Abstract
Free full text

Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML
Associated Data
Abstract
A hallmark of cancer is the disruption of differentiation within tumor cells. Internal tandem duplication mutations of the FLT3 kinase (FLT3/ITD) occur commonly in acute myeloid leukemia (AML) and are associated with poor survival, leading to efforts to develop FLT3 kinase inhibitors. However, FLT3 inhibitors have thus far met with limited success, inducing only a clearance of peripheral blasts with minimal BM responses. Quizartinib is a novel potent and selective FLT3 inhibitor currently being studied in clinical trials. In 13 of 14 FLT3/ITD AML patients with normal karyotype treated with quizartinib, we observed terminal myeloid differentiation of BM blasts in association with a clinical differentiation syndrome. The single patient whose blasts failed to differentiate had a preexisting C/EBPα mutation and another developed a C/EBPα mutation at disease progression, suggesting a mechanism of resistance to FLT3 inhibition. In vitro, in primary blasts cocultured with human BM stroma, FLT3 inhibition with quizartinib induced cell-cycle arrest and differentiation rather than apoptosis. The present study is the first description of terminal differentiation of cancer cells in patients treated with a tyrosine kinase inhibitor. These data highlight the importance of the differentiation block in the patho-genesis of AML.
Introduction
A characteristic of cancer cells is their ability to avoid terminal differentiation,1 and acute myeloid leukemia (AML) has been recognized as representing a paradigm for this phenomenon.2 Disruption of a differentiation-inducing transcription factor can be identified in more than one-third of newly diagnosed cases of AML.3–6 Although carcinogenesis is generally believed to be a process requiring multiple mutations, some have proposed that AML develops from a central core of 2 classes of mutations, one blocking differentiation and the other promoting proliferation via effects on growth factor pathways.7 Internal tandem duplication mutations of the FLT3 receptor tyrosine kinase (FLT3/ITD), one of the most common mutations in AML,8 constitutively activate this cytokine receptor and are members of the “growth factor” class in this 2-step model.
Because FLT3/ITD mutations confer a negative prognostic effect on the clinical outcome of AML, FLT3 tyrosine kinase inhibitors (TKIs) have been investigated as a potential targeted therapy for this disease. Clinical responses to FLT3 inhibitors were initially confined to clearance of peripheral blasts with minimal effects on the BM.9–13 However, the use of more potent FLT3 inhibitors such as sorafenib and quizartinib has resulted in a higher rate of BM blast reduction in FLT3/ITD AML patients.14
In an exploratory group of relapsed/refractory FLT3/ITD AML patients treated with quizartinib (as part of a phase 2 trial), a remarkably high clinical response rate of 71% was reported recently.15 The BM and peripheral blood specimens from these patients, particularly those with normal cytogenetics, displayed an unexpected finding. Instead of the typical rapid loss of cellularity seen after conventional cytotoxic chemotherapy, the BM remained hypercellular, continued to express the FLT3/ITD mutation, and displayed progressive myeloid differentiation over time. This is similar to the response of acute promyelocytic leukemia (APL) to all-trans retinoic acid (ATRA), in which a cellular BM and persistence of the leukemia-specific mutation PML-RARα is observed as the ATRA promotes differentiation from immature leukemic promyelocytes to mature neutrophils.16
To study this phenomenon further, we developed an in vitro model of FLT3 inhibitor–induced differentiation using coculture of leukemia cells with human BM stroma. We conclude that in FLT3/ITD AML, aberrant signaling from the mutant FLT3 receptor is responsible for the block in differentiation that is so characteristic of this disease. Previous in vitro data suggested a potential role of FLT3/ITD in myeloid differentiation.17,18 In the present study, we provide the first evidence that TKI therapy induces terminal differentiation of human cancer cells in vivo. Our data provide new insight into the role of tyrosine kinases in the pathogenesis of cancer and help to define the clinical and biologic consequences of FLT3 inhibition in FLT3/ITD AML.
Methods
Patients
This is a laboratory and clinical correlative study using BM and blast samples from a subset of patients enrolled on protocol AC220-002 (National Cancer Institute clinical trial no. NCT00989261). AC200-002 is a phase 2 multicenter, international study of quizartinib administered as monotherapy to patients with relapsed or refractory FLT3/ITD AML (as well as a smaller cohort of patients with wild-type FLT3) that accrued 333 patients. Although interim results from a safety subset of patients from this trial have been presented,15 final analysis of the clinical data are not yet available. This correlative study is based on 28 patients with FLT3/ITD mutations meeting eligibility requirements who were accrued to the trial at 2 institutions. In addition, patient blasts were collected and banked separately as part of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Tumor and Cell Procurement Bank, supported by a Regional Oncology Research Center Grant (2 P30 CA 006973-44). Whole blood and BM aspirates from healthy donors were also collected under this procurement protocol. All patients gave informed consent in accordance with the Declaration of Helsinki.
Clinical specimens
BM aspirates were collected on days 0, 15, and 29. Slides were fixed in methanol and stained with Wright-Giemsa stain (Harleco/VGD). A 100-cell manual differential was performed using standard clinical protocols. Unstimulated cultures of BM were performed overnight and slides were prepared. G-banding was carried out according to standard clinical protocols. Twenty metaphases were analyzed for each case. No additional growth factors were used for conventional cytogenetic analysis. For analysis of cell morphology, cytospins were prepared using 1 × 105 cells, which were centrifuged onto a glass slide and then fixed and stained with modified Wright-Giemsa stain (Sigma-Aldrich). Cells were analyzed by light microscopy using an Olympus BX41 clinical microscope. Photographs were taken using an attached Olympus DP72 digital camera with Olympus cellSens Version 1.3 software. For isolation of neutrophils, whole blood was centrifuged over a layer of Ficoll-Paque PLUS (GE Healthcare), and then the monolayer was extracted, overlaid onto Histopaque (Sigma-Aldrich), and centrifuged a second time. The monolayer was then isolated, washed, and cytospins were prepared. Light microscopy was used to confirm that the isolated cells were more than 95% neutrophils.
Cell culture and reagents
All cell lines and primary blast samples were cultured as described previously.19 Molm14 cells were obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). Sorafenib and quizartinib were dissolved in DMSO at stock concentrations of 10mM. Quizartinib was supplied by Ambit Biosciences. Sorafenib was obtained from LC Laboratories.
BM stroma coculture
Leftover BM from healthy donor harvests was collected, resuspended in RPMI medium (Invitrogen), and cells were centrifuged over a layer of Ficoll-Paque PLUS (GE Healthcare). Mononuclear cells were collected, washed twice with RPMI medium, and counted (Beckman Coulter). Cells were resuspended in stroma medium consisting of RPMI medium, 10% FBS, 1% L-glutamine, 1% penicillin-streptomycin (both Invitrogen), and 1μM hydrocortisone (Sigma-Aldrich). Cells were then plated into a 75-cm2 tissue culture flask with an average number of 50 × 106 cells/flask and were grown at 33°C in 5% CO2. After 24-48 hours, medium containing nonadherent cells was removed and replaced with 20 mL of fresh stroma medium. Cells were cultured until 95%-100% confluence was reached. Cultures were passaged every 4-8 weeks after harvesting with 0.25% trypsin-EDTA (Sigma-Aldrich). For BM stroma coculture experiments, stromal cells were plated into 6-well plates (BD Biosciences) and allowed to grow until 95%-100% confluence was reached. Adherent cells were then washed with PBS 3 times and medium was replaced with culture medium. Molm14 cells or primary patient blasts were then added to the culture medium and plates were incubated at 37°C in 5% CO2.
DNA isolation and sequencing
DNA was isolated from primary patient blasts using the DNeasy Mini Kit (QIAGEN). DNA sequencing, PCR for FLT3, and estimation of FLT3 mutant allelic burden were performed as described previously.19 Direct sequencing of the PCR products covering the entire C/EBPα coding sequence was with primers described previously.20,21
Flow cytometry
Flow cytometric immunophenotyping was performed as described previously22 on primary patient blasts grown on stromal coculture in the presence or absence of quizartinib. For cell-cycle experiments, cells were seeded in 6-well plates containing a confluent stromal cell monolayer at a density of 2 × 106 suspension cells/mL and incubated with or without quizartinib. After 24 hours (Molm14 cells) or 48 hours (primary patient blasts), cells were harvested and analyzed for propidium iodide staining, as described previously.23
Immunoblotting
Electrophoresis and immunoblotting for FLT3 and ERK were performed as described previously.19 Abs to MMP-9 and to lactoferrin were obtained from Cell Signaling Technology. Anti–C/EBPα and anti–phospho-C/EBPα Abs were obtained from Santa Cruz Biotechnology and Cell Signaling Technology, respectively.
NBT reduction
For nitroblue tetrazolium (NBT) reduction assays, reagents were obtained from Sigma-Aldrich. Whole blood taken from quizartinib-treated patients and healthy controls was collected in heparinized tubes and incubated with NBT solution with or without stimulant solution (bacterial extract) per the manufacturer's instructions. Blood smears were prepared using the manual wedge method and were stained according to the manufacturer's instructions. Cells treated with quizartinib in vitro were also incubated as described above, cytospins were prepared as described under “Clinical specimens,” and staining was performed per the manufacturer's instructions.
C/EBPα knockdown
For C/EBPα knockdown experiments, Silencer Select C/EBP-α siRNA and the Amaxa Nucleofector V kit were used (Invitrogen). For each sample, 2 million cells were incubated with 320pM siRNA (C/EBP-α or scrambled control) and electroporated using program T-019. Cells were lysed after 24 and 48 hours for analysis of C/EBPα protein levels by Western blotting.
Results
Peripheral blood and BM responses to quizartinib
AC220-002 is a multicenter, international phase 2 study of quizartinib administered as a single agent to adult patients with relapsed or refractory AML. Although the trial has completed accrual and interim clinical results from a safety subset of patients have been presented in abstract form,24 final clinical results are not yet available. The primary outcome of the trial was composite complete response rate (complete remission plus complete remission with incomplete platelet recovery plus complete remission with incomplete count recovery) with a secondary outcome of survival. Results regarding the primary outcomes (eg, overall remission rates and survival) will be presented elsewhere. The data presented herein are derived from a laboratory correlative study of patients enrolled at 2 centers.
AML is a molecularly heterogeneous disease. Patients are classified for prognostic purposes according to both cytogenetic and molecular risk groups and FLT3/ITD AML, which has a diploid karyotype, represents a unique category that has been well characterized from a prognostic standpoint.25,26 Of the 28 patients enrolled at the 2 centers in the present study, 17 had normal diploid cytogenetics, 7 had an intermediate-risk karyotype, and 4 had a poor-risk/complex karyotype according to conventional classification.26 Because we noted the differentiation effect occurring predominantly in those patients with diploid karyotype, and because FLT3/ITD mutations are most closely associated with a normal karyotype (in non-M3 AML),8,27 we restricted our evaluation to include only these patients. We had genomic DNA samples, BM aspirates, and BM core biopsies from pretreatment, day 15, and day 29 time points available from 14 of the 17 patients. These 14 patients (with FLT3/ITD mutation, diploid karyotype, and samples available for correlative studies) represent the study set for this laboratory correlative evaluation. The treatment regimens given to the 14 patients are summarized in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patients treated with quizartinib displayed rapid clearance of peripheral blasts, which is consistent with responses observed with other FLT3 inhibitors.9,10,12,13 However, although neutrophil levels tended to be very low in these patients at the start of treatment, we noted a surge of neutrophils in the peripheral blood after several weeks of therapy (Figure 1A) in 13 of the 14 study subjects. This increase in the absolute neutrophil count typically peaked around day 40 and then decreased again (Table 1), remaining below 1000/mm3 for the duration of the trial. Although formal outcome results from this trial are not yet available for publication, the patients experiencing this surge of neutrophils typically achieved a complete remission with incomplete count recovery, as reported in the interim results.15 In general, the peripheral blood neutrophils circulating in these patients during this period appeared morphologically normal (Figure 1B). In parallel with this neutrophil surge, several patients developed fever and inflammatory infiltrates in the lungs, soft tissue, or skin. No infectious etiologies were identified, and treatment with steroids led to their rapid resolution. Biopsy of the skin lesions revealed neutrophilic lobular panniculitis, consistent with Sweet syndrome. This finding can occasionally be observed in patients with APL treated with ATRA.28 In general, the fevers and steroid-responsive lung and skin nodules that accompanied the neutrophil surge were reminiscent of retinoic acid differentiation syndrome.29
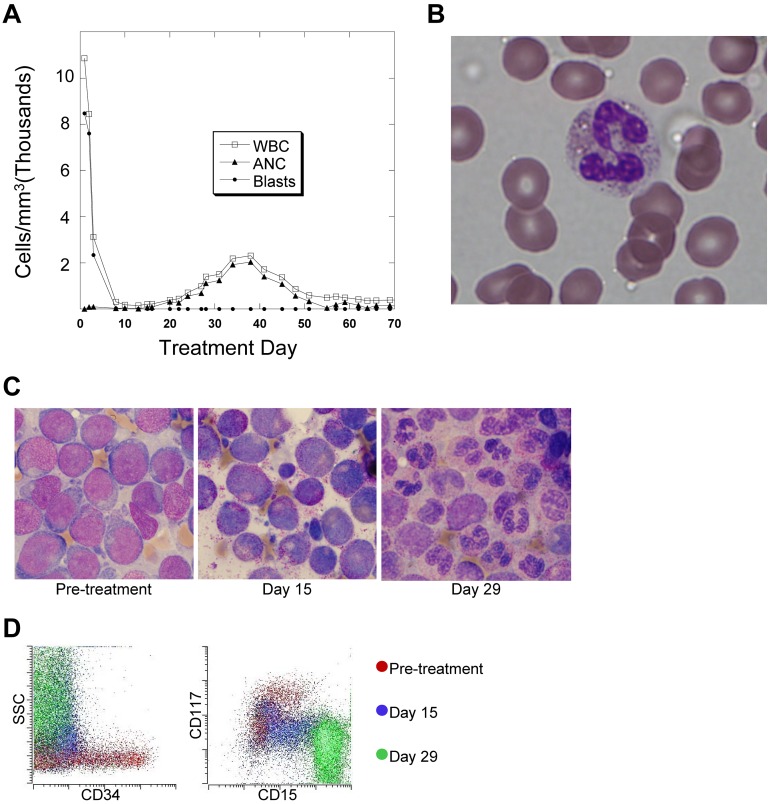
In vivo terminal myeloid differentiation in patients treated with quizartinib. (A) Graph of peripheral total WBC count, absolute neutrophil count (ANC), and absolute blast count in a representative patient (Table 1 patient 2) during the first 2 months of treatment with quizartinib. (B) Photomicrograph of a peripheral blood neutrophil from a whole blood sample collected from the patient shown in panel A on day 35 of treatment. (C) BM aspirates collected from a representative patient (Table 1 patient 7) 1 week before and on days 15 and 29 of treatment with quizartinib. (D) Flow cytometry analysis of BM aspirate specimens collected pretreatment and on days 15 and 29 from patient 7. The analysis was performed on the day of collection, and the dot plots are overlaid, organized by color.
Table 1
Clinical characteristics and peripheral blood neutrophil and blast counts for patients treated with quizartinib
| Patient | Age/Sex | Karyotype | RL/RF/PR | Baseline ANC | Peak ANC | Days to peak ANC | Baseline PB blasts | Blast count at peak ANC |
|---|---|---|---|---|---|---|---|---|
| 1 | 32/M | Diploid | PR | 520 | 3124 | 63 | 35 | 0 |
| 2 | 70/F | Diploid | RL | 0 | 2040 | 38 | 8471 | 0 |
| 3 | 29/F | Diploid | RL | 0 | 280 | 29 | 269 | 0 |
| 4 | 68/M | Diploid | PR | 224 | 3497 | 63 | 3556 | 0 |
| 5 | 55/M | Diploid | RF | 0 | 770 | 36 | 57 825 | 0 |
| 6 | 23/F | Diploid | PR | 100 | 3488 | 36 | 9552 | 0 |
| 7 | 61/M | Diploid | PR | 0 | 2760 | 36 | 64 501 | 0 |
| 8 | 72/M | Diploid | RL | 0 | 720 | 58 | 14 062 | 0 |
| 9 | 54/F | Diploid | RF | 40 | 4500 | 40 | 10 370 | 0 |
| 10 | 64/F | Diploid | PR | 0 | 5290 | 27 | 83 808 | 0 |
| 11 | 56/M | Diploid | PR | 0 | 3560 | 60 | 14 204 | 0 |
| 12 | 33/F | Diploid | RF | 330 | 3350 | 38 | 22 272 | 0 |
| 13 | 62/F | Diploid | RF | 50 | 5540 | 41 | 23 409 | 0 |
| Mean | 97 | 2994 | 43 | 24 026 | 0 | |||
| 14 | 66/F | Diploid | PR | 460 | 200* | 37* | 33 642 | 0* |
All counts are in cells/mm3. “ANC baseline” refers to the lowest absolute neutrophil count (ANC) during the first week of therapy. “ANC peak” refers to the highest ANC observed after the first week of therapy. “PB blasts baseline” refers to the absolute peripheral blood (PB) blast count at the start of therapy. Mean values are shown for patients 1-13.
RL indicates relapsed; RF, refractory to most recent therapy; and PR, primary refractory (ie, patient was refractory to induction therapy at diagnosis).
Based on these observations, we hypothesized that quizartinib was inducing differentiation of BM blasts. BM core biopsies and aspirates were obtained within 2 weeks of treatment initiation and on days 15 and 29 of the first cycle of quizartinib. Most patients began therapy with hypercellular BM (> 70% cellularity). Table 2 compares cellularity between pretreatment and day 29 specimens for the 14 patients. In the majority, there was little or no difference in overall cellularity of the BM among the pretreatment, day 15 (not shown), and day 29 BM samples, indicating that no significant cell killing had occurred throughout the first 4 weeks of quizartinib therapy. However, in 13 of 14 patients, there were striking changes in the cellular morphology. Examination of day 15 aspirates revealed that the majority of the BM cells were myelocytes, and by day 29 most were mature-appearing neutrophils. An example is shown in Figure 1C (from patient 2). For the 13 patients who exhibited differentiation, the mean BM cellularity was 75% before treatment and 68% on day 29, whereas blasts decreased from 77% to 6% over the same time period, and maturing myeloid cells (ie, myelocytes, metamyelocytes, and neutrophils) increased from 9% to 57% (Table 2). Interestingly, the FLT3/ITD mutant allelic ratios changed minimally between the pretreatment and day 29 samples. For example, in patients 2, 7, 8, 9, 10, and 11, whose BM on day 29 displayed predominantly mature neutrophils, very little wild-type FLT3 allele was detectable by PCR on either pretreatment or day 29, indicating that the maturing myeloid elements were derived directly from leukemic precursors, and the cells retained their ITD allele. Samples with low allelic ratios, which likely harbored heterozygous ITD mutations, also showed minimal changes in ratio over the treatment period. Shown in Figure 1D are flow cytometric analyses of the clinical BM samples from patient 7, who demonstrated the typical myeloid differentiation we observed. During treatment, the cells within the BM displayed increased side scatter, decreased expression of the immature surface markers CD34 and CD117, and increased expression of the maturation marker CD15.
Table 2
BM cellularity, differentials, and FLT3 and C/EBPα mutations for patients treated with quizartinib
| Patient | Cellularity, Pre | Blasts, Pre | Myelocytes and neutrophils, Pre | Cellularity, D.29 | Blasts, D.29 | Myelocytes and neutrophils, D.29 | FLT3 allelic ratio, Pre | FLT3 allelic ratio, D.29 | C/EBPα mutation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 70% | 15% | 25% | 70% | 7% | 43% | 0.1 | 0.2 | None |
| 2 | 95% | 84% | 8% | 40% | 0% | 83% | 4.1 | 1.6 | TAD* |
| 3 | 80% | 86% | 5% | 60% | 29% | 36% | 0.2 | 0.1 | None |
| 4 | 40% | 71% | 11% | ND | 3% | 83% | 0.3 | 0.2 | None |
| 5 | 90% | 80% | 2% | 50% | 2% | 51% | 4.9 | 3.8 | None |
| 6 | 90% | 87% | 8% | 80% | 1% | 55% | 0.2 | 0.2 | None |
| 7 | 90% | 87% | 6% | 90% | 2% | 75% | 16.4 | 12.9 | None |
| 8 | 90% | 89% | 1% | 60% | 24% | 20% | 4 | 1.5 | None |
| 9 | 50% | 95% | ND | 85% | 2% | 40% | 7 | 13 | None |
| 10 | 60% | 92% | 3% | 80% | 4% | 73% | 3 | 4 | None |
| 11 | 75% | 75% | 12% | 70% | 6% | 53% | 3.7 | 1.3 | None |
| 12 | 70% | 77% | 14% | 85% | 0% | 63% | 0.8 | 1.3 | None |
| 13 | ND | 59% | 15% | 40% | 0% | 66% | 1.3 | 1 | None |
| Mean | 75% | 77% | 9% | 68% | 6% | 57% | 3.5 | 3.2 | |
| 14 | 90% | 85% | 7% | 90% | 59% | 9% | 0.4 | 0.3 | bZIP |
BM core biopsy samples (decalcified, slide mounted, and stained with H&E) were examined by light microscopy to visually estimate cellularity within 2 weeks prior (Pre) and after 4 weeks of continuous therapy (D.29) with quizartinib. BM aspirates (obtained from the same procedure as the core biopsies) were stained with Wright-Giemsa stain and examined by light microscopy. For each specimen, 100 cells were counted and scored by morphology as blasts, myelocytes, metamyelocytes, bands, or neutrophils and are expressed as a percentage of the total. FLT3 allelic ratios were derived from the aspirate specimens. C/EBPα mutation status was determined as described in “DNA isolation and sequencing.” Mean values are shown for patients 1-13.
Neutrophils are derived from the FLT3/ITD blasts
As patients accrued to this clinical protocol, it became apparent that quizartinib might be inducing the blasts to undergo differentiation. We therefore isolated and characterized the neutrophils produced during a surge. We prepared purified peripheral blood neutrophils from a patient at the peak of the surge (Figure 2A). We then prepared genomic DNA from the isolated neutrophils and from blasts collected from this same patient before beginning quizartinib therapy. PCR of the FLT3 juxtamembrane domain from both samples yielded fragments that migrated with an identical pattern on ethidium-stained agarose (Figure 2B). DNA sequencing of the mutant bands (not shown) confirmed that these were identical 75-bp ITD mutations, establishing that the neutrophils were derived from leukemic progenitors. We next analyzed these same purified neutrophils for expression of FLT3, lactoferrin, and MMP-9 (gelatinase) by immunoblotting. On normal mature neutrophils, FLT3 is not expressed30 and lactoferrin and MMP-9 are found within the granules.31 Figure 2C shows the expression of constitutively activated FLT3 in the pretreatment blasts of this patient but not in the isolated neutrophils, whereas lactoferrin and MMP-9 were appropriately expressed in both the patient-derived and control neutrophils. The leukemia-derived neutrophils exhibited normal oxidative burst activity in an NBT assay (Figure 2D).
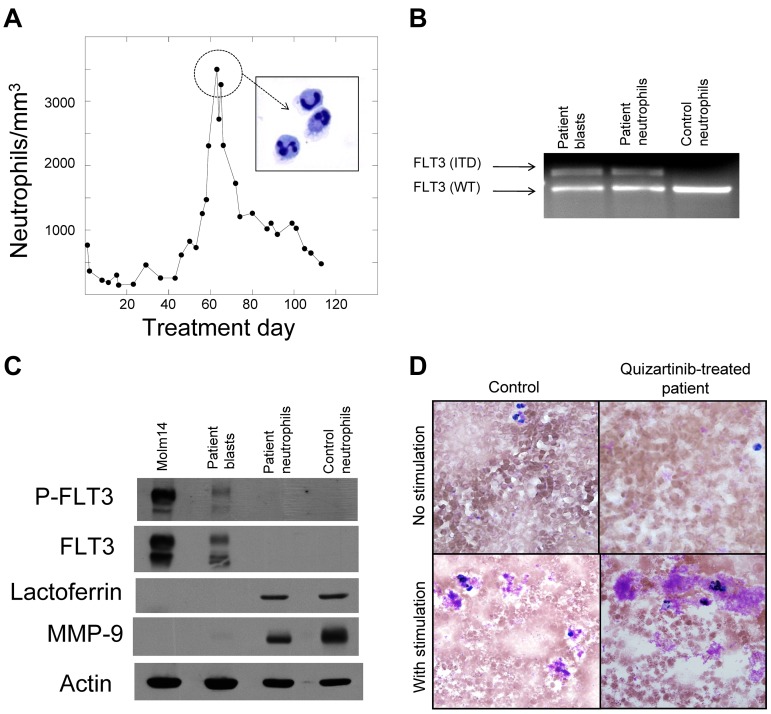
Neutrophils are derived from the leukemic blasts. (A) Graph of peripheral blood absolute neutrophil count from patient 4 during treatment with quizartinib. Neutrophils from day 60 were isolated to > 95% homogeneity as described in “Methods,” which was confirmed by cytospin (inset). (B) Genomic DNA samples isolated from pretreatment blasts, day 60 neutrophils, and neutrophils from a healthy donor were analyzed by PCR for the FLT3/ITD mutation, as described in “Methods.” Shown is an ethidium bromide–stained agarose gel. WT indicates wild-type. (C) Whole-cell lysates were prepared from Molm14 cells, pretreatment blasts from patient 4, day 60 neutrophils from patient 4, and neutrophils from a healthy donor. Lysates were analyzed for total FLT3, phosphorylated FLT3, lactoferrin, and MMP9, as described in “Methods.” (D) NBT reduction assay of peripheral blood collected from a healthy donor (“Control”) compared with a quizartinib-treated patient during the neutrophil surge. Cells “with stimulation” were exposed to bacterial extract to induce respiratory burst activity.
These data indicate that in 13 of 14 FLT3/ITD AML patients, treatment with quizartinib resulted in terminal granulocytic differentiation of BM blasts. In one patient (Table 2 patient 14), however, there was no evidence of differentiation at any time during treatment. Although this patient experienced complete clearance of peripheral blasts, no neutrophil surge occurred (Table 1), and there was no clinically significant reduction in BM blasts on day 15 or 29. The patient had a normal karyotype, so the ongoing block in differentiation could not be explained by translocation of a transcription factor such as core binding factor. However, mutation analysis of the CEPBα coding sequence revealed a b-ZIP domain mutation (insertion of 4 base pairs, ACCG, at position 1449 of the reference sequence U34070, leading to a frameshift at R287), expected to result in loss of C/EBPα-mediated cell-cycle control.6,32 CEPBα is a well-described transcription factor central to the induction of granulocyte development and is associated with transcriptional activation of genes such as lactoferrin.33 Mutations in CEPBα occur in approximately 10%-15% of normal karyotype AML,34 and are known to act in a dominant-negative manner to disrupt granulocytic differentiation.6 The presence of this CEPBα mutation offered a potential explanation for this patient's lack of differentiation in response to FLT3 inhibition. In a second patient (Table 2 patient 2), a trans-activation domain CEPBα mutation (deletion of 17 base pairs, CCGCCCCGGAGCCGCTG, at position 731 of the reference sequence U34070, leading to a frameshift at P46) was detected at disease progression 4 months after beginning quizartinib treatment.
Differentiation of Molm14 cells in vitro
FLT3 inhibition has been reported to induce granulocytic differentiation in FLT3/ITD AML cells.17,18 These studies postulated that FLT3/ITD signaling leads to phosphorylation and potential inactivation of C/EBPα on residue ser21 via P-ERK1/2, resulting in a differentiation block that could be overcome with FLT3 inhibition. However, these observations were made using in vitro models with FLT3/ITD-expressing cell lines in suspension culture. The differentiation that we observed in our patients occurred within the BM. Therefore, we sought to develop an in vitro model of FLT3/ITD AML differentiation that more closely mimicked the conditions of the BM microenvironment, with direct interactions between the leukemia and BM stromal cells. Monolayers of stromal cells from healthy BM donors were cocultured with Molm14 cells, an FLT3/ITD-expressing AML line used previously to study differentiation in response to FLT3 inhibition.18,35 This type of model has been used to study hematopoiesis and chemoresistance conferred by the BM microenvironment.36,37
To determine whether differentiation in response to FLT3 inhibition represents a class effect of FLT3 TKIs, we cultured Molm14 cells on stromal layers with increasing doses of 2 different FLT3 TKIs, sorafenib and quizartinib.19,38–40 In suspension culture, Molm14 cells undergo apoptosis on exposure to sustained, potent FLT3 inhibition.13 In contrast, whereas the cells cocultured on stroma arrested in G1 in response to the inhibitors, the degree of apoptosis induced by either drug was diminished (Figure 3A-B). After 24 hours of treatment, morphologic differentiation of the cells was evident in the presence of either quizartinib (Figure 3C) or sorafenib (not shown) at an equipotent FLT3-inhibitory concentration,19 and respiratory burst activity by NBT assay was present (Figure 3D). This difference in response to FLT3 inhibition in suspension culture compared with stromal coculture parallels the responses observed clinically, in which peripheral blasts are rapidly cleared whereas BM blasts remain viable but undergo differentiation. It should be noted that although quizartinib inhibits both FLT3 and c-KIT, the IC50 for inhibition of c-KIT is 35nM (data not shown), whereas the IC50 for FLT3 is approximately 1nM.40 Accordingly, the differentiation effects from this drug are most likely because of FLT3 inhibition and, although FLT3 autophosphorylation was fully inhibited under the conditions of this experiment (Figure 3E), P-ERK was only partially inhibited, in contrast to what is routinely observed with FLT3/ITD cell lines in suspension culture.18,41 C/EBPα was readily detectable and phosphorylated despite FLT3 inhibition (Figure 3E). Because a previous study in murine 32D cells expressing a FLT3/ITD receptor indicated that C/EBPα levels increased in response to FLT3 inhibition,17 we examined C/EBPα levels over 24 hours of quizartinib exposure (Figure 3F). C/EBPα (and P-C/EBPα) was maximally expressed during the first 4-8 hours of stromal contact and then decreased as the cells differentiated.
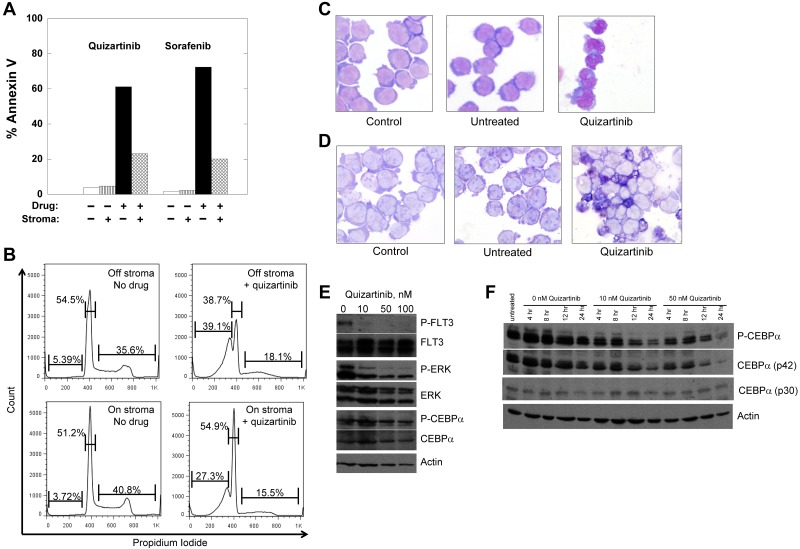
Treatment of Molm14 cells with FLT3 inhibitors. (A-B) Molm14 cells were exposed to quizartinib (10nM) or sorafenib (100nM) in suspension culture or coculture with BM stroma and analyzed for annexin V binding (A) and propidium iodide staining (B) by flow cytometry. (C-D) Molm14 cells were cocultured with stroma in the presence and absence of 10nM quizartinib for 24 hours, and then cells were collected and analyzed for morphology (C) and NBT reduction activity (D). (E) Molm14 cells were cocultured on stroma in the presence and absence of 10nM quizartinib. After 1 hour, cells were collected and lysates were analyzed by immunoblotting as described in “Immunoblotting.” (F) Molm14 cells were cocultured with stroma and quizartinib at the indicated concentrations. Cells were collected after 0, 4, 8, 12, and 24 hours of drug exposure and analyzed by immunoblotting for phosphorylated and total C/EBPα.
C/EBPα knockdown impedes FLT3 inhibitor-induced differentiation
Because of the central role of C/EBPα in myeloid differentiation and because the single patient in our series who failed to display differentiation harbored a C/EBPα mutation, we predicted that the loss of C/EBPα in FLT3/ITD cells would prevent FLT3 inhibitor–induced differentiation. Molm14 cells cocultured with stroma differentiated in a dose-responsive fashion to quizartinib (Figure 4A). We then used siRNA treatment of the Molm14 cells to knockdown C/EBPα levels (Figure 4B), which resulted in a significant decrease in differentiation induced by quizartinib (Figure 4C). This experiment demonstrates the importance of C/EBPα in mediating differentiation induced by FLT3 inhibition.

Knockdown of C/EBPa blocks the differentiation induced by FLT3 inhibition. (A) Molm14 cells were cocultured with stroma in the presence of increasing concentrations of quizartinib for 24 hours, and then cells were collected and analyzed for morphology. For each condition, 100 viable cells were counted and categorized as undifferentiated or differentiated. (B) Molm14 cells (2 million per sample) were incubated with siRNA for C/EBP-α (or scrambled control), electroporated, and then cocultured with stroma. After 24 hours, the cells were harvested, lysed, and analyzed for C/EBPα protein levels by Western blotting. Incubation for 48 hours led to identical results (not shown). (C) Molm14 cells were subjected to siRNA C/EBPa knockdown as in panel B, cocultured with stroma for 24 hours, and then treated with 5nM quizartinib. After 24 hours, the cells were harvested and examined and scored for morphologic changes as in panel A.
Differentiation of primary FLT3/ITD AML patient blasts in vitro
We had sufficient viable blasts from patient 7 (Figure 1D and Tables 1–2), who exhibited the typical differentiation response while on treatment, to study in our stromal coculture system. When treated in suspension culture, these blasts displayed a cytotoxic response to quizartinib (not shown), resembling the typical response we and others have described previously with numerous FLT3 inhibitors including quizartinib.19,40,42 The blasts were plated onto stroma with or without 200nM quizartinib, a concentration of drug chosen to approximate the steady-state levels (accounting for plasma protein binding) achieved in patients treated with 135 mg/d of quizartinib.43 The cells were examined daily by light microscopy to monitor viability and morphology. Viable cell number, as assessed by trypan blue staining, did not decrease over the duration of the experiment in either treated or untreated samples (not shown). However, the proliferative fraction decreased significantly during the first 48 hours of quizartinib treatment, which is indicative of a cell-cycle arrest (Figure 5A). Differentiation was evident in the quizartinib-treated samples, but the time course was very different compared with Molm14 cells. Not until day 14 of coculture with stroma and quizartinib did the cells display morphologic changes consistent with differentiation (Figure 5B and quantified in C), although functional evidence (ie, NBT) was present by day 5 (not shown). This time course is consistent with that observed in the clinical specimens from patients, in which the maturation occurred over 2-4 weeks (Figure 1C). We confirmed that the ITD mutation present in the blasts at the start of the experiment was also detectable in the differentiated cells at an identical ratio (not shown). Flow cytometric analysis of the cells from patient 7 after 14 days of drug exposure on stroma revealed loss of CD34 and CD117 and gain of CD15 in the blasts treated with quizartinib (Figure 5D). The blasts cocultured on stroma without quizartinib also showed some flow cytometric evidence of differentiation, but less than the quizartinib-treated cells and there was little evidence of morphologic differentiation (Figure 5B and D). As with the Molm14 cells, the primary cells displayed only partial inhibition of P-ERK despite complete inhibition of FLT3 autophosphorylation (Figure 5E). Likewise, C/EBPα was expressed at the protein level (increased in cells on stroma compared with suspension cells) and remained phosphorylated despite quizartinib treatment (Figure 5E).
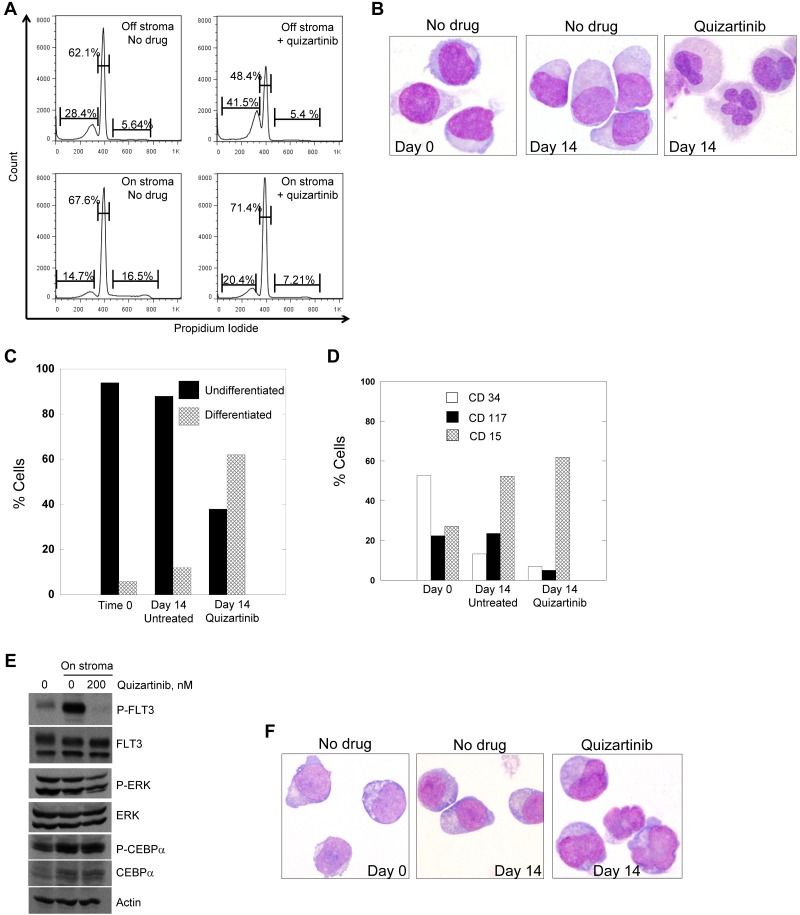
Treatment of primary patient blasts with FLT3 inhibitors. (A) Blasts from patient 7 were cocultured with stroma or grown in suspension culture overnight and then 200nM quizartinib was added to both cultures. Cells were incubated with drug for 48 hours and then stained with propidium iodide and analyzed by flow cytometry. Blasts from patient 7 were cocultured with stroma in the presence and absence of 200nM quizartinib for 14 days, and cells were collected and analyzed by light microscopy (B) and scored for differentiation (C). (D) Cells from panel B were also analyzed for the presence of differentiation markers by flow cytometry as described in “Flow cytometry.” (E) Blasts from patient 7 were cocultured with stroma overnight and then 200nM quizartinib was added. After 1 hour, cells were collected and lysates were analyzed by immunoblotting as described in “Flow cytometry.” (F) Blasts from a 52-year-old man with relapsed, refractory FLT3/ITD AML were cocultured on stroma as in panel B. Shown are cells harvested after 9 days of culture with or without quizartinib.
We repeated the experiment using a second FLT3/ITD AML sample (with a mutant to wild-type allelic ratio of 19) and again achieved morphologic differentiation of the blasts, but this time in 9 days (Figure 5F).
Discussion
The present study is the first to report in vivo terminal differentiation of leukemia cells occurring as a result of therapeutic inhibition of an oncogenic tyrosine kinase. Two previous studies have used in vitro models to predict that FLT3 inhibition would relieve the differentiation block that characterizes AML cells.17,18 Although clinically apparent differentiation has not been seen with other FLT3 inhibitors, quizartinib is 10-50 times more potent in vivo than previously studied drugs,19 and the reported interim response rate is significantly higher than that seen with other inhibitors.15 The formal trial results for all patients, including the exploratory group, are not yet available.
The release of the differentiation block appears to be an on-target effect of FLT3 inhibitors, because we induced differentiation in vitro with sorafenib and with quizartinib and 2 prior studies used lestaurtinib and tandutinib.17,18 In general, therefore, the clinical response to potent sustained FLT3 inhibition appears to consist of clearance of peripheral blasts over a few days and terminal differentiation of BM blasts over several weeks. This difference in the response of blasts in the peripheral blood compared with the BM highlights the influence of the BM microenvironment on the pathogenesis of leukemia.
In previous studies in FLT3/ITD-expressing cells in suspension culture, both cell lines and primary patient blasts responded to FLT3 inhibition in vitro with rapid apoptosis,40,42,44–47 which parallels the clinical peripheral blood response. However, in our stromal coculture system, the response mimicked the differentiation observed in the BM of the quizartinib-treated patients. The protective effect that BM stroma confers on leukemia cells is well described,48 so it is not surprising that apoptosis was not induced with FLT3 inhibition in cells on stroma. Our in vitro model was successful in reproducing the differentiation observed in vivo and so provided the opportunity to investigate some of the mechanisms regulating this process.
Differentiation is tightly coupled with cell-cycle arrest, and in normal myelopoiesis, C/EBPα is generally considered to play a central role in both processes.33,49 The function of C/EBPα is frequently perturbed in AML through a variety of mechanisms,17,50–52 and FLT3/ITD mutations have been reported to inhibit C/EBPα function through P-ERK–mediated phosphorylation.18 In the present study, whereas the level of C/EBPα protein was increased in the blasts on stroma compared with the cells in suspension, it remained phosphorylated, possibly because of the persistent activation of ERK; ERK, in turn, remained active presumably because of stromal-derived factors acting outside of the FLT3 signaling pathway. This failure of FLT3 inhibition to inhibit ERK in blasts on stroma has been reported by others,37 and stands in distinct contrast to the consistent findings in suspension cells.13,44 In blasts on stroma, FLT3 inhibition results in a cell-cycle arrest, which appears to be sufficient to trigger differentiation. Given that the single patient in our series who failed to differentiate with quizartinib harbored a C/EBPα b-ZIP mutation, and that clinical resistance to quizartinib in another patient was associated with the emergence of a C/EBPα trans-activation domain mutation, it appears that FLT3 inhibitor–induced differentiation requires the presence of a functional C/EBPα.
ATRA is effective in treating APL by induction of terminal differentiation, but fails to permanently clear the leukemic clone (as can be achieved by combining ATRA with anthracyclines or arsenic).29 Only through these combination therapies is the eradication of the leukemia achieved. With quizartinib, it would seem that truly potent in vivo FLT3 inhibition can finally be accomplished, offering the hope that this drug, combined with chemotherapy, may result in significant cure rates for FLT3/ITD AML.
The detection of leukemia-derived neutrophils and of a clinical differentiation syndrome represent striking parallels between FLT3/ITD AML and APL treated with molecularly targeted therapy. However, there are important differences. The increase in the WBC count that commonly occurs during the treatment of APL is presumably because of a much larger fraction of the leukemic burden undergoing differentiation. In FLT3/ITD AML, the majority of the malignant cells undergo apoptosis relatively rapidly, followed by gradual differentiation of the residual BM blasts. The “surge” of the absolute neutrophil count after this differentiation is therefore much more modest than that seen in APL.
The results of the present study highlight an important role for differentiation therapy, but suggest an incomplete understanding of relevant targets in most cases of AML, including those with FLT3/ITD. In summary, FLT3 inhibition by quizartinib induces terminal differentiation of FLT3/ITD AML blasts and demonstrates a largely unrecognized role of tyrosine kinase mutations in generating the maturation arrest so characteristic of AML.
Acknowledgments
This work was supported by grants from the National Cancer Institute (Specialized Programs of Research Excellence [SPORE] leukemia grants P50 CA100632-06 and R01 CA128864) and the American Society of Clinical Oncology (to M.L.). M.L. is a Clinical Scholar of the Leukemia & Lymphoma Society.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S. and M.L. designed the study, performed the experiments, enrolled the patients, analyzed the data, and wrote the manuscript; A.P. contributed to the study design, analyzed the data, enrolled the patients, and helped to edit the manuscript; X.Y. and T.R. performed the experiments; M.B. contributed to the study design and helped to analyze the flow cytometry data; C.G. and G.E.N. helped to analyze the data; C.T. analyzed the samples for C/EBPα mutations; and M.F., K.P., J.K., and B.D.S. enrolled the patients.
Conflict-of-interest disclosure: A.P., C.T., and M.L. are on the clinical advisory board for Ambit Biosciences, which makes quizartinib. The remaining authors declare no competing financial interests.
Correspondence: Mark Levis, MD, PhD, Kimmel Cancer Center at Johns Hopkins, 1650 Orleans St, Rm 243, Baltimore, MD 21231; e-mail: ude.imhj@amsivel.
References
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2012-01-402545
Read article for free, from open access legal sources, via Unpaywall:
https://ashpublications.org/blood/article-pdf/120/20/4205/1360241/zh804612004205.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1182/blood-2012-01-402545
Article citations
Quizartinib: a potent and selective FLT3 inhibitor for the treatment of patients with FLT3-ITD-positive AML.
J Hematol Oncol, 17(1):111, 13 Nov 2024
Cited by: 0 articles | PMID: 39538314 | PMCID: PMC11558990
Review Free full text in Europe PMC
Bone marrow stromal cells enhance differentiation of acute myeloid leukemia induced by pyrimidine synthesis inhibitors.
Am J Physiol Cell Physiol, 327(5):C1202-C1218, 16 Sep 2024
Cited by: 0 articles | PMID: 39279497 | PMCID: PMC11559649
A phase 1 study of the irreversible FLT3 inhibitor FF-10101 in relapsed or refractory acute myeloid leukemia.
Blood Adv, 8(10):2527-2535, 01 May 2024
Cited by: 2 articles | PMID: 38502195 | PMCID: PMC11131057
Autophagy Inhibition-induced Cytosolic DNA Sensing Combined with Differentiation Therapy Induces Irreversible Myeloid Differentiation in Leukemia Cells.
Cancer Res Commun, 4(3):849-860, 01 Mar 2024
Cited by: 0 articles | PMID: 38466568 | PMCID: PMC10953625
FLT3 tyrosine kinase inhibition modulates PRC2 and promotes differentiation in acute myeloid leukemia.
Leukemia, 38(2):291-301, 05 Jan 2024
Cited by: 2 articles | PMID: 38182819 | PMCID: PMC11141246
Go to all (100) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00989261
Nucleotide Sequences
- (2 citations) ENA - U34070
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The role of quizartinib in the treatment of acute myeloid leukemia.
Expert Opin Investig Drugs, 22(12):1659-1669, 26 Sep 2013
Cited by: 11 articles | PMID: 24070241
Review
Glucocorticoids enhance the antileukemic activity of FLT3 inhibitors in FLT3-mutant acute myeloid leukemia.
Blood, 136(9):1067-1079, 01 Aug 2020
Cited by: 17 articles | PMID: 32396937 | PMCID: PMC7453151
Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase.
Br J Haematol, 164(1):61-72, 10 Oct 2013
Cited by: 77 articles | PMID: 24116827 | PMCID: PMC4076672
Concurrent Inhibition of Pim and FLT3 Kinases Enhances Apoptosis of FLT3-ITD Acute Myeloid Leukemia Cells through Increased Mcl-1 Proteasomal Degradation.
Clin Cancer Res, 24(1):234-247, 26 Oct 2017
Cited by: 24 articles | PMID: 29074603 | PMCID: PMC6444348
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: R01 CA128864
Grant ID: P50 CA100632
Grant ID: P50 CA100632-06
 1
1




