Abstract
Free full text

Disease and the dynamics of extinction
Abstract
Invading infectious diseases can, in theory, lead to the extinction of host populations, particularly if reservoir species are present or if disease transmission is frequency-dependent. The number of historic or prehistoric extinctions that can unequivocally be attributed to infectious disease is relatively small, but gathering firm evidence in retrospect is extremely difficult. Amphibian chytridiomycosis and Tasmanian devil facial tumour disease (DFTD) are two very different infectious diseases that are currently threatening to cause extinctions in Australia. These provide an unusual opportunity to investigate the processes of disease-induced extinction and possible management strategies. Both diseases are apparently recent in origin. Tasmanian DFTD is entirely host-specific but potentially able to cause extinction because transmission depends weakly, if at all, on host density. Amphibian chytridiomycosis has a broad host range but is highly pathogenic only to some populations of some species. At present, both diseases can only be managed by attempting to isolate individuals or populations from disease. Management options to accelerate the process of evolution of host resistance or tolerance are being investigated in both cases. Anthropogenic changes including movement of diseases and hosts, habitat destruction and fragmentation and climate change are likely to increase emerging disease threats to biodiversity and it is critical to further develop strategies to manage these threats.
1. Introduction
The role of infectious disease in the history of human society is well known. In contrast, until the pioneering work of Anderson and May [1–4] in the late 1970s, the perception among most ecologists was that ‘well-adapted’ parasites do not harm their hosts. The role of infectious disease as a driver of host population dynamics was therefore underappreciated, and infectious diseases or parasites were rarely considered as significant extinction threatening processes.
In theory, however, simple models show that parasites and infectious diseases may, in some circumstances, be capable of being significant contributors to extinction [5]. From first principles, a population declines when there are more deaths than there are births and extinction occurs when there continue to be more deaths than births even as the population declines towards zero. It is therefore necessary to first distinguish between situations in which infectious disease may reduce population size to such an extent that other factors may lead to extinction and cases in which infectious disease contributes to there being an excess of deaths over births even in a vanishingly small population.
The most fundamental concept in epidemiology is the basic reproductive number, R0, which is the number of secondary infections per primary infection when disease is introduced into a naive population [6,7]. For many (but not all) pathogens or parasites, R0 is an increasing or saturating function of population size or density [8], leading to the existence of a threshold host population size below which the pathogen cannot persist.
The simplest host pathogen models are those in which there is a single host and a single pathogen, no spatial structure, and transmission occurs as a binary collision process between infected and susceptible hosts. In this situation, R0 is directly proportional to host population size, which means that at a sufficiently small population size R0 will decrease to below one and the pathogen will no longer be able to persist in the host population. A host-specific pathogen should therefore itself become extinct before it is capable of driving its sole host to extinction [9]. However, such a pathogen maybe able to reduce a population to a sufficiently low level that other factors may lead to the ultimate extinction of the host.
Sexually transmitted diseases are particularly likely to have severe effects on their host population and potentially to be able to cause host extinction. Not only are they often transmitted in a frequency-dependent rather than in a density-dependent fashion [10,11], meaning that R0 depends weakly, if at all, on host density, but also in many cases they affect fecundity rather than mortality. Whereas very high mortality reduces R0 because infected animals are rapidly removed from the population, a reproductively suppressed host may remain in the population for an extended period, continuing to spread infection. The number of sexually transmitted diseases in non-human animals is much larger than is commonly supposed [12] and they may be important drivers of extinction.
Finally, in many populations where disease is an important threatening process, it is one of several multiple stressors. For example, many local populations of the koala Phascolarctos cinereus are affected by chlamydia (Chlamydia pecorum and Chlamydia pneumoniae [13]), which is primarily sexually transmitted, causing both reproductive suppression through cystitis and increased mortality through keratoconjunctivitis-related blindness [13–15]. However, while prevalence in a population of clinical signs of chlamydia does appear to be associated with lower koala recruitment, it may be a relatively weak predictor of overall population change (figure 1), suggesting that other factors may be more important in driving population dynamics. It seems that chlamydia is particularly important in combination with multiple stressors such as habitat loss and distress caused by overbrowsing [17].
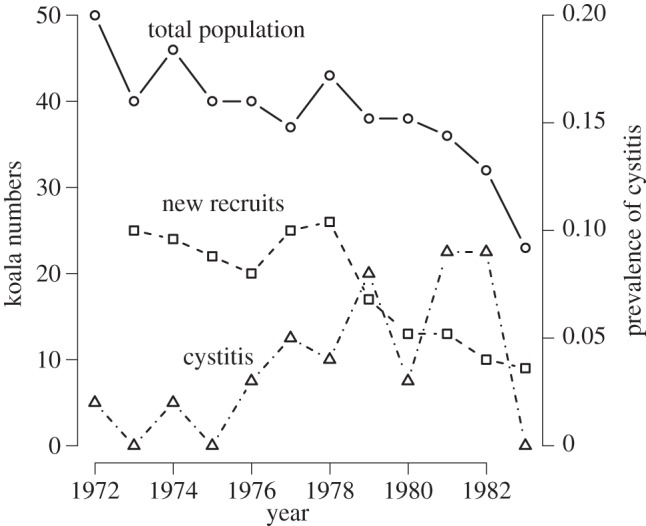
Effects of chlamydial infection on an almost completely censused population of koalas at Inverness, Queensland. The number of new recruits in the subsequent year, as a proportion of the number of adults in the current year, is inversely related to the prevalence of cystitis owing to chlamydial infection in the current year (quasi-binomial model, t = −2.23, p = 0.05, d.f. = 9), but there is no evidence that the finite population growth rate ln(Nt+1/Nt) is related to the prevalence of cystitis at time t (linear model r = −0.133, p = 0.27, d.f. = 9). Data from [16].
2. Role of disease in historical and prehistoric extinctions
Verifying that infectious disease has been responsible for a historical or prehistoric extinction is difficult. The extinct host is, of course, no longer available for any experimental work and it is rare for good-quality data to have been collected as the species involved was in terminal decline. Further, the mere presence of a pathogen or parasite in a declining host population is not evidence that the parasite was a significant contributor to decline.
In some cases, the role of infectious disease in extinction is nevertheless fairly clear. There is very good evidence that avian malaria and birdpox were responsible for the extinction of a substantial proportion of the Hawaiian avifauna in the late nineteenth century [18,19]. While there is no direct evidence that any of the 25 [18] species of Hawaiian land birds that have become extinct since the documented arrival of Culex quinquefasciatus in 1826 [19] were even susceptible to malaria and there is limited anecdotal information suggesting they were affected by birdpox [19], the observation that several remaining species only persist either on islands where there are no mosquitoes or at altitudes above those at which mosquitoes can breed and that these same species are highly susceptible to avian malaria and birdpox [18,19] is certainly very strong circumstantial evidence. An important contributor to the potential of avian malaria and birdpox to cause extinctions of highly susceptible species is the existence of a range of birds in Hawaii, both native and non-native, that are able to tolerate infections with these pathogens and therefore act as reservoirs [20,21].
A second example of extinction of an island species in which disease is strongly implicated is less commonly known. The formerly abundant endemic rats Rattus macleari and Rattus nativitas disappeared from Christmas Island in the Indian Ocean (10°29′ S 105°38′ E) around the turn of the twentieth century. Their disappearance was apparently abrupt, and shortly before the final collapse sick individuals were seen crawling along footpaths [22]. At that time, trypanosomiasis transmitted by fleas from introduced black rats R. rattus was suggested as the causative agent. Recently, Wyatt et al. [22] managed to isolate trypanosome DNA from both R. rattus and R. macleari specimens collected during the period of decline, whereas no trypanosome DNA was present in R. nativitas specimens collected before the arrival of black rats. While this is good circumstantial evidence, direct evidence that trypanosomes caused the mortality is limited, except that the one specimen described at the time of collection as suffering trypanosome infection did in fact test positive for trypanosome DNA. As with the Hawaiian birds, the role of a reservoir species able to tolerate infection, in this case the introduced black rat, is critical.
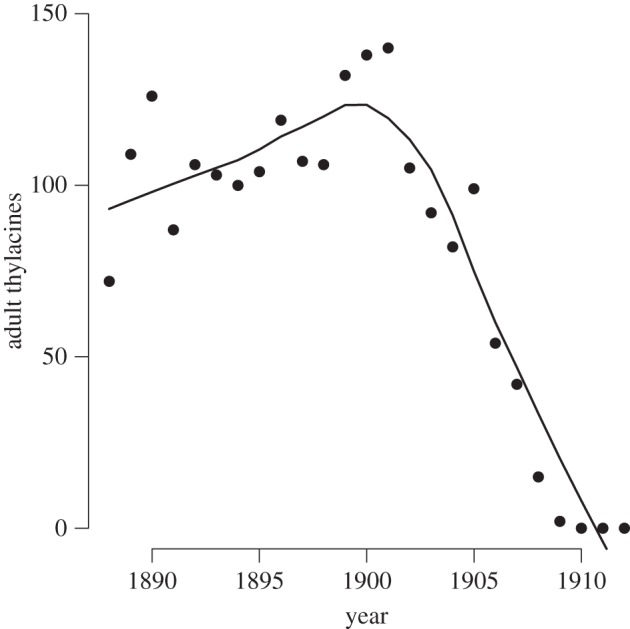
For other historical extinctions that have been blamed on disease, evidence is much weaker. It has been suggested that disease was responsible for the most notorious of Australia's mammal extinctions, that of the thylacine or Tasmanian tiger Thylacinus cynocephalus. The suggestion was first made by Guiler [23], who claimed that the decline in thylacine scalps brought in through the then bounty programme (figure 2) was too rapid to have been a result of overhunting but was consistent with what might be observed as a result of epidemic disease. There were also some anecdotal records of a ‘distemper like’ disease among both thylacines [24] and other marsupial carnivores (dasyurids) [25]. Canine distemper certainly poses an extinction threat to some populations of placental carnivores [26,27] but I can find no published evidence to confirm that dasyurids are susceptible to canine distemper.
Attributing prehistoric extinctions to infectious disease has become almost a cottage industry in the last two decades. Table 1 summarizes some of these proposed disease-induced extinctions. In all cases, the evidence is far from convincing, although several cases are plausible.
Table 1.
Prehistoric extinctions attributed to infectious disease.
| extinction event | hypothesis/argument in favour proposed by authors | objection |
|---|---|---|
| moa [28] | genetic evidence suggests that there were at least 3–12 million moa 1000 years ago and decline occurred before human settlement. Disease introduced by migrant birds from Australia may have caused decline to the approximately 160 000 moa present at the time of human settlement | no direct evidence of the presence of a virulent infectious disease. Why would migrant birds bringing disease have arrived only as recently as 1000 years ago? |
| dinosaurs [29] | endothermic vertebrates are relatively resistant to fungal diseases in comparison with ectotherms and elevated environmental temperature can clear fungal infections in ectotherms. Events at the K-T boundary may have led to a massive fungal proliferation | no direct evidence. Is the assumption that most dinosaurs were ectothermic correct? |
| Neanderthals [30] | after Neanderthals had persisted in Europe for 200 000 years, anatomically modern humans eliminated them 28 000 years ago, after the arrival of modern humans 40 000 years ago. Perhaps modern humans brought in infectious agents with which they had co-evolved in Africa | no direct evidence. 15 000 years of coexistence would appear unlikely if epidemic disease was responsible for Neanderthal extinction |
| Late Quaternary extinctions, especially North American mega-fauna [31] | ‘hyperdisease’ transmitted from humans or their commensals has led to extinction in numerous species. Such a hyperdisease should have one or more reservoir species, potential for causing infection and very high mortality in ‘new hosts’, but not to seriously threaten humans. Large bodied species were particularly vulnerable because low reproductive capacity reduced ability to recover from epidemics | no existing disease meets the necessary criteria and there is no evidence for size-based declines or extinctions in modern multi-species epizootics such as West Nile virus and avian malaria in Hawaii [32]. Rapid extinctions within a few generations of human contact would be expected, but has not been observed [33] |
3. Current extinctions
Disease is listed as a key threatening process for a relatively small proportion of endangered or vulnerable species in the IUCN Red List. In an analysis based on the 2004 Red List, Smith et al. [34] found that of the 833 listed extinctions (animals and plants), only 31 were attributed even in part to infectious disease. In the 2006 Red List, 54 mammal species have infectious disease listed as a threatening process [35]. Mammals threatened by infectious disease are clustered in two orders, with 13 per cent of the 218 artiodactyl species and 5.3 per cent of the 281 carnivores listed as threatened.
However, it is possible that disease may be underestimated as a cause of extinction. For example, Western Australia has recorded a large number of extinctions and dramatic range contractions among mammals since European settlement (of the 72 non-flying mammals present on the Western Australia mainland in 1900, 11 are extinct and 20 have experienced major declines [36]. These declines are usually attributed to predation by European red foxes and domestic cats [37,38]). Abbott [39] describes nearly 50 first-hand reports from between 1840 and 1920 of disease (possibly mange) causing large-scale mortality amongst native mammals and reports declines prior to the arrival of the red fox in Western Australia around 1910 [38]. He also suggests that there is evidence of a spatial trend in declines, consistent with the introduction of disease somewhere in the northwest.
In this paper, I consider two case studies of infectious diseases threatening to cause extinction in Australia, Tasmanian devil facial tumour disease (DFTD) and the amphibian chytrid fungus Batrachochytrium dendrobatidis (hereafter Bd). These are very different diseases in terms of their epidemiology and ecology. Tasmanian DFTD is entirely host-specific and is almost uniformly lethal [40]. It appears to be capable of causing the extinction of Tasmanian devil populations because the rate of transmission depends weakly, if at all, on host population density [41]. The amphibian chytrid fungus, in contrast, has a very broad host range and while it is highly pathogenic in some populations of some frog species [42,43], other species (and even some populations of frogs that have declined as a result of the disease [44]) appear to be able to persist in the presence of the disease with little overt impact on either individuals or at a population level, creating the potential for spillover from tolerant reservoir species even as intolerant species become rare.
What both diseases have in common is that the host–pathogen relationship is very recent in evolutionary time, the amphibian chytrid fungus being apparently a relatively recent introduction worldwide [45] and Tasmanian DFTD being the result of a mutation in a single individual, probably in the last 20 years [46,47]. In both cases, active evolutionary processes are currently underway, and the best prospect for managing disease may well lie in artificially accelerating these evolutionary processes.
(a) Batrachochytrium dendrobatidis
An infectious disease was first suggested in 1996 as the agent responsible for a series of declines and extinctions in stream-dwelling frogs in Queensland [48], although no agent had been identified and the suggestion was highly controversial at the time [49,50]. Several years later, the amphibian chytrid fungus Bd was described, shown to be highly pathogenic in some frogs and suggested as the agent responsible for these declines [51,52]. Since then, it has become increasingly clear that the fungus is a primary causative agent for widespread amphibian declines in Australia [53,54], Central America [42,55,56] and North America [57–59].
Nevertheless, the situation is substantially more complex than simply arrival of an exotic pathogen leading to widespread declines and extinctions. Some frog species are clearly highly susceptible to the disease, whereas others are relatively tolerant [60–62]. This much falls well within the standard paradigms of disease-induced extinction: the existence of reservoir hosts within which the disease has limited effect enables a strong force of infection to be maintained on to highly susceptible and intolerant species, even as their population size declines towards extinction [9]. What is more puzzling is that some populations of particular species appear able to persist with the disease, whereas other populations of the same species decline to extinction [63]. Temperature certainly plays an important role. As a parasite of a poikilothermic host, it is not surprising that the growth rate of Bd is strongly influenced by environmental conditions. In culture, the fungus grows fastest at temperatures between 10°C and 23°C and it can be eliminated from frogs by exposing them to temperatures in excess of 30°C [64]. Several Australian tropical frog species have disappeared at altitudes over 1000 m, but persist at lower altitudes [65]. Environmental temperature is an important predictor of both the distribution of the fungus in Australia and the extent to which it has caused population declines [66].
Relatively little is known about the transmission dynamics of Bd. An experiment using caged Rana muscosa tadpoles in infected natural lakes [67] showed that transmission occurred from the water in the absence of close contact with infected individuals, but that transmission increased with increasing density of infected tadpoles in the cages. The standard compartmental SIR framework of most infectious disease models is insufficient to adequately understand the dynamics of the disease, as the intensity of infection determines the mortality rate of individual frogs and intensity is highly variable within infected populations, both seasonally and at any particular point in time. Unlike typical microparasites, Bd does not multiply rapidly within an individual host. The life cycle of the fungus involves growth within the epidermis of the frog, followed by external release of zoospores [68]. These may reinfect the same host, but the extent to which this happens is likely to depend on whether the frog is in water or, if not, the humidity and microclimate occupied by the frog at the time of zoospore release. The dynamics of the parasite therefore share some features of ‘macroparasite’ models developed by Anderson & May [3], in which the parasite burden and distribution within the host population are of critical importance. Recent work by Briggs et al. [68] suggests that understanding this feature of the dynamics may be critically important in understanding how the pathogen can display both endemic and epidemic behaviour.
(b) Tasmanian devil facial tumour disease
Tasmanian DFTD is an infectious cancer threatening to cause the extinction of the largest surviving marsupial carnivore, the Tasmanian devil Sarcophilus harrisii [40,46,69]. The tumour cells themselves are the infectious agent [70], an aetiology shared in nature by only one other cancer, canine transmissible venereal tumour [71,72]. DFTD is spread by biting, which occurs frequently in sexual encounters and disputes over food. Tumour cells appear not to be rejected by recipient devils because of the very low genetic diversity in devil populations, particularly even in the major histocompatibility complex (MHC), to the extent that cells from another devil are not recognized as ‘foreign’ [47,73], although recent work suggests that the tumour cells also have some ability to evade the host's immune system [74]. Since its first appearance in northeastern Tasmania in 1996, the infection has spread across most of the range of Tasmanian devils, causing population declines of up to 90 per cent in affected populations [69]. The disease manifests as large tumours, particularly around the head of the affected animal, and is inevitably fatal once clinical signs are apparent [75,76]. In populations with MHC types similar to the tumour [73], prevalence increases rapidly after the initial arrival of the disease, affecting the majority of the adult population within 3–4 years (figure 3). Prevalence remains very high (in excess of 50% in susceptible age classes) even after major population declines (figure 4), suggesting that the disease has a very low density threshold [41]. Although no local population has yet completely disappeared, the disease could drive populations to low levels where individuals cannot find mates or stochastic extinction becomes inevitable.
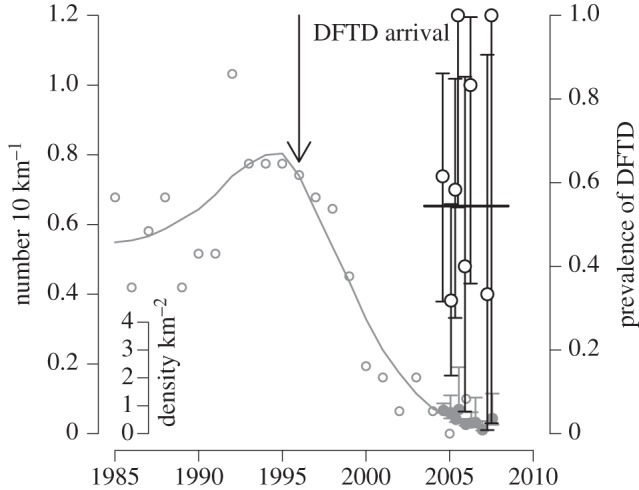
Tasmanian devil population size and DFTD prevalence in northeastern Tasmania. The grey open circles and the grey line are the number of Tasmanian devil sightings from spotlight counts in the northeastern region of Tasmania, with a locally weighted regression fit, scaled on the main left hand axis. Data from [69]. The solid grey circles with CIs, scaled on the inset axis, are the population density from a mark recapture study at Mt William, the national park in northeastern Tasmania where DFTD was first observed. The open black circles are of the prevalence of DFTD in adult devils captured during the mark recapture study, with corresponding exact binomial 95% CIs. The solid line is the mean prevalence of DFTD throughout the study. Data from [41].
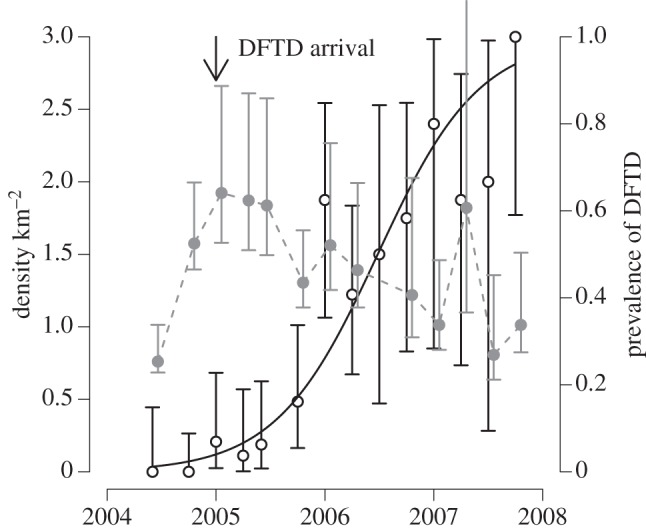
Tasmanian devil population size and DFTD prevalence at Fentonbury, central Tasmania, where monitoring commenced just before the time of disease arrival in January 2005. The solid grey circles with CIs are the population density from a mark recapture study, scaled on the axis at the left of the figure. The open black circles are the prevalence of DFTD in adult devils captured during the mark recapture study, with corresponding exact binomial 95% CIs, and the solid black line is the best fit logistic model of prevalence as a function of time. Data from [41].
4. Predicting the emergence of extinction threatening diseases
One clear lesson from both the amphibian chytrid fungus and Tasmanian DFTD is that the early detection of a disease capable of causing host extinction is critical in managing disease threats [40]. If action to manage disease is delayed until unequivocal evidence of the threat is obtained, it is likely to be too late. Two questions arise. First, are there characteristics of some wildlife populations that are likely to make them particularly susceptible to emerging disease threats? Second, what are the signs that declines may be attributable to an infectious disease?
(a) Identifying particularly susceptible populations
Almost all cases of disease-threatening extinction are a result of a host encountering a pathogen to which it has had no previous exposure in evolutionary time. We clearly need to be particularly concerned with ‘pathogen pollution’ [77,78], in which pathogens are introduced into naive populations or communities. Populations in habitats that have been relatively isolated until recently are therefore likely to be particularly at risk from disease. Habitat fragmentation and ecological transformation are also likely to bring susceptible host populations in contact with novel pathogens [79]. Although DFTD is a counter example, most pathogens threatening to cause extinction have one or more disease-tolerant reservoir hosts that can maintain a high force of infection even as the threatened host declines towards extinction. Low genetic diversity in host populations not only may make them initially more susceptible to disease, but also reduces their potential to respond evolutionarily.
(b) Identifying incipient disease threats
High prevalence of a parasite in a declining population or among morbid individuals is not in itself evidence that the pathogen is responsible for decline. In fact, parasites that cause little mortality are more likely to occur at high prevalence than those that are highly pathogenic [9]. Other criteria that indicate a pathogen may be responsible for population decline are needed. A possible approach is to apply the Bradford Hill criteria [80], which have also been suggested as being useful in understanding the emergence of infectious wildlife diseases [81]. Bradford Hill developed nine criteria to suggest that environmental factors have a causal relationship to human disease. He discussed these primarily in relation to smoking as a cause of lung cancer, a non-infectious disease causation, but they can be applied in other contexts. The criteria (table 2) are essentially a series of circumstantial associations, which suggest a causal relationship if several are satisfied.
Table 2.
The Bradford Hill criteria for establishing causation, explained in the context of identifying pathogen related conservation threats, as applied to chytridiomycosis (Bd) and/or Tasmanian DFTD.
| no. | criterion | explanation | application |
|---|---|---|---|
| 1 | strength of association | should expect substantial differences in rate of population decline depending on whether the pathogen is present or absent | clearly the case for both Bd and DFTD |
| 2 | consistency | the association between a pathogen and population decline should be repeatedly observed in different places and circumstances and by different observers | clearly fulfilled by Bd, which has been associated with population declines in many species and on several continents |
| 3 | specificity | declines should occur only in association with the postulated disease agent. (One of the more problematical of Hill's criteria: he suggests that if the specificity criterion is fulfilled, it is indicative, but if it is not fulfilled, it is not strong evidence against a particular causation) | a frog population declining because of habitat destruction is not evidence that others are not declining due to Bd. Nevertheless, if there were widespread amphibian population declines in otherwise pristine environments in the absence of Bd, this would indicate that other causes for such declines needed to be investigated |
| 4 | temporality | the putative action of the cause should not precede its presence. | extensive investigation of museum specimens has failed to identify Bd in frog populations in Australia and the Americas prior to broad-scale population declines. For DFTD, devil populations had not been declining prior to disease emergence |
| 5 | biological gradient | there should be something resembling a dose response curve | difficult to establish at a population level in wildlife. If a population has been monitored through time, the rate of population decline should be associated with disease prevalence (as is the case with DFTD), but one would not necessarily expect to see prevalence of Bd across sites and species being associated with rate of decline |
| 6 | plausibility | the causation should make biological sense | both DFTD and Bd cause mortality in individuals |
| 7 | coherence | essentially the converse of plausibility: the proposed causation should not conflict with knowledge of the ecology of the host and parasite | |
| 8 | experiment | experimental reduction in the prevalence of the infectious agent or of its effects on individual hosts should produce a response at the population level in comparison with control populations | |
| 9 | analogy | does the same or a similar parasite produce population impacts in other species, other habitats or at other times? |
The criteria may even be of use in suggesting the influence of infectious disease in population decline, without having identified a putative disease agent. Laurance et al. [48,82] postulated that an infectious disease was responsible for precipitous declines in several frog species in tropical and subtropical Australia. At that time, no agent was suggested. Of their lines of evidence (many of which were refuted at the time [49,50]), there was limited experimental evidence that some mortality-causing agent could be transferred in water (criterion 8), evidence that declines were widespread in similar habitats (criterion 2), evidence that moribund individuals could be collected from affected populations (criterion 6) and a suggestion of a wavelike progression of extinctions from south to north (perhaps evidence supporting criterion 4). Their final line of evidence, ‘absence of plausible alternatives’, could perhaps be aligned with criterion 7.
5. Managing extinction-threatening diseases
The options available to manage extinction-threatening diseases in wild populations are limited [83,84]. They can broadly be considered as: (i) isolating uninfected populations from disease; (ii) culling either infected animals only or all animals to reduce the force of infection; (iii) reducing transmission by habitat modification to reduce foci of infection; (iv) genetic management to spread resistance alleles within the host population; and (v) vaccination. Treating individuals will rarely be practical. A fallback option to preserve some individuals of species threatened with extinction is to bring them into captivity, with the possibility of reintroduction following extinction in the wild, if the disease problem has been solved.
(a) Isolating infected populations
In almost all cases, an extinction-threatening disease will be a relatively recent introduction into the population under threat: otherwise, that population would no longer exist. Preventing further spread of infection into currently unaffected populations or establishing new free-ranging populations in areas that cannot be reached by disease is an obvious management strategy. For chytridiomycosis, bioclimatic models have an important role to play in identifying currently uninfected areas into which the disease could be expected to spread [66]. On a global level, these include Madagascar, Borneo and New Guinea [85]. Concerningly for the latter two, the fungus has recently been isolated from Indonesia [86].
For Tasmanian DFTD, two possible ways of isolating uninfected free-ranging populations are introducing devils to offshore islands currently unoccupied by devils and fencing off peninsulas in western Tasmania, which the disease has yet to reach [87]. Fencing to prevent disease spread will inevitably be extremely difficult when potential hosts live on either side of the fence, although there has been some success of this strategy in preventing foot and mouth disease spread in South Africa [88]. Proposals to establish disease-free populations of Tasmanian devils on offshore islands have proved surprisingly controversial [87], it appears because of a deep-seated reluctance to undertake ex situ translocations. This is despite the success of similar strategies in protecting the related northern quoll from cane toads in Australia's Northern Territory [89] and increasing suggestions that ex situ translocations may be necessary to preserve species from climate change threats [90–92].
(b) Culling
Culling of all individuals that have potentially been in contact with disease is a standard approach for controlling incursions of exotic diseases into livestock populations (for example, foot and mouth disease [93]). However, it is unlikely to often be appropriate for control of extinction threatening diseases in wildlife populations. In principle, rapid ‘stamping out’ would be applicable to control the incursion of a known highly pathogenic disease into a population of conservation significance, provided it could be done early enough. However, in the cases of both DFTD and Bd, disease was widely spread in most affected populations before the extinction threat was confirmed so that stamping out would be more likely to lead to extinction than to prevent it. For a pathogen affecting multiple host species, non-selective culling of a non-endangered reservoir species may be an appropriate means to limit disease transmission into an endangered species. For example, North American bullfrogs (Rana catesbeiana) have frequently become feral in South America, Europe and Japan, where they consistently carry Bd, with the potential to transmit the pathogen to endangered native anurans [94]. Although there is limited evidence that it has been effective, non-selective culling of bullfrogs to control Bd has been proposed [95]. Culling of grey squirrels, which are a reservoir for a pox virus that threatens red squirrel populations, has also been suggested as a strategy to maintain red squirrel populations [96]. Non-selective culling of hosts can contribute to disease control in two potential ways. First, if it is intense, it may increase mortality of infected hosts (even though they are not specifically targeted) sufficiently to reduce R0 to below one. Second, non-selective culling can reduce host density and thus disease transmission. This effect relies on the shape of the relationship between host density and transmission [8]. There can, however, be unexpected results of culling, particularly in territorial species, which may increase movements following culling. For example, there is evidence that culling at some spatial scales can lead to an increase in bovine tuberculosis in both badgers and cattle in the United Kingdom [97,98].
An alternative culling strategy is to remove infected individuals only, often referred to as ‘test and cull’ in the veterinary literature. This approach is immediately more appealing than ‘stamping out’ when dealing with infectious disease in an endangered species. However, it has rarely been successful in practice. An attempt was made to control DFTD on the Tasman Peninsula, which is connected to the Tasmanian mainland only by a narrow isthmus cut by a canal. Frequency-dependent transmission makes control by culling more difficult than if transmission is density-dependent, but in other respects, DFTD is a disease one might expect to be amenable to this control strategy. Devils are highly trappable, clinical disease is apparent on external examination and it is likely that transmission occurs primarily from individuals with large, visible tumours. Unfortunately, the trial was not successful and has now been terminated. There was no evidence that the rate at which uninfected individuals acquire disease has been decreased by the removal since late 2004 of all infected devils captured [99]. Models [100] suggest that it is unlikely that any feasible culling strategy would have been successful: more than 90 per cent of infected animals would need to be removed during each trapping bout, which cannot be achieved in practice. These models assumed that culling commenced very early in the epidemic and that transmissibility and detectability occurred after the same time delay (i.e. the incubation and latent periods were of the same length). A test that enabled the identification and removal of infected animals before they become infectious would increase the likelihood that a test and cull strategy would be successful. ‘Test and cull’ has also not proved to be successful in controlling brucellosis in bison populations in the USA [101], and modelling suggests that culling sufficient to eliminate chronic wasting disease in deer would probably cause extinction of the deer populations [102].
(c) Genetic management
Natural selection on hosts in response to a highly pathogenic emerging infectious disease can be expected to be very strong. Selection can be expected to occur for either increased resistance (hosts are less likely to acquire infection when exposed) or increased tolerance (hosts still become infected, but with fewer effects on mortality or fecundity), or both. Provided sufficient genetic variation in susceptibility to the disease exists in populations under threat, the host population should become progressively more resistant or tolerant. For example, in the case of myxoma and Australian rabbits, case fatality from the original myxoma strain in an area with annual epizootics decreased from 99 per cent to less than 70 per cent within a decade of the introduction of the pathogen [103], indicating increased tolerance. In comparison with most species likely to be threatened with emerging infectious disease, rabbits have very high reproductive output, permitting rapid response to selective pressure. Nevertheless, the rabbit–myxoma example is not unique. Hawaiian Amakihi Hemignathus virens, a bird species that originally declined following the introduction of avian malaria, now are increasing in numbers at low altitudes despite high levels of infection by Plasmodium relictum, strongly suggesting that they have evolved tolerance of the parasite [21].
If this process of evolution of resistance or tolerance in the host could be accelerated or facilitated, it would represent a powerful means to control emerging disease threats. In the case of Tasmanian devils, the cancer has been able to spread through populations in the eastern part of the island because of extraordinarily low MHC diversity [47], such that recipient devils are unable to recognize tumour cells from another animal as ‘non-self’. In the northwest of the island, which is only now being invaded by the tumour, there is a greater variety of MHC types [73]. Furthermore, there is evidence that the epidemiology of the tumour is different in the northwest from eastern Tasmania, with slower increase in prevalence through time and fewer effects on the age structure of the devil population [104]. However, it is unclear at this stage whether this is related to differences in the host population or differences in tumour strain. If northwestern populations do contain alleles resistant to the tumour, then it may be possible to introduce animals with these genotypes into the currently depleted east coast populations.
It may also prove possible to manage for genetic resistance to chytridiomycosis. Some populations of frogs that were apparently brought close to extinction when chytridiomycosis was first detected now appear to coexist with the fungus with little detectable impact on survivorship [44]. However, Briggs et al. [68] have shown both from empirical evidence and the modelling that some populations of the same species can coexist with Bd whereas others are driven to extinction, without there necessarily being any difference in innate susceptibility between the populations. They suggest that this is a result of differences in parasite load between populations, as a result of differing population density and differences in self-reinfection rates.
An intriguing possibility for genetic management of Bd is that some frog species excrete antimicrobial peptides that prevent establishment of Bd [105]. In principle, it might be possible to use genetic modification techniques to insert genes producing these peptides into susceptible species. This would raise a series of ethical questions about the appropriateness of using genetic modification on endangered species, which then would be released into the wild. One can imagine that spectre of ‘Frankenfrogs’ might be raised.
An option that is less likely to be successful is genetic management of the pathogen population. It is certainly the case that selective pressure can act to reduce the pathogenicity of emerging diseases: for example, the dominant strain of myxomatosis in Australia changed from a case fatality rate of 99 per cent in 1950–1951 to a case fatality rate between 70 and 95 per cent in 1975–1981 [103] (see also [106]). This has usually been thought of as a result of a trade-off between virulence and transmissibility [107]: a parasite that kills its host too quickly has less opportunity to transmit. Furthermore, when a pathogen is first introduced into a naive population, there may be transient selection for high virulence in the initial epidemic phase compared with the final endemic state [106]. However, developing strategies for virulence management of pathogens has proved elusive [108,109].
(d) Habitat modification
Wobeser [83] suggests that habitat modification to interfere with disease transmission is ‘potentially of great value’ in managing diseases of wild animals. As is the case with human diseases (for example, draining swamps to limit malaria transmission), this is likely to be of most value when dealing with infectious diseases transmitted by vectors.
(e) Vaccination
Delivery of an oral rabies vaccine through baits has been effective in controlling rabies infection in foxes in Europe [110] and raccoons in the USA in order to safeguard human health [111]. Targeted delivery of rabies vaccine by injection to Ethiopian wolves (Canis simensis) in habitat corridors between subpopulations has also proved effective in limiting the size of rabies epidemics in this endangered species [112]. Rabies is, however, a rather unusual case in that its impact on human health means that there has been considerable investment in developing a vaccine. There has been some success in vaccinating American robins against West Nile virus [113], another wildlife disease with serious zoonotic effects, although this was not done with a conservation aim. Developing vaccines for pathogens that affect only wildlife is likely to be prohibitively expensive in most cases. There has been research into developing a vaccine for DFTD [114,115], but it has yet to result in an effective vaccine.
Some microbes are effective in limiting mortality and morbidity from Bd in captive populations [116], and it has been proposed that bioaugmentation of such microbes might be used to control chytridiomycosis [117]. While this is not vaccination in the usual sense of the word, functionally it is quite similar. Potentially, these microbes might be self disseminating in wild populations, although effects on non-target species would need to be considered.
6. Conclusion
It is relatively unusual for infectious disease to be the sole cause of endangerment for a species [34]. In most cases, there will be multiple stressors contributing to decline, such as habitat destruction and fragmentation or overexploitation. Chytridiomycosis is unusual in that it has led to declines in frog species in otherwise pristine environments. Although there have been suggestions that climate change has been responsible for increasing the impact of the disease in central and South America [118], this hypothesis has not stood up to detailed analysis [119]. Tasmanian DFTD is threatening a species that, until the appearance of disease, appeared to be secure and increasing in numbers. However, extremely low genetic diversity, possibly as a result of a previous selective sweep [47,73], has predisposed the species to be susceptible to an allograft.
Loss of genetic diversity and pathogen pollution are increasing, with concomitant increase in risk of novel disease threats. The effects of anthropogenic climate change on infectious diseases are complex [120], but climate change is likely to lead to disease emergence in at least some cases [121], including through host range shifts and changes in migration patterns [122]. While approaches to address these threats are being developed, the range of tools currently available is limited. There is an urgent need both to address the factors likely to cause disease emergence in wildlife populations and to develop new approaches to manage the disease threats to biodiversity that will inevitably arise in the future.
Acknowledgements
I am grateful to the organizers of the joint Zoological Society of London—Royal Society symposium ‘Disease Invasion’ for the invitation to present at the meeting. This research has been supported by grants from the Australian Research Council (DP0772644, LP0561120 and DP110102656).
References
Articles from Philosophical Transactions of the Royal Society B: Biological Sciences are provided here courtesy of The Royal Society
Full text links
Read article at publisher's site: https://doi.org/10.1098/rstb.2012.0224
Read article for free, from open access legal sources, via Unpaywall:
https://royalsocietypublishing.org/doi/pdf/10.1098/rstb.2012.0224
Citations & impact
Impact metrics
Article citations
Pitfalls and windfalls of detecting demographic declines using population genetics in long-lived species.
Evol Appl, 17(7):e13754, 14 Jul 2024
Cited by: 0 articles | PMID: 39006005 | PMCID: PMC11246600
Virome of Australia's most endangered parrot in captivity evidenced of harboring hitherto unknown viruses.
Microbiol Spectr, 12(1):e0305223, 04 Dec 2023
Cited by: 2 articles | PMID: 38047696 | PMCID: PMC10783009
Seroprevalence of canine distemper virus (CDV) in the free-roaming dog (Canis familiaris) population surrounding Chitwan National Park, Nepal.
PLoS One, 18(2):e0281542, 27 Feb 2023
Cited by: 2 articles | PMID: 36848365 | PMCID: PMC9970093
Viruses of Atlantic Bonefish (Albula vulpes) in Florida and the Caribbean show geographic patterns consistent with population declines.
Environ Biol Fishes, 106(2):303-317, 05 Aug 2022
Cited by: 6 articles | PMID: 35965638 | PMCID: PMC9362051
Impact of multiple small and persistent threats on extinction risk.
Conserv Biol, 36(5):e13901, 05 May 2022
Cited by: 3 articles | PMID: 35212024 | PMCID: PMC9790556
Go to all (48) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
BIODIVERSITY. Averting a North American biodiversity crisis.
Science, 349(6247):481-482, 01 Jul 2015
Cited by: 53 articles | PMID: 26228132
The ecology and impact of chytridiomycosis: an emerging disease of amphibians.
Trends Ecol Evol, 25(2):109-118, 14 Oct 2009
Cited by: 189 articles | PMID: 19836101
Review
Parallels in amphibian and bat declines from pathogenic fungi.
Emerg Infect Dis, 19(3):379-385, 01 Mar 2013
Cited by: 11 articles | PMID: 23622255 | PMCID: PMC3647649
Review Free full text in Europe PMC
Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction.
Ecology, 90(12):3379-3392, 01 Dec 2009
Cited by: 129 articles | PMID: 20120807