Abstract
Free full text

Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans
SUMMARY
Pheromone responses are highly context-dependent. For example, the C. elegans pheromone ascaroside C9 (ascr#3) is repulsive to wild-type hermaphrodites, attractive to wild-type males, and usually neutral to “social” hermaphrodites with reduced activity of the npr-1 neuropeptide receptor gene. We show here that these distinct behavioral responses arise from overlapping push-pull circuits driven by two classes of pheromone-sensing neurons. The ADL sensory neurons detect C9, and in wild-type hermaphrodites, drive C9 repulsion through their chemical synapses. In npr-1 mutant hermaphrodites, C9 repulsion is reduced by the recruitment of a gap junction circuit that antagonizes ADL chemical synapses. In males, ADL sensory responses are diminished; in addition, a second pheromone-sensing neuron, ASK, antagonizes C9 repulsion. The additive effects of these antagonistic circuit elements generate attractive, repulsive or neutral pheromone responses. Neuronal modulation by circuit state and sex, and flexibility in synaptic output pathways, may permit small circuits to maximize their adaptive behavioral outputs.
INTRODUCTION
Connectomics, the description of neuronal circuits based on anatomically defined synapses, is an ongoing venture in neuroscience (White et al., 1986; Lichtman and Denk, 2011; Sporns, 2011). A question that is unanswered by such studies is the extent to which these synapses are functionally, as opposed to anatomically, stable in their properties. In many animals, pheromone detection results in behaviors that are highly sensitive to context (Wyatt, 2003). Here, we examine circuits for pheromone-dependent behaviors and show that a small set of common sensory inputs can give rise to multiple behavioral outputs through flexible circuit interactions.
The nematode worm Caenorhabditis elegans releases a pheromone mixture composed of derivatives of the dideoxysugar ascarylose (ascarosides) each of which has characteristic effects on development and behavior (Edison, 2009; Srinivasan et al., 2012). Two pheromones that have been characterized in multiple assays are C3 (ascr#5; asc ωC3) and C9 (ascr#3; asc ΔC9) ascarosides. C3 and C9 potently regulate larval entry into and exit from the alternate dauer developmental stage (Butcher et al., 2007; Butcher et al., 2008; Kim et al., 2009), and also elicit a variety of behavioral effects in adults. Adult wild-type males accumulate in low concentrations of C9, suggesting a role in sex attraction (Srinivasan et al., 2008; Macosko et al., 2009). Hermaphrodites with low activity alleles of the npr-1 neuropeptide receptor gene (henceforth ‘npr-1’) are weakly attracted to ascaroside mixtures of C3 and C9, but not to either single compound alone (Macosko et al., 2009). Hermaphrodites from the standard laboratory strain N2 (henceforth ‘wild-type’) strongly avoid C9 alone or together with C3 (Srinivasan et al., 2008; Macosko et al., 2009). The differential pheromone response in hermaphrodites correlates with aggregation behaviors: social npr-1 animals usually aggregate into groups on food, consistent with attraction to pheromones, whereas solitary wild-type animals rarely aggregate (de Bono and Bargmann, 1998). The npr-1 genotype appears to be a surrogate for a stress-related behavioral state, as aggregation and other npr-1-associated behaviors are stimulated regardless of genotype by stressful conditions (de Bono et al., 2002; Cheung et al., 2005; Rogers et al., 2006). Thus, pheromone responses in C. elegans depend on sex and neuromodulatory state.
The bilateral pair of ASK sensory neurons acts with different partners in different pheromone responses. In dauer formation, ascaroside pheromones are sensed by ASK and ASI sensory neurons (Hu, 2007; Kim et al., 2009; McGrath et al., 2011). In adult males, attraction to hermaphrodite pheromones requires ASK and the male-specific CEM sensory neurons (Srinivasan et al., 2008). In npr-1 hermaphrodites, the ASK neurons sense pheromones and promote aggregation by cooperating with URX, ASH, and ADL sensory neurons, all of which are connected by gap junctions to the RMG inter/motorneurons in a hub-and-spoke circuit (White et al., 1986; de Bono et al., 2002; Macosko et al., 2009). The integrated input from spoke sensory neurons drives synaptic outputs from RMG and ASK to promote aggregation (Macosko et al., 2009). In wild type animals, high NPR-1 activity in RMG inhibits this circuit (Macosko et al., 2009).
Wild-type hermaphrodites are repelled by ascarosides (Srinivasan et al., 2008; Macosko et al., 2009) but the underlying circuit mechanisms are unknown. Here we ask how repulsion from pheromones is mediated, and how repulsion is transformed into neutral or attractive pheromone responses in males and in npr-1 mutants. We find that the ADL sensory neurons promote repulsion from C9 in a sex and npr-1 state-dependent manner, and that alternative pheromone-dependent behaviors rely differentially upon antagonistic activities of ADL chemical synapses, the RMG gap junction circuit, and ASK. Our results describe a mechanism by which overlapping, flexible circuits allow animals to integrate pheromone signals with sex and neuromodulatory state to generate a biologically appropriate behavioral response.
RESULTS
ADL neurons detect the repulsive pheromone ascaroside C9
To identify neurons responsible for pheromone avoidance behavior, we first examined the acute responses of wild-type hermaphrodites to individual ascarosides using the drop test assay (Hilliard et al., 2002). In this assay, a chemical diluted in buffer is presented to an animal that is moving forward, and reversal responses are compared to those to buffer alone (see Experimental Procedures). Using this behavioral response, we found that wild-type hermaphrodites specifically avoided nanomolar concentrations of ascaroside C9, but not ascarosides C3 or C6 (Figure 1A). These responses were enhanced in the presence of food, resulting in a ~10-fold increase in sensitivity (Figure S1A).
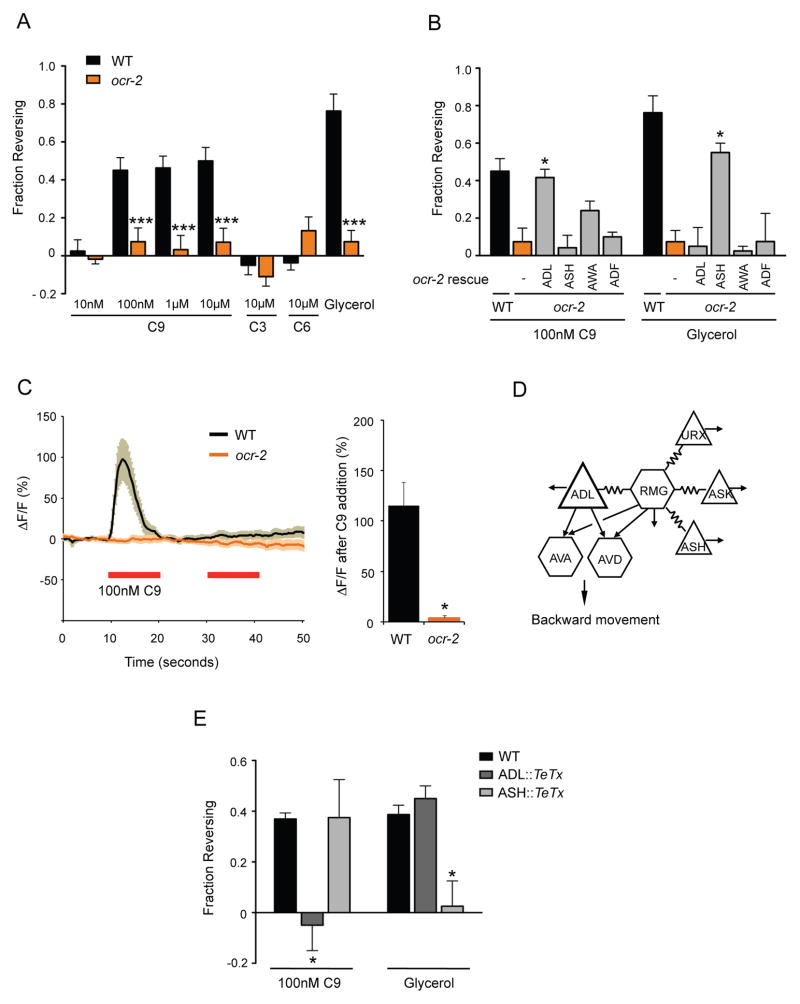
ADL sensory neurons mediate C9 avoidance.
A. Wild-type hermaphrodites avoid C9 in the drop test assay, but ocr-2(ak47) mutants do not. Assays were performed in the absence of food. *** indicates responses different from wild type at P<0.001. n=20–100 animals each. See Figure S1A for assays on food, and Figure S1B for behaviors of osm-9 mutants.
B. ocr-2 acts in ADL to mediate C9 avoidance. Assays were performed in the absence of food. * indicates responses different from ocr-2 at P<0.05. n=20–100 animals each. ocr-2 expression in AWA showed a trend toward rescue, but odr-7 mutants with developmental defects in the AWA neurons (Sengupta et al., 1994) retained the ability to respond to C9 (Figure S1C).
C. C9-induced pheromone responses in ADL. (Left) Intracellular Ca2+ dynamics in GCaMP3-expressing ADL neurons in wild-type and ocr-2(ak47) mutant hermaphrodites upon addition of pulses of 100 nM C9 (red horizontal bars). n≥10 neurons each. Shading around the lines represents SEM. (Right) Average peak percentage changes in fluorescence upon addition of the first pulse of C9. * indicates amplitude different from wild-type at P<0.001. Also see Figure S1D.
D. ADL has chemical synapses onto AVA and AVD command interneurons, and is electrically coupled with the RMG hub-and-spoke circuit (adapted from (White et al., 1986). Triangles represent sensory neurons and hexagons represent interneurons. Additional synapses (not shown) are indicated by arrows.
E. C9 avoidance requires ADL chemical synapses. Avoidance assays in the presence of food. * indicates responses different from wild type at P<0.05. n=20–80 animals each. Error bars in all panels represent standard error of the mean (SEM).
The neurons required for C9 avoidance were identified by examining sensory transduction mutants. C. elegans detects many chemical repellents with ciliated sensory neurons that signal through OSM-9 and OCR-2 TRPV channels (Bargmann, 2006). We found that both osm-9 and ocr-2 mutants exhibited strong defects in C9 avoidance (Figure 1A and Figure S1B). These two genes are co-expressed in four classes of head sensory neurons (Colbert et al., 1997; Tobin et al., 2002), which were individually tested for transgenic rescue of the ocr-2 behavioral defect. The C9 avoidance defects were rescued upon expression of ocr-2 in ADL, but not in other neurons (Figure 1B; also see Figure S1C). In control experiments, ocr-2 expression in ADL did not rescue avoidance of high osmotic strength glycerol, a sensory response characteristic of ASH neurons (Bargmann et al., 1990; Hilliard et al., 2005) (Figure 1B). These results indicate that OCR-2 acts in the ADL neurons to mediate C9 avoidance.
To ask whether ADL responds to C9, we expressed the genetically encoded calcium (Ca2+) sensor GCaMP3 (Nakai et al., 2001; Tian et al., 2009) in ADL neurons and monitored intracellular Ca2+ dynamics in response to C9. A pulse of 100 nM C9 induced a rapid, transient increase in ADL intracellular Ca2+ levels (Figure 1C). ADL Ca2+ transients adapted quickly, returning to baseline within 10s of C9 addition, and recovering ~120s later (Figure 1C and data not shown). The response to C9 was abolished in ocr-2 mutants that disrupt the sensory TRPV channel (Figure 1C). The ascaroside-evoked Ca2+ transients matched the behavioral results showing ADL-specific, chemically-selective responses: ASH neurons did not respond to C9 or other ascarosides with Ca2+ transients, and no changes in Ca2+ dynamics were observed in the ADL neurons upon addition of C3 and C6 ascarosides (Figure S1D).
The anatomical wiring diagram of C. elegans hermaphrodites indicates that the ADL neurons are connected by chemical synapses to the AVA and AVD backward command interneurons, as well as other neurons (Figure 1D) (Chalfie et al., 1985; White et al., 1986). In addition, ADL neurons are spoke neurons connected by gap junctions to the RMG hub-and-spoke circuit that promotes aggregation (Figure 1D) (de Bono et al., 2002; Macosko et al., 2009a). In the simplest model, ADL-mediated avoidance behavior could be driven by synaptic output of the ADL neurons and activation of the backward command interneurons. To examine this possibility, we inhibited ADL chemical synapses by cell-specific expression of the tetanus toxin light chain (TeTx) that cleaves the synaptic vesicle protein synaptobrevin (Schiavo et al., 1992). Blocking synaptic transmission in ADL significantly suppressed C9 avoidance responses (Figure 1E), but not osmotic avoidance behavior mediated by the ASH neurons. Conversely, expression of similar transgenes in ASH blocked high-osmolarity glycerol avoidance but did not affect C9 avoidance (Figure 1E). Thus, the ADL neurons drive C9 avoidance through their chemical synapses.
npr-1 action in the RMG hub-and-spoke circuit suppresses C9 avoidance
npr-1 animals show reduced avoidance of repulsive pheromones in accumulation assays (Macosko et al., 2009), consistent with their increased aggregation behaviors. As the aggregation behaviors are most prominent on food (de Bono and Bargmann, 1998; de Bono et al., 2002), we included food when comparing npr-1 and wild-type responses to C9. npr-1 mutants did not avoid 10 nM C9 in the drop test, although they avoided higher concentrations (Figure 2A, 2B). High-osmolarity glycerol avoidance was unaffected by npr-1 (Figure 2A). Silencing ADL synaptic output by TeTx expression in npr-1 mutants did not further affect their behavioral responses to 10 nM C9, but reduced their avoidance of 100 nM C9 (Figure 2B). These results suggest that ADL chemical synapses can drive C9 avoidance in npr-1 animals, but with reduced sensitivity compared to wild type.
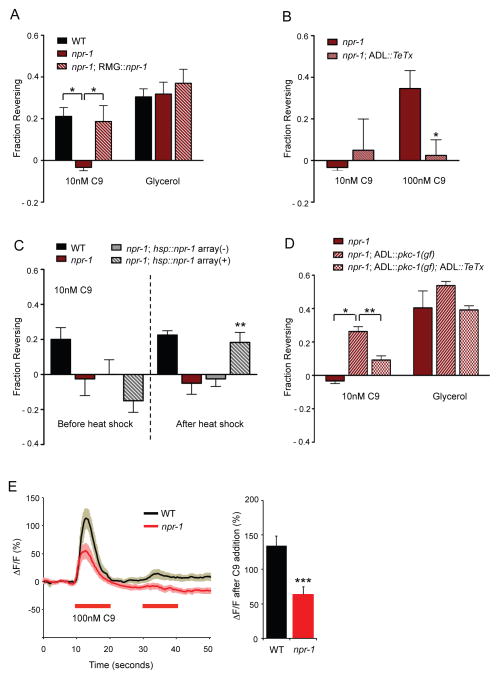
NPR-1 acts in RMG to antagonize ADL chemical synapses.
A. npr-1 animals show decreased C9 avoidance. Avoidance assays in the presence of food. * indicates responses different from values indicated by brackets at P<0.05. n=40–100 animals each.
B. ADL chemical synapses can drive C9 avoidance in npr-1. Avoidance assays in the presence of food. * indicates responses different from wild type at P<0.05. n=40–100 animals each.
C. Heat shock-driven expression of npr-1 during the adult stage rescues the C9 avoidance defect of npr-1 animals. ** indicates responses different from the responses of the same animals before heat shock at P<0.01. Array(-) indicates animals from the hsp16.2::npr-1-expressing transgenic strain that have lost the extrachromosomal array, representing sibling controls for the array(+) transgenic animals. Behavioral assays were performed in the presence of food immediately after heat-shock at 33 C for 30 minutes. n=40–60 animals each.
D. Strengthening ADL chemical synapses enhances C9 avoidance in npr-1. Avoidance assays in the presence of food. * and ** indicate responses different from values indicated by brackets at P<0.05 and P<0.01, respectively. n=40–100 animals each. Also see Figure S2A.
E. (Left) Changes in G-CaMP fluorescence in response to C9 in ADL neurons of wild-type and npr-1(ad609) hermaphrodites. Wild-type and all experimental conditions (see Figure 4C and Experimental Procedures) were examined together on multiple days. n=12 neurons each. Shading around the lines represents SEM. (Right) Average peak percentage changes in fluorescence upon addition of C9. *** indicates responses different from wild-type at P<0.001. Also see Figures S2B and S2C. Error bar in all panels represent SEM.
Previous studies have indicated that the ADL neurons promote aggregation in npr-1 mutants, in apparent contradiction to their role in C9 avoidance in wild type (de Bono et al., 2002). A possible explanation of this paradox is provided by the proposed circuit for aggregation, which involves gap junctions between ADL and RMG neurons rather than ADL chemical synapses (Figure 1D) (White et al., 1986; Macosko et al., 2009). Aggregation through the RMG circuit is inhibited by npr-1 expression in RMG (Macosko et al., 2009). We hypothesized that this gap junction circuit might antagonize or inactivate C9 avoidance mediated by ADL chemical synapses. Indeed, the C9 avoidance defects in npr-1 animals were fully rescued by a transgene expressing an npr-1 cDNA in RMG (Figure 2A), indicating that NPR-1 acts in RMG to block C9 avoidance behaviors initiated by ADL.
To determine whether NPR-1 acts during development to affect connectivity, or in adults to regulate circuit function, we asked whether expression of NPR-1 during the adult stage could rescue C9 avoidance in npr-1 animals. An npr-1 cDNA expressed under a heat-shock promoter (Coates and de Bono, 2002) restored C9 avoidance fully after 30 minutes of heat shock (Figure 2C), suggesting that NPR-1 acts acutely to regulate neuronal responses and circuit output.
The model that npr-1 in RMG antagonizes ADL chemical synapses predicts that increased ADL synaptic function might restore avoidance. To test this prediction, we used an ADL-specific promoter to drive pkc-1(gf), a constitutively active protein kinase C isoform that enhances neuronal synaptic outputs (Okochi et al., 2005; Sieburth et al., 2007; Tsunozaki et al., 2008; Macosko et al., 2009). Expression of pkc-1(gf) in ADL enhanced C9 avoidance in npr-1 animals (Figure 2D), and blocking ADL chemical synapses with TeTx eliminated C9 avoidance in the pkc-1(gf) strain (Figure 2D). Expression of pkc-1(gf) in ADL neurons of wild-type animals had little effect on C9 avoidance (Figure S2A). These results suggest that strengthening ADL chemical synapses can override the effect of the npr-1 mutation.
ADL Ca2+ transients were slightly but significantly reduced in amplitude in npr-1 as compared to wild type animals (Figures 2E, S2B). Two results suggest that this small change in amplitude is due to indirect effects of RMG on ADL. First, ADL Ca2+ responses were rescued by expressing npr-1 under a promoter that is expressed in RMG (as well as a few other neurons) but not in ADL (Figure S2B). Second, the effect of npr-1 on ADL Ca2+ responses was reversed in animals mutant for unc-9, which encodes a gap junction subunit that is broadly expressed in muscles and neurons (Liu et al., 2006; Starich et al., 2009) (Figure S2C). This observation suggests that gap junctions are required for NPR-1 to affect ADL, as predicted by the hub-and-spoke model. However, unc-9 has stronger effects on ADL Ca2+ responses than npr-1 (Figure S2C), and acts at multiple sites, so it may have either direct or indirect effects on ADL. In summary, npr-1 has a strong effect on C9 avoidance behavior that is mediated by RMG, and an indirect effect on ADL Ca2+ responses. Our results suggest that npr-1 functions primarily by changing activity of the RMG gap junction circuit relative to ADL chemical synapses, and not solely by changing ADL sensory properties.
Reduced ADL C9 responses and ADL-ASK antagonism abolish C9 avoidance in wild-type males
Unlike wild-type hermaphrodites, wild-type C. elegans males accumulate in low concentrations of C9, a behavior that requires the ASK neurons and the male-specific CEM sensory neurons (Srinivasan et al., 2008). In agreement with this result, we found that wild-type males did not avoid either 10 nM or 100 nM C9 in the drop test, although they exhibited robust avoidance of high-osmolarity glycerol (Figure 3A).
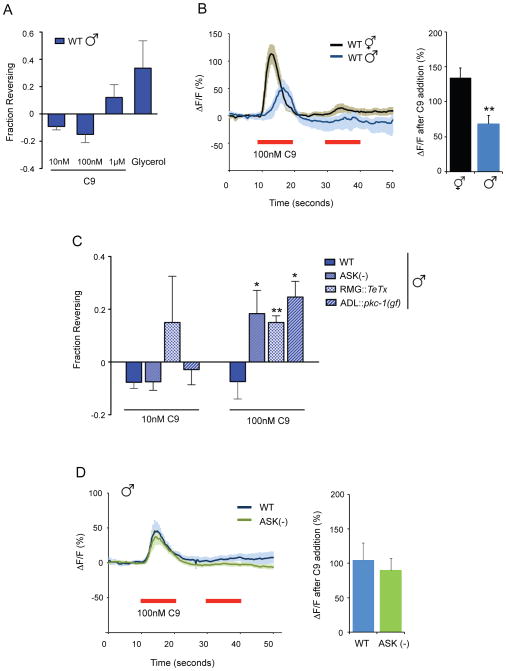
ASK antagonism of ADL decreases C9 avoidance in males.
A. Wild-type males do not avoid C9. Avoidance assays were performed in the presence of food. n=20–70 animals each.
B. (Left) Changes in intracellular fluorescence in GCaMP3-expressing ADL neurons of wild-type hermaphrodites and males upon addition of 100 nM C9. n≥12 neurons each. Shading around the lines represents SEM. (Right) Average peak percentage change in fluorescence upon C9 addition. ** indicates responses different from wild-type at P<0.01. Also see Figures S3A and S3B.
C. ASK and RMG antagonize ADL in wild-type males. Avoidance of C9 in the presence of food. * and ** indicate responses different from wild type at P<0.05 and P<0.01, respectively. n=20–120 animals each. Also see Figure S3C.
D. C9 responses in wild-type male ADL neurons are unaffected by ASK ablation. Ca2+ transients upon C9 addition (red horizontal bar) in ADL in wild-type and ASK-ablated animals. n≥10 neurons each. Error bars in all panels represent SEM.
This sexually dimorphic behavioral response to C9 was accompanied by sexually dimorphic Ca2+ responses in ADL neurons. C9-induced Ca2+ transients in male ADL neurons were delayed by several seconds and reduced in amplitude compared to responses in hermaphrodites (Figures 3B, Figure S3A). Because slow changes in neuronal responses are often associated with neuropeptide signaling, we investigated ADL C9 responses in egl-3 and egl-21 mutants, which lack processed neuropeptides (Kass et al., 2001; Jacob and Kaplan, 2003). Both males and hermaphrodites showed sex-appropriate ADL Ca2+ transients in neuropeptide mutant backgrounds (Figure S3A), suggesting that classical neuropeptide signaling is not essential for sexual dimorphism. Thus altered male behaviors are associated with decreased and delayed pheromone signaling by the ADL neurons, which might or might not be intrinsic to ADL.
We next probed the roles of other sexually dimorphic neurons in C9 avoidance. The male-specific CEM sensory neurons are required for male accumulation at low C9 concentrations (Srinivasan et al., 2008), but were not central to C9 avoidance: sex-appropriate behaviors to C9 were observed both in males lacking CEM neurons (ceh-30(lf)), and in hermaphrodites with ectopic CEM neurons (ceh-30(gf)) (Schwartz and Horvitz, 2007) (Figure S3B). The ASK neurons are pheromone-sensing neurons that participate in the RMG gap junction circuit (Macosko et al., 2009a) (Figure 1D), and these neurons are functionally dimorphic between males and hermaphrodites (Srinivasan et al., 2008; Srinivasan et al., 2012). Males whose ASK neurons were killed with a mouse caspase gene (Kim et al., 2009) exhibited significant avoidance of 100 nM C9, unlike wild-type males (Figure 3C). Ablation of ASK had little effect on wild-type hermaphrodite C9 avoidance (Figure S3C). Thus, ASK effectively antagonizes ADL-mediated C9 avoidance in wild-type males, but not in wild-type hermaphrodites. ASK ablation did not affect C9-induced Ca2+ transients in male ADL neurons (Figure 3D), suggesting that ASK acts at a circuit level to suppress C9 avoidance.
Reasoning by analogy to the npr-1 circuit, we asked whether synaptic output of the RMG gap junction circuit antagonizes C9 avoidance in males. Indeed, expression of TeTx in the RMG neurons led to robust C9 avoidance behavior in wild-type males (Figure 3C). Expression of pkc-1(gf) in ADL also led to C9 avoidance, indicating that a strongly activated ADL neuron can drive repulsion in males (Figure 3C), as it can in npr-1 hermaphrodites (Figure 2D). These results suggest that ADL has a latent ability to drive C9 avoidance in males, but this activity is inhibited by ASK and RMG.
Male sexual identity and npr-1 have additive effects on C9 responses
Both males and npr-1 hermaphrodites have decreased C9 avoidance (compare Figures 2A and and3A),3A), and males also resemble npr-1 hermaphrodites in their avoidance of high oxygen, their rapid movement on food, and their propensity to aggregate (Figures S4A, S4B). Despite this similarity, behavioral analysis of npr-1 males suggest that npr-1 mutations and male sex have independent effects on C9 responses. First, in npr-1 males C9 failed to induce reversals as it did in npr-1 hermaphrodites and wild-type males, but instead suppressed spontaneous reversals (Figure 4A). Based on the biased random walk model for C. elegans chemotaxis, the suppression of reversals suggests that npr-1 males are attracted to C9 (Pierce-Shimomura et al., 1999; Luo et al., 2008). The suppression of reversals was eliminated by each of the genetic manipulations that increased C9 repulsion in wild-type males: killing ASK with the caspase transgene, reducing RMG synaptic output with TeTx, or enhancing ADL output with pkc-1(gf) (Figure 4B). Like other effects of npr-1, the effect on males was rescued by npr-1 expression in RMG neurons (Figure 4B), and was rapidly reversed after acute expression of npr-1 in adults (Figure S4C).
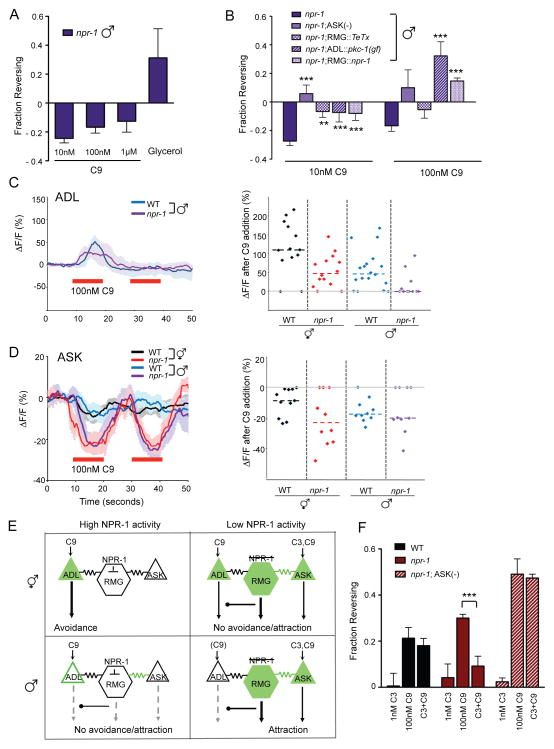
Masculinization and NPR-1 exert additive effects on a common circuit.
A. Spontaneous reversals are suppressed in the presence of C9 in npr-1 males. Avoidance assays were performed in the presence of food. n=30–120 animals each. Responses to 10 nM and 1 μM C9 are different from those of wild-type males at P<0.01 (see Figure 3A). Also see Figure S4.
B. C9-induced suppression of spontaneous reversals in npr-1 males is eliminated by circuit manipulations that enhance C9 avoidance. ** and *** indicate responses different from npr-1 at P<0.01 and P<0.001, respectively. n=40–120 animals each.
C. (Left) Intracellular Ca2+ dynamics in G-CaMP-expressing ADL neurons of wild-type and npr-1(ad609) mutant males in response to 100 nM C9. Only animals with positive responses were included in the averages at left: n=14 (of 17) neurons for wild-type males and n=4 (of 11) neurons for npr-1 males. Shading around the lines represents SEM. (Right) Scatter plot showing peak percentage changes in fluorescence in individual ADL neurons of animals of the indicated genotypes in response to a pulse of 100 nM C9. Data are included from Figure 2E for comparison. Dotted lines indicate the median. The percentage of ADL neurons that failed to exhibit significant changes in fluorescence were 14% in wild-type hermaphrodites (n=14); 14% in npr-1 hermaphrodites (n=14); 12% in wild-type males (n=17) and 64% in npr-1 males (n=11).
D. ASK responses to C9 are enhanced in npr-1 mutants. (Left) Intracellular Ca2+ dynamics in GCaMP3-expressing ASK neurons (Macosko et al., 2009b) of wild-type and npr-1(ad609) mutant males and hermaphrodites in response to 100 nM C9 (red horizontal bars). Only animals with quantifiable changes were included in these averages; n≥6 neurons for each. Shading around the lines represents SEM. (Right) Scatter plot showing peak percentage changes in fluorescence in individual ADL neurons of animals of the indicated genotypes in response to a pulse of 100 nM C9. Dotted lines indicate the median.
E. Inferred activities of ADL, ASK, and RMG neurons in animals of different sex and npr-1 genotype. High NPR-1 activity inhibits RMG. C9 responses are high in ADL and low in ASK in wild-type hermaphrodites; ADL drives C9 avoidance via its chemical synapses. In males, ASK C9 responses remain low and ADL C9 responses are decreased due to sexual dimorphism. ASK and the RMG circuit antagonize ADL output to further reduce ADL-driven C9 avoidance. In npr-1 animals, ASK C9 responses are increased in both males and hermaphrodites, and ADL C9 responses are significantly decreased in npr-1 males. ASK and RMG antagonize ADL chemical synapses and decrease avoidance (hermaphrodites) or promote attraction (males).
F. Integration of pheromone blend information in npr-1 hermaphrodites requires ASK. Assays were performed in the presence of food. *** indicates responses different from values indicated by brackets at P<0.001. n=60–80 animals each. Error bars in all panels represent SEM.
Additive effects of npr-1 and male sex were also observed in Ca2+ imaging. The majority of ADL neurons in npr-1 mutant males failed to modulate Ca2+ after C9 addition (Figure 4C, right panel). This reduction in ADL Ca2+ responses far exceeded that of wild-type males or npr-1 hermaphrodites, even considering only the small subset of npr-1 males that did modulate ADL Ca2+ in response to C9 (Figure 4C, left panel).
The strong reduction in ADL Ca2+ transients might explain the loss of C9 avoidance in npr-1 males, but would not predict the appearance of the new behavior of C9 attraction (strictly speaking, reversal suppression). Therefore, we sought another sensory neuron that enhances C9 attraction in npr-1 males. ASK was a plausible candidate to drive C9 attraction based on the behavioral analysis (Figure 4B), so we asked if its pheromone sensitivity was altered by npr-1. Indeed, ASK neurons showed much stronger C9-evoked Ca2+ transients in npr-1 males than in wild-type males (Figure 4D). A similar enhancement of ASK responses was present in npr-1 hermaphrodites, whose C9 avoidance is also antagonized by ASK (Figures 4D, S3C).
Together, these results indicate that npr-1 males have enhanced ASK C9 responses and decreased ADL C9 responses compared to wild-type males, and that these changes drive attraction to C9 through RMG chemical synapses. Circuit changes driving sexually dimorphic and NPR-1-dependent C9 pheromone responses are summarized in Figure 4E.
Pheromone blends are integrated by the RMG circuit
The results described above suggest that antagonism between repulsive signaling from ADL chemical synapses and attractive signaling mediated by ASK and the RMG gap junction circuit determine whether C9 is repulsive, neutral, or attractive. We considered what this might mean for the pheromone-dependent behaviors of npr-1 hermaphrodites, which are weakly attracted to mixtures of ascarosides, including C9 and C3, but not to either C3 or C9 alone (Srinivasan et al., 2008; Macosko et al., 2009). By analogy with the detection of pheromone blends in other animals (Slessor et al., 1988; Kaissling, 1996; Matsuura et al., 2010), synergistic attraction to ascaroside blends could result from cooperation of multiple pheromone-sensing neurons. Hermaphrodite ASK neurons detect C3 at nanomolar concentrations (Kim et al., 2009), and ASK pheromone responses are stronger in npr-1 than in wild type hermaphrodites (Macosko et al., 2009). If antagonism between ASK and ADL applies to pheromone blends, C3 detection by ASK should suppress repulsion to C9 detected by ADL. To address this hypothesis, the drop test assay was used to detect behavioral interactions between pheromones.
In agreement with the observation that C3 is not highly attractive or repulsive on its own (Macosko et al., 2009), C3 did not induce or suppress reversals in wild-type or npr-1 hermaphrodites (Figure 4F). However, C3 did modify the response to C9 in npr-1 hermaphrodites, suppressing their avoidance of 100 nM C9 almost to baseline levels (Figure 4F). No suppression was observed in wild-type hermaphrodites, indicating that the interaction depends on npr-1 and the gap junction circuit. Genetic ablation of the ASK neurons in npr-1 hermaphrodites abolished the interaction between C3 and C9 (Figure 4F). These results support the model that ASK suppresses ADL-mediated avoidance, and additionally are consistent with a circuit that can evaluate pheromone blends, so that the combination of C3 detected by ASK and C9 detected by ADL is less repulsive than C9 alone.
Discussion
Sex and NPR-1 neuropeptide signaling converge on a common neural circuit to regulate behavioral responses to the ascaroside C9. In each case, alternative behaviors are initiated by the ADL and ASK sensory neurons, but specific behavioral outcomes are determined by antagonism between ADL chemical synapses that promote repulsion, and the RMG gap junction circuit that promotes attraction. These two antagonistic elements form a push-pull circuit motif, in which a single sensory input can give rise to opposite behaviors (Figure 4E). On the repulsive arm of the circuit, wild-type hermaphrodites avoid C9 through ADL chemical synapses, whose predicted targets include the backward command interneurons. Although this effect is diminished in npr-1 mutants and males, all genotypes retain a covert ability to avoid C9. On the attractive arm, the RMG gap junction circuit suppresses C9 avoidance via RMG chemical synapses, which converge with ADL chemical synapses on the command interneurons (see Figure 1D). NPR-1 inhibits RMG through unknown molecular mechanisms; in one model, it could close the RMG gap junctions to disengage the entire hub-and-spoke circuit. The ASK neurons also sense C9, and drive attractive behavioral responses more strongly in males, in npr-1 mutants, or in the presence of C3. ASK and ADL form gap junctions with RMG; both behavioral results and functional imaging indicate that RMG potentiates ASK signaling and inhibits ADL signaling (Macosko et al., 2009, and this work). The attractive arm of the circuit dominates in npr-1 males, which have minimal C9 responses in ADL, strong C9 responses in ASK, and the ability to propagate these changes through the RMG circuit.
It is likely that the alternative circuits in wild-type and npr-1 mutants are representative of alternative neuromodulatory states that exist in all genotypes to differing degrees. The behaviors of npr-1 animals resemble the behaviors of wild-type animals under metabolic or crowding stress, and conversely, npr-1 animals placed in low oxygen environments behave like wild-type animals in most respects (de Bono and Bargmann, 1998; de Bono et al., 2002; Cheung et al., 2005; Rogers et al., 2006). The differential modulation of ADL chemical synapses and gap junctions in overlapping circuits by npr-1 is reminiscent of the flexible circuit states of crustacean stomach central pattern generators and vertebrate spinal cord motor circuits, which are also controlled by neuromodulatory inputs (Hooper and Moulins, 1989; Dickinson et al., 1990; Grillner, 2006). In males, sexual dimorphism in sensory neuron responses and circuit properties further expand this behavioral flexibility.
The RMG hub-and-spoke circuit has both similarities to and differences from the recently described RIH hub-and-spoke circuit for mechanosensation (Chatzigeorgiou and Schafer, 2011). A central hub neuron coordinates responses via gap junctions in both circuits, but RIH appears to facilitate the transfer of mechanosensory information through the circuit (Chatzigeorgiou and Schafer, 2011), whereas RMG antagonizes ADL synaptic output while facilitating ASK synaptic output, generating a consensus behavior that can be distinct from that generated by either sensory neuron. Thus, a common network motif can perform distinct computations in ways that are not evident solely from anatomical wiring diagrams.
Pheromone blends with defined concentrations of individual pheromone components elicit sex- and context-specific behaviors in many organisms (Kaissling, 1996; Wyatt, 2003). The RMG circuit coordinates sensory responses via gap junctions to generate coherent responses to specific pheromones and pheromone blends. Spoke sensory neurons in the RMG circuit also respond to non-pheromone cues (Bargmann, 2006), allowing the circuit to integrate pheromones with other environmental signals. At the same time, each sensory neuron also has other outputs; for example, ASK can promote attraction to indole ascarosides via its chemical synapses in an RMG-independent manner (Srinivasan et al., 2012), and ADL alone can drive repulsion. These results reveal a multifunctional, multiplexed sensory circuit, whose compact structure integrates external context with internal states to generate a variety of adaptive behaviors.
EXPERIMENTAL PROCEDURES
Detailed protocols are listed in Supplemental Experimental Procedures.
Behavioral assays
The drop test was performed essentially as previously described (Hilliard et al., 2002). “Fraction reversing” represents (fraction of animals reversing in 4s with pheromone) – (fraction reversing in 4s to buffer).
Ca2+ imaging
Ca2+ imaging experiments were performed as previously described (Kim et al., 2009; Macosko et al., 2009; Chalasani et al., 2010) using microfluidic devices custom-designed to restrain adult hermaphrodites (Chalasani et al., 2007) or adult males (this study) (Microfluidics Facility, Brandeis Materials Research Science and Engineering Center).
Acknowledgments
We are grateful to Eugene Kim and Andrew Gordus for assistance with ADL imaging experiments and analysis, the Caenorhabditis Genetics Center for strains and Eve Marder for discussion. This work was supported by the NSF (IOS 0542372 – P.S., DMR-0820492 – D.K. (MRSEC program), the HFSP (RGY0042-P.S.), the NIH (core grant P30 NS45713 to the Brandeis Biology Department; F31 DC011467 – D.M.Z., R00 GM87533 – R.A.B.), the DGIST MIREBrain and Convergence Science Center and Basic Science Research Program (2012009385) of the Ministry of Education, Science and Technology (K.K.), the Natural Sciences and Engineering Research Council of Canada (PGS-D3) and by the Brandeis National Committee (S.J.N.), a gift from the Jensam Foundation (C.I.B.), and the Howard Hughes Medical Institute (C.I.B.). C.I.B. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Author contributions: H.J., K.K., S.J.N., E.M. and D.M.Z. performed the experiments; D.K. and R.B. provided reagents; H.J., K.K., C.I.B. and P.S. analyzed and interpreted data; C.I.B. and P.S. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bargmann CI. Chemosensation in C. elegans. In: Chalfie M, editor. Wormbook: A review of C. elegans biology. 2006. pp. 1–29. [Abstract] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harbor Symp Quant Biol. 1990;LV:529–538. [Abstract] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J. Small molecule signaling of dauer formation in C. elegans. Nat Chem Biol. 2007;3:420–422. [Abstract] [Google Scholar]
- Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci USA. 2008;105:14288–14292. [Europe PMC free article] [Abstract] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a neural circuit for food-seeking behavior in Caenorhabditis elegans. Nature. 2007;450:63–70. [Abstract] [Google Scholar]
- Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. [Europe PMC free article] [Abstract] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. [Abstract] [Google Scholar]
- Chatzigeorgiou M, Schafer WR. Lateral facilitation between primary mechanosensory neurons controls nose touch perception in C. elegans. Neuron. 2011;70:299–309. [Europe PMC free article] [Abstract] [Google Scholar]
- Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–917. [Abstract] [Google Scholar]
- Coates JC, de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–929. [Abstract] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. [Abstract] [Google Scholar]
- de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. [Abstract] [Google Scholar]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. [Europe PMC free article] [Abstract] [Google Scholar]
- Dickinson PS, Mecsas C, Marder E. Neuropeptide fusion of two motor-pattern generator circuits. Nature. 1990;344:155–158. [Abstract] [Google Scholar]
- Edison AS. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr Opin Neurobiol. 2009;19:378–388. [Europe PMC free article] [Abstract] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. [Abstract] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. [Europe PMC free article] [Abstract] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol. 2002;12:730–734. [Abstract] [Google Scholar]
- Hooper SL, Moulins M. Switching of a neuron from one network to another by sensory-induced changes in membrane properties. Science. 1989;244:1587–1589. [Abstract] [Google Scholar]
- Hu PJ. Dauer. WormBook. 2007:1–19. [Europe PMC free article] [Abstract] [Google Scholar]
- Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci. 2003;23:2122–2130. [Abstract] [Google Scholar]
- Kaissling KE. Peripheral mechanisms of pheromone reception in moths. Chem Senses. 1996;21:257–268. [Abstract] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci. 2001;21:9265–9272. [Abstract] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. [Europe PMC free article] [Abstract] [Google Scholar]
- Lichtman JW, Denk W. The big and the small: challenges of imaging the brain’s circuits. Science. 2011;334:618–623. [Abstract] [Google Scholar]
- Liu Q, Chen B, Gaier E, Joshi J, Wang ZW. Low conductance gap junctions mediate specific electrical coupling in body-wall muscle cells of Caenorhabditis elegans. J Biol Chem. 2006;281:7881–7889. [Abstract] [Google Scholar]
- Luo L, Gabel CV, Ha HI, Zhang Y, Samuel AD. Olfactory behavior of swimming C. elegans analyzed by measuring motile responses to temporal variations of odorants. J Neurophysiol. 2008;99:2617–2625. [Abstract] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behavior in C. elegans. Nature. 2009;458:1171–1175. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsuura K, Himuro C, Yokoi T, Yamamoto Y, Vargo EL, Keller L. Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA. 2010;107:12963–12968. [Europe PMC free article] [Abstract] [Google Scholar]
- McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. [Europe PMC free article] [Abstract] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. [Abstract] [Google Scholar]
- Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–2137. [Europe PMC free article] [Abstract] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–9569. [Abstract] [Google Scholar]
- Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–659. [Abstract] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. [Abstract] [Google Scholar]
- Schwartz HT, Horvitz HR. The C. elegans protein CEH-30 protects male-specific neurons from apoptosis independently of the Bcl-2 homolog CED-9. Genes Dev. 2007;21:3181–3194. [Europe PMC free article] [Abstract] [Google Scholar]
- Sengupta P, Colbert HA, Bargmann CI. The C. elegans gene odr-7 encodes an olfactory-specific member of the nuclear receptor superfamily. Cell. 1994;79:971–980. [Abstract] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10:49–57. [Abstract] [Google Scholar]
- Sporns O. The human connectome: a complex network. Ann N Acad Sci. 2011;1224:109–125. [Abstract] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. [Europe PMC free article] [Abstract] [Google Scholar]
- Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O’Doherty OG, Edison AS, Sternberg PW, Schroeder FC. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001237. [Europe PMC free article] [Abstract] [Google Scholar]
- Starich TA, Xu J, Skerrett IM, Nicholson BJ, Shaw JE. Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev. 2009;4:16. [Europe PMC free article] [Abstract] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. [Europe PMC free article] [Abstract] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann CI. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. [Abstract] [Google Scholar]
- Tsunozaki M, Chalasani SH, Bargmann CI. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron. 2008;59:959–971. [Europe PMC free article] [Abstract] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Transact R Soc Lond B. 1986;314:1–340. [Abstract] [Google Scholar]
- Wyatt TS. Pheromones and Animal Behavior: Communication by Smell and Taste. Cambridge: Cambridge University Press; 2003. [Google Scholar]
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.neuron.2012.06.034
Article citations
Meloidogyne incognita genes involved in the repellent behavior in response to ascr#9.
Sci Rep, 14(1):25706, 28 Oct 2024
Cited by: 0 articles | PMID: 39465253 | PMCID: PMC11514155
RNAi-dependent expression of sperm genes in ADL chemosensory neurons is required for olfactory responses in <i>Caenorhabditis elegans</i>.
Front Mol Biosci, 11:1396587, 11 Jul 2024
Cited by: 0 articles | PMID: 39055986 | PMCID: PMC11269235
Peripheral peroxisomal β-oxidation engages neuronal serotonin signaling to drive stress-induced aversive memory in C. elegans.
Cell Rep, 43(4):113996, 24 Mar 2024
Cited by: 0 articles | PMID: 38520690
C. elegans males optimize mate-preference decisions via sex-specific responses to multimodal sensory cues.
Curr Biol, 34(6):1309-1323.e4, 11 Mar 2024
Cited by: 3 articles | PMID: 38471505
G protein-coupled receptor-based thermosensation determines temperature acclimatization of Caenorhabditis elegans.
Nat Commun, 15(1):1660, 23 Feb 2024
Cited by: 2 articles | PMID: 38396085 | PMCID: PMC10891075
Go to all (95) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans.
Nature, 458(7242):1171-1175, 06 Apr 2009
Cited by: 310 articles | PMID: 19349961 | PMCID: PMC2760495
Decoding a neural circuit controlling global animal state in C. elegans.
Elife, 4, 11 Mar 2015
Cited by: 34 articles | PMID: 25760081 | PMCID: PMC4440410
Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans.
Curr Biol, 16(7):649-659, 01 Apr 2006
Cited by: 91 articles | PMID: 16581509
Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions.
Int J Mol Sci, 20(16):E3898, 09 Aug 2019
Cited by: 16 articles | PMID: 31405082 | PMCID: PMC6719183
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NCATS NIH HHS (1)
Grant ID: UL1 TR000064
NIDCD NIH HHS (1)
Grant ID: F31 DC011467
NIGMS NIH HHS (2)
Grant ID: R00 GM87533
Grant ID: R00 GM087533
NIMH NIH HHS (1)
Grant ID: R25 MH094612
NINDS NIH HHS (2)
Grant ID: P30 NS45713
Grant ID: P30 NS045713




