF-α administration. (A) leukocyte rolling; (B) adhesion; and (C) extravasation were determined at 180 minutes after TNF-α (**P < .01, ***P < .001 compared with vehicle control, n = 3 mice per group).
Consistent with the idea that HU and BAY73-6691 alter cGMP signaling, plasma cGMP levels were significantly increased in SCD animals that received the combined treatment (Figure 2E). No significant differences in hemodynamic parameters were detected in either C57BL/6 WT or SCD inflammatory mice after HU and/or BAY73-6691, suggesting that these drugs did not induce vasodilation at the time of intravital microscopy (supplemental Tables 1-3). A considerable increase in the circulating WBC count was observed in those SCD mice that received HU and BAY73-6691 (supplemental Table 2), possibly reflecting the substantial decrease in leukocyte recruitment to the vessel wall and tissues. In addition, significant alterations were not found in leukocyte recruitment when HU and BAY73-6691 were administered to mice at 180 minutes after TNF-α stimulation, at a time when vaso-occlusive events were fully installed (data not shown). Our results suggest that acute modulation of cGMP-dependent signaling by HU and/or a PDE inhibitor, at the time of TNF-α inflammatory induction, alters leukocyte recruitment in vivo in SCD mice.
HU and BAY73-6691 reduce TNF-α–induced endothelial activation and heterotypic interactions in SCD mice
To examine the effect of drug treatment on the vascular endothelium, we quantified expression levels of the major endothelial adhesion molecules mediating leukocyte recruitment in the cremaster muscle (ie, ICAM-1, P-selectin, and E-selectin) using immunofluorescence confocal microscopy of cremaster muscle tissue sections. Although TNF-α administration was found to significantly increase the expression of these adhesion receptors in skeletal muscle, coadministration of HU and BAY73-6691 together with TNF-α abrogated this up-regulation in both chimeric SCD mice (Figure 5A-C) and native SCD mice (data not shown), suggesting that these agents can prevent endothelial cell activation.
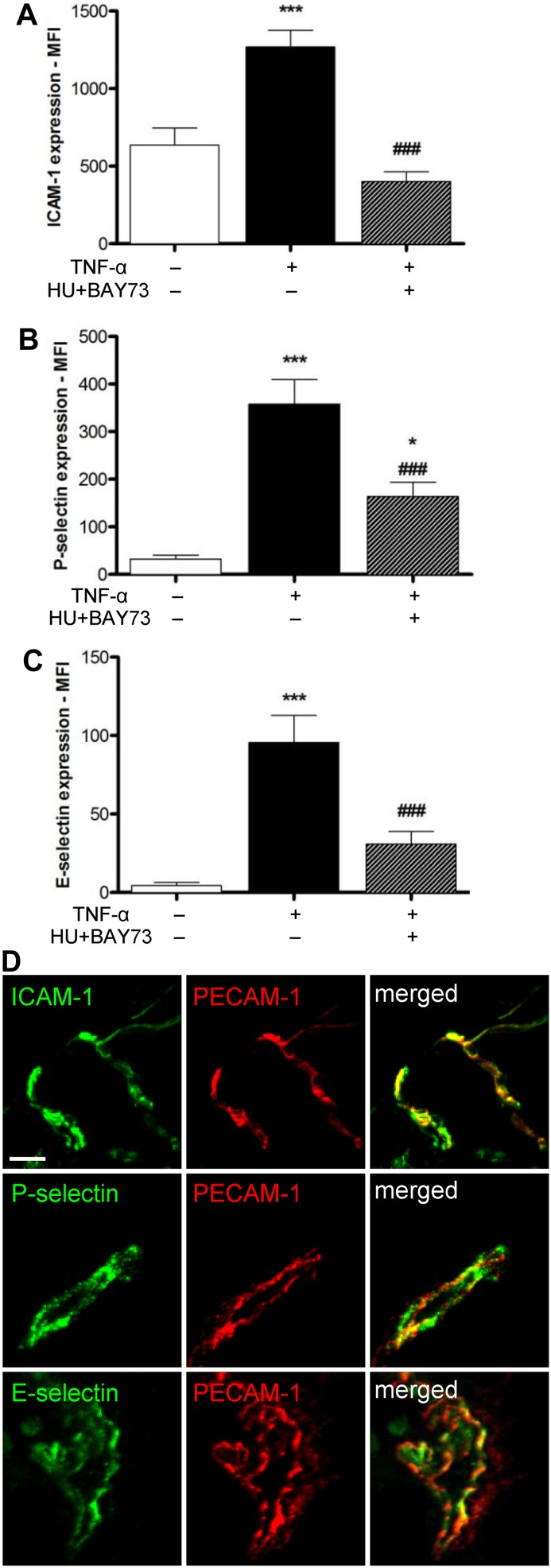
Expression of endothelial surface adhesion molecules in exteriorized cremaster muscle tissues of TNF-α–treated SCD mice. Endothelial cell expression of (A) ICAM-1, (B) P-selectin, and (C) E-selectin in cremaster muscle tissues isolated from SCD mice in steady state or after TNF-α administration in the presence or absence of HU and BAY 73-6691 or drug vehicle, n = 37-69 venules PECAM-1+ vascular areas quantified from 4-6 mice per group (*P < .05, ***P < .0001 compared with SCD mice in steady state; ###P < .0001 compared with TNF-α–stimulated SCD mice without HU and BAY-73-6691). (D) Representative images of ICAM-1, P-selectin, and E-selectin expression in cremaster muscle isolated from TNF-α–stimulated SCD mice at the end of the in vivo observation period. Scale bar: 20 μm.
We next investigated whether the 2 drugs could prevent the activation of leukocytes. Consistent with this idea, we found that oral HU and BAY73-6691 by itself or in combination with intravenous HU significantly decreased heterotypic WBC-RBC interactions (Figures 3D and and6A).6A). In addition, coadministration of HU and BAY73-6691 significantly decreased the activity of the leukocyte integrin Mac-1 (αMβ2, CD11b/CD18), as measured by the reduced ability of adherent leukocytes of treated SCD mice to capture in vivo fluorospheres coated with denatured albumin (Figure 6B-D).9 Taken together, these data demonstrate the extensive anti-inflammatory properties of HU and BAY73-6691.
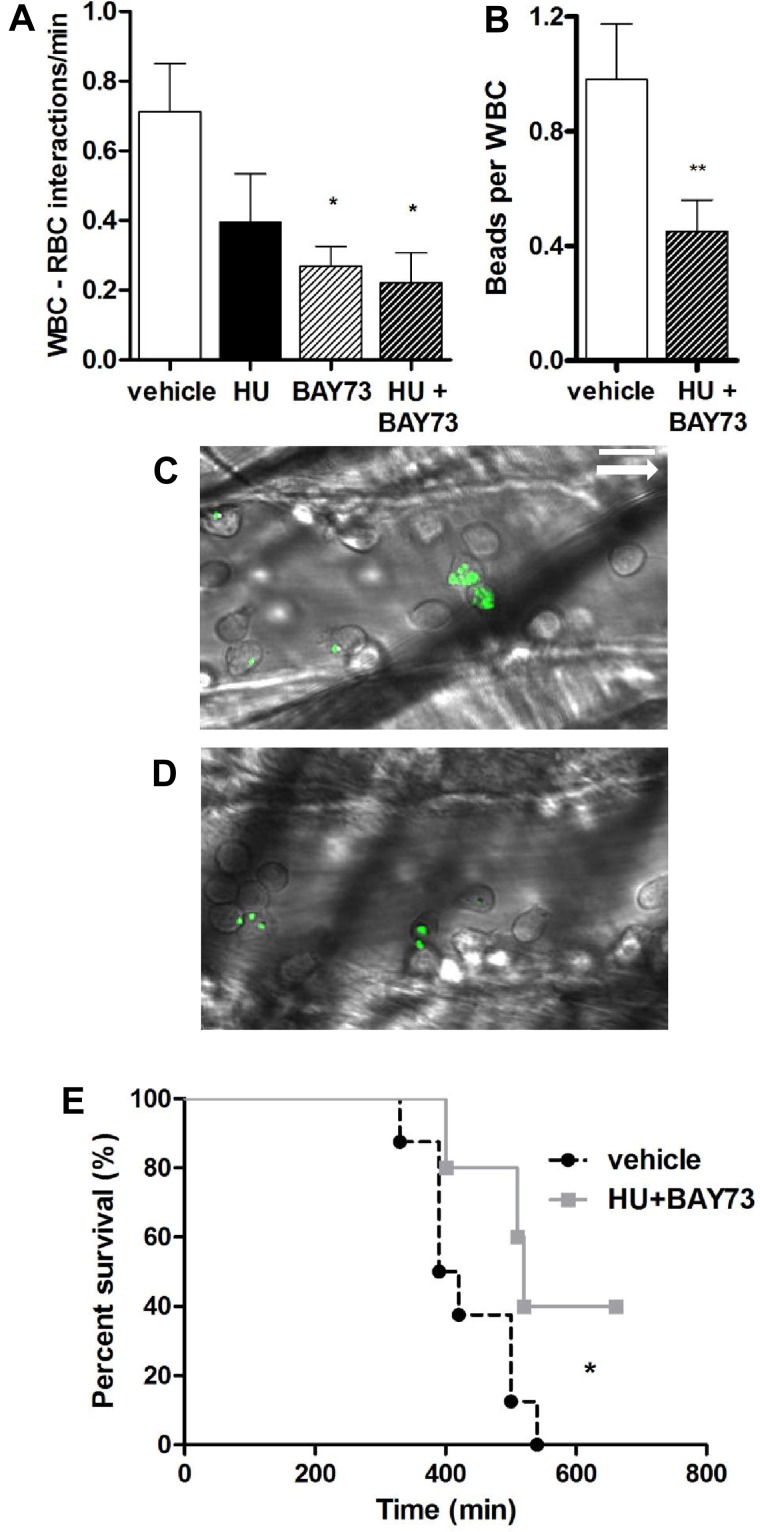
Effect of HU and BAY73-6691 treatment on leukocyte activation and survival of TNF-α–treated SCD mice. (A) Quantification of RBC-WBC interactions, n = 5-7 mice per group (*P < .05 compared with vehicle). (B) Mac-1 integrin activity on adherent leukocytes as assessed by capture of fluorescent microspheres, n = 3 to 5 mice per group (**P < .01 compared with vehicle, Mann-Whitney test). Representative images of fluorescent spheres bound to adherent leukocytes after treatment with vehicle (C) or HU and BAY-73-6691 (D). The white arrow indicates the direction of blood flow. (E) Kaplan-Meier survival curve after treatment with HU and BAY73-6691 or vehicle. Log-rank (Mantel-Cox) test (*P < .05 compared with vehicle, n = 5-7 mice per group). Scale bar: 10 μm.
HU and BAY73-6691 prolong survival in TNF-α–inflamed SCD mice
TNF-α–induced inflammation in SCD mice is known to substantially increase mortality in these animals.11 Accordingly, SCD mice presented elevated mortality after TNF-α administration, with an average survival time of just 423 minutes (Figure 6E). In contrast, coadministration of HU and BAY73-6691 significantly improved the survival of SCD mice (550 minutes; Figure 6E). No significant effect of either drug on survival was observed when given (intravenously) alone in this model (data not shown).
HU exerts its beneficial effects via NO production and subsequent stimulation of the cGMP-signaling pathway
To further elucidate the mechanisms of HU-induced signaling in leukocyte recruitment, we investigated the effects of the drug in TNF-α–treated C57BL6 WT mice. In a manner similar to observations in SCD animals, HU exhibited no significant effect on leukocyte rolling, but reduced leukocyte adhesion and extravasation (Figure 7A-C). To determine whether the effects of HU were caused by NO release or by the activation of NOS, HU was administered together with either L-NAME (a NOS enzyme inhibitor; see scheme Figure 1) or PTIO (a NO scavenger that does not affect NOS activity). Although treatment with L-NAME did not have significant effects, the administration of PTIO reversed the beneficial properties of HU with respect to leukocyte adhesion and extravasation (Figure 7A-C). These results suggest that, in this inflammatory scenario, HU acts as a NO donor rather than via stimulation of NOS activity. Downstream signaling of HU, after NO production, probably occurred via a cGMP-dependent pathway because administration of the soluble guanylate cyclase (sGC) inhibitor, ODQ (see scheme Figure 1), was able to abrogate the effects of HU (Figure 7A-C).
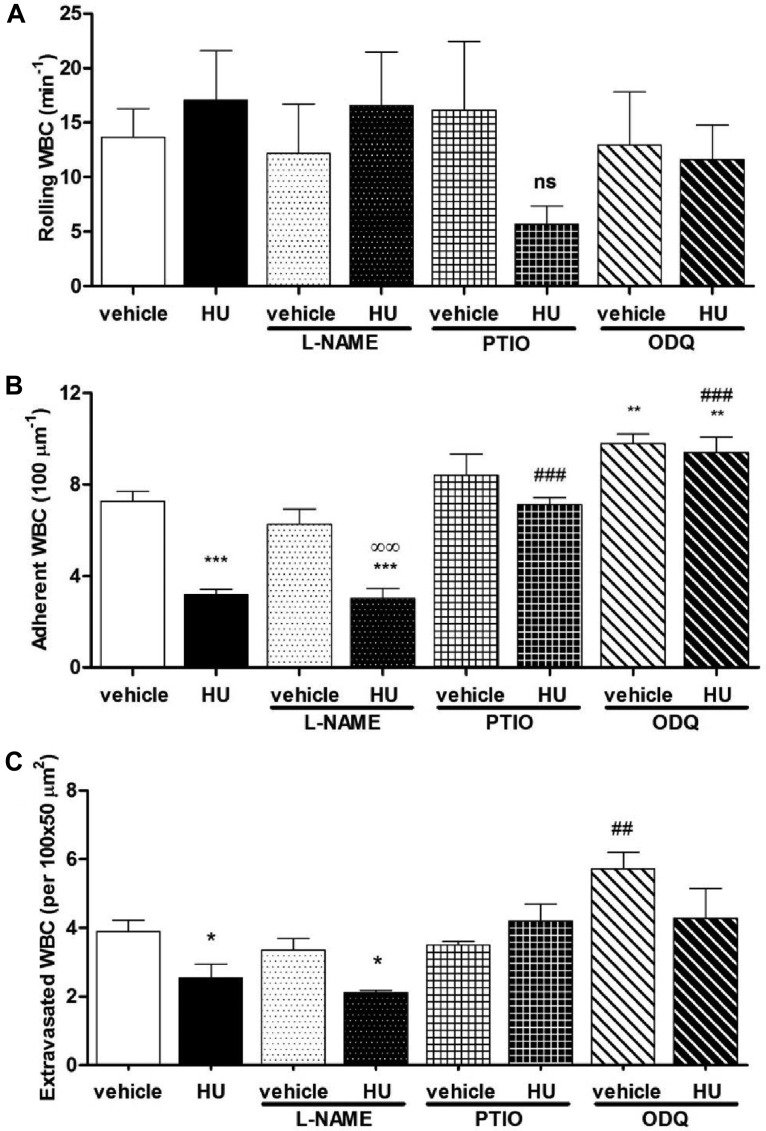
HU reduces leukocyte adhesion and extravasation via NO production and a cGMP-dependent pathway. (A) Leukocyte rolling, (B) adhesion and (C) extravasation in TNF-α–treated C57BL6 WT mice after administration of vehicle or HU in the presence or absence of L-NAME, PTIO, or ODQ, n = 4-10 mice per group (*P < .05, **P < .01, ***P < .001, compared with vehicle alone; ##P < .01, ###P < .001 compared with HU alone; ∞∞P < .01, compared with vehicle + L-NAME).
BAY73-6691 reduces leukocyte recruitment via a cGMP-dependent pathway
To assess whether BAY73-6691 acted via cGMP-dependent signaling mechanisms, as seen for HU, we evaluated the effect of BAY73-6691 in TNF-α–stimulated C57BL/6 WT mice. We found that BAY73-6691 administration significantly reduced leukocyte adhesion and extravasation, but did not alter leukocyte rolling (Figure 8A-C), corroborating our results obtained in SCD mice (Figure 2). To confirm the specificity of BAY73-6691 as a modulator of cGMP-dependent signaling, we coadministered KT5823 (cGMP-dependent protein kinase [PKG] inhibitor, see scheme Figure 1). KT5823 prevented the beneficial effects of BAY73-6691 on leukocyte adhesion and extravasation (Figure 8A-C), indicating that BAY73-6691 indeed mediates its actions via a sGC/PKG-dependent pathway and suggesting that BAY73-6691 may act in synergy with HU by amplifying the cGMP signaling cascade, and thereby reducing leukocyte recruitment and inflammation.
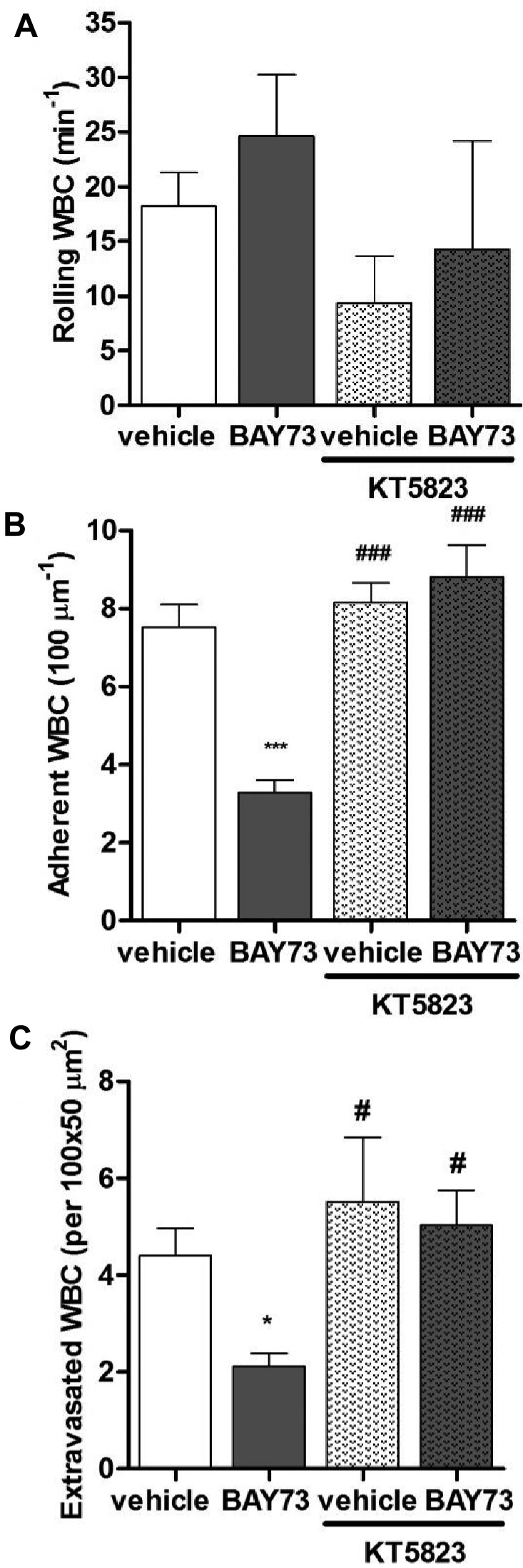
BAY73-6691 reduces leukocyte adhesion and extravasation via a cGMP-dependent pathway. (A) Leukocyte rolling, (B) adhesion, and (C) extravasation in TNF-α–treated C57BL6 WT mice after administration of vehicle or BAY73-6691, in the presence or absence of KT5823 (n = 4-10 mice per group; *P < .05, ***P < .001, compared with vehicle alone; #P < .05, ###P < .001, compared with BAY73-6691 alone).
Discussion
Acute vaso-occlusive crises account for much of the morbidity observed in SCD patients,1,2 and better therapeutic approaches to prevent or reduce these events are needed. Experiments in mouse models of SCD indicate that vascular obstruction can be triggered by hypoxia, trauma, and/or inflammatory events.5,9,32,33 In the humanized mouse model of SCD, the administration of TNF-α initiates the recruitment of leukocytes to the microvascular endothelium, followed by the capture of sickle RBCs to adherent leukocytes, leading to a reduction in blood flow, and frequently, to the death of the animal.9 Mouse models of SCD, including the Berkeley mouse and chimeric SCD mice, although presenting low levels of baseline events, and potentially graft-versus-host disease, respectively, currently represent an important means for the experimental investigation of the mechanisms of SCD and potential approaches to attenuate its manifestations.
HU, used in a chronic regimen, can improve SCD pathologies by increasing HbF production, in addition to other effects that include the reduction of leukocyte and platelet counts and some inflammatory parameters.2,20,34 HU's effects are generally believed to result mainly from its HbF-elevating capacity, as well as its marrow cytotoxicity, which decreases neutrophil and reticulocyte counts.22 In a recent study, transgenic Berkeley SCD mice (which do not express HbF during adulthood) were treated for 20 weeks with a maximally tolerated dose of HU. Although significant reductions in the neutrophil and platelet counts were observed, no improvements in hemolytic anemia and end-organ damage were found in treated mice.35 In contrast, mice expressing high levels of HbF, because of gene transfer, showed improvements in anemia and organ damage, suggesting that the inhibition of RBC sickling by HbF may be a major factor in long-term HU therapy. However, HU is also reported to exhibit NO donor properties,23 and may induce NOS activity and NO production in endothelial cells.36 Thus, given the contribution of reduced NO bioavailability to SCD pathophysiology,37 we hypothesized that HU may exert immediate beneficial effects that are not mediated by HbF elevation or other long-term effects. Herein, we present data indicating, for the first time in an in vivo setting, that HU may have acute and immediate benefits. Furthermore, we show that these effects can be further amplified by the cGMP-elevating agent, BAY73-6691.
Using a model of TNF-α–induced acute vaso-occlusion in SCD mice, our data indicate that the administration of either HU or BAY73-6691 is able to reduce leukocyte adhesion to the microvascular endothelium. Notably, coadministration of both drugs had a synergetic effect and resulted in significant reductions in leukocyte adhesion and RBC/WBC interactions in SCD mice; events that were also observed in similarly treated native SCD mice. The beneficial effects of these drugs on vaso-occlusive–like events were reduced when administered after installation of full experimental vaso-occlusion in the SCD mouse model; as such, further studies are necessary to establish drug doses required for the reversal of vaso-occlusion, once established.
Improvements in leukocyte/endothelial cell and RBC/WBC interactions in TNF-α–stimulated SCD mice, after the coadministration of HU and BAY73-6691 were accompanied by a very significant increase in circulating WBCs, possibly reflecting the attenuation of leukocyte margination and extravasation at the vessel wall. These changes were associated with abrogated endothelial adhesion molecule expression and leukocyte Mac-1 integrin activity, which together probably account for the significant increase in animal survival. In nonsickle C57BL/6 mice, our data indicate that HU-mediated alterations in leukocyte behavior, after the induction of inflammation, are mediated via activation of soluble guanylate cyclase (as shown by the reversal of the effects of HU by ODQ). Similarly, in the same model, the effects of BAY73-6691 were prevented by the inhibition of PKG. Thus, our data indicate that, under these experimental conditions, both drugs exert their effects via a central cGMP-dependent pathway. HU reportedly stimulates sGC in vitro by direct nitrosylation of the enzyme, by NO donation as well as by stimulation of nitric oxide synthase.36,38,39 When we administered HU together with a NO scavenger, PTIO, its beneficial effect on TNF-α–stimulated mice was abolished; in contrast, coadministration of a NOS inhibitor (L-NAME) did not affect HU-mediated effects. These data indicate that, in this acute inflammatory setting, HU generates intravascular NO, providing further support to the hypothesis that HU can act as a NO donor in vivo.
Importantly, HU, when given orally, was able to reproduce the effect of intravenously administered HU in TNF-α–stimulated SCD mice and even demonstrated greater efficiency in increasing leukocyte rolling and reducing WBC-RBC interactions, possibly because of the slightly higher concentration used in these experiments. Thus, although the administration of HU by the intravenous route is possible, it does not appear to present any clear advantages over oral administration with regard to bioavailability40 and capacity to alter leukocyte recruitment in vivo. Careful clinical studies to evaluate a potential novel use for oral HU for the treatment of acute vaso-occlusive episodes, alone or in combination with other drugs that amplify its effects, may be called for. BAY73-6691 inhibits PDE9, an enzyme that degrades cGMP. In SCD mice, some of the NO donated by HU is probably scavenged by cell-free hemoglobin, possibly explaining why its immediate NO-dependent effects require amplification by BAY73-6691 to achieve the full cGMP-elevating benefit in these mice. Accordingly, circulating cGMP levels were found to be significantly elevated in the circulation of SCD mice after the administration of the 2 drugs. The PDE9 enzyme constitutes an attractive therapeutic target, because of its relatively restricted tissue distribution profile, being highly expressed in the brain41 and hematopoietic cells,28 and with very low-level basal expression in other tissues.28 BAY73-6691, a potent inhibitor of PDE9,42 has demonstrated significant effects in vitro, inducing erythroid cell HBG gene production and decreasing leukocyte adhesive properties and adhesion molecule expression.28,29
In our SCD mouse model, BAY73-6691 amplified the cGMP-dependent effects of HU in the absence of any apparent vasodilatory effect (possibly because of the limited tissue expression of PDE9). TNF-α–stimulated SCD mice that received HU and BAY73-6691 presented a diminished expression of endothelial surface adhesion molecules in venules that may have contributed to reduced leukocyte recruitment to the vessel wall. In human endothelial cells, gene expression of PDE9 appears to be low, but is up-regulated after cell activation (C.B.A. and N.C., unpublished observations, November 2010), conceivably explaining the effect of BAY73-6691 on endothelial activation and function, when used in association with HU. Moreover, a recent study in SCD mice reported that treatment with HU for 4 weeks attenuated neutrophil recruitment to the lung, after challenge with pneumococci, mediated by a down-modulation of E-selectin expression.43 Our data further indicate that the prevention of endothelial inflammation and expression of adhesion molecules, such as E-selectin, may be one of the major, and immediate effects of HU therapy and that BAY-736691 may amplify this effect. The increase in leukocyte rolling velocities observed reflects the inhibition of E-selectin–mediated slow rolling.44 Studies in inflammatory mouse models have demonstrated the importance of the activation of secondary leukocyte integrin-signaling events, induced by interactions with endothelial E-selectin, for heterotypic cell interactions.9 Although the mechanism for the HU/BAY73-6691–mediated decrease in RBC-WBC interactions observed is not clear, results indicate that a decrease in the activity of the major leukocyte integrin, Mac-1, may play a role in this mechanism. Leukocyte integrin activity is mediated by changes both in the surface expression of the integrin, as well as by alterations in its affinity and avidity45; interestingly, the in vitro inhibition of PDE9 in human neutrophils from SCD individuals was found to decrease surface expression of Mac-1, which was associated with a decrease in their capacity to adhere to recombinant ICAM-1.29 Such a rapid down-regulation of Mac-1 activity could occur by shedding (as occurs with other adhesion molecules, including L-selectin),29 or by its association with uPAR, resulting in reduced Mac-1 ligand-binding activity.46
Inhibition of phosphodiesterase 5 (PDE5) has been proposed as a potential therapeutic option for the treatment of SCD patients with pulmonary hypertension. However, a recent clinical trial to evaluate the use of sildenafil in such patients was terminated prematurely after a reportedly increased incidence of hospitalization for pain in SCD patients treated with sildenafil for elevated tricuspid regurgitant velocity (TRV) and low exercise capacity47; the authors of this study suggested a role for NO-cGMP signaling in this effect. It is notable that the tissue distributions of PDE5 and PDE9 are very different; PDE5 is highly expressed in vascular smooth muscle, platelets, brain, lung, heart, kidney, and skeletal muscle, but has a very low expression in hematopoietic cells, whereas PDE9 is mostly expressed in the brain and hematopoietic cells.25,28 This suggests that the potential side effects of drugs inhibiting either enzymes, are probably very different. PDE9 inhibitors are currently undergoing clinical trials (eg, NCT00930059 and NCT00736528) for potential use in the treatment of Alzheimer disease, because they have been shown to improve cognition and memory48,49; although no data are currently available regarding the side effects of these drugs.
Our data suggest that drugs that stimulate the NO/cGMP pathway may be capable of diminishing vaso-occlusive processes, at least in SCD mice, when administered acutely with a significant outcome on animal survival. Notably, our findings indicate that HU could have important, immediate, and direct beneficial effects on the microvasculature that are mediated by cGMP-dependent mechanisms. HU is administered in a chronic therapeutic regimen for SCD, with proven results that are often attributed solely to its HbF-elevating properties. Previous studies have shown that nitrosylation of hemoglobin can occur within 1 to 2 hours after the administration of a single oral dose of HU in individuals with SCD,50 indicating that the production of NO from HU in humans is also extremely rapid and that the acute and immediate beneficial effects of HU, observed herein, may also occur in humans. Based on these promising preclinical results, the use of oral HU as an option for the treatment of acute vaso-occlusive crisis should be considered for phase 2/3 clinical trials. Because PDE9 is highly expressed in hematopoietic cells, this enzyme represents a potential tissue-specific target to enhance cGMP activity and intensify the NO-mediated acute beneficial effects of HU.
Acknowledgments
C.B.A. is supported by a fellowship from FAPESP, Brazil (Proc 07/58533-3 and Proc 11/50959-7). C.S. received fellowship support from the German Academic Exchange Service (DAAD). This work was also supported by the National Institutes of Health (R01HL69438, R01HL097700, and RC1HL099545 to P.S.F.)
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.B.A. conceived the study, designed and performed experiments, analyzed and interpreted data, and wrote the paper; C.S. and J.-E.J. designed and performed experiments, analyzed and interpreted data, and revised the paper; C.P. generated chimeric sickle animals; F.F.C. interpreted data and revised the paper; N.C. conceived the study, designed experiments, interpreted data, and wrote the paper; and P.S.F. conceived the study, designed experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The use of a pharmaceutical composition of hydroxyurea and a PDE9 inhibitor for the control of vaso-occlusive processes is currently being registered for patent protection by the Brazilian Institute of Property–INPI (Instituto Nacional de Propriedade Industrial) with The University of Campinas and Albert Einstein College of Medicine named as applicants. The authors declare no other competing financial interests.
Correspondence: Paul S. Frenette, Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research, Albert Einstein College of Medicine, Bronx, NY 10461; e-mail: [email protected]; or Nicola Conran, Hemocentro, Rua Carlos Chagas 480, Cidade Universitária, UNICAMP, Campinas, São Paulo, 13083-970, Brazil; e-mail: rb.pmacinu@narnoc.
References
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2012-02-409524
Read article for free, from open access legal sources, via Unpaywall:
https://ashpublications.org/blood/article-pdf/120/14/2879/1357145/zh804012002879.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Renal and cardiac effects of the PDE9 inhibitor BAY 73-6691 in 5/6 nephrectomized rats.
Pflugers Arch, 476(5):755-767, 02 Feb 2024
Cited by: 0 articles | PMID: 38305876
Persistence of chronic inflammation after regular blood transfusion therapy in sickle cell anemia.
Blood Adv, 7(3):309-313, 01 Feb 2023
Cited by: 1 article | PMID: 35834752 | PMCID: PMC9898595
Impact of Abnormal Leukocyte Count in the Pathophysiology of Sickle Cell Anemia.
J Blood Med, 13:673-679, 16 Nov 2022
Cited by: 3 articles | PMID: 36415590 | PMCID: PMC9675987
Neutrophils as drivers of vascular injury in sickle cell disease.
Immunol Rev, 314(1):302-312, 17 Oct 2022
Cited by: 3 articles | PMID: 36251624 | PMCID: PMC10132504
Review Free full text in Europe PMC
Effects of Hydroxyurea on Skeletal Muscle Energetics and Function in a Mildly Anemic Mouse Model.
Front Physiol, 13:915640, 15 Jun 2022
Cited by: 0 articles | PMID: 35784862 | PMCID: PMC9240423
Go to all (61) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT00736528
- (1 citation) ClinicalTrials.gov - NCT00930059
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
HU for acute treatment of sickle VOC?
Blood, 120(14):2777-2778, 01 Oct 2012
Cited by: 3 articles | PMID: 23043024
Beneficial Effects of Soluble Guanylyl Cyclase Stimulation and Activation in Sickle Cell Disease Are Amplified by Hydroxyurea: In Vitro and In Vivo Studies.
J Pharmacol Exp Ther, 374(3):469-478, 06 Jul 2020
Cited by: 6 articles | PMID: 32631869 | PMCID: PMC7445859
High expression of the cGMP-specific phosphodiesterase, PDE9A, in sickle cell disease (SCD) and the effects of its inhibition in erythroid cells and SCD neutrophils.
Br J Haematol, 142(5):836-844, 17 Jun 2008
Cited by: 22 articles | PMID: 18564357
Leukocyte adhesion and the pathophysiology of sickle cell disease.
Curr Opin Hematol, 13(1):40-44, 01 Jan 2006
Cited by: 54 articles | PMID: 16319686
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (6)
Grant ID: RC1HL099545
Grant ID: R01HL097700
Grant ID: RC1 HL099545
Grant ID: R01HL69438
Grant ID: R01 HL097700
Grant ID: R01 HL069438
 1 and
1 and 




