Abstract
Free full text
Translational Research in OCD: Circuitry and Mechanisms
Although the pathophysiology of obsessive-compulsive disorder (OCD) remains unknown, converging lines of evidence point to abnormalities in the orbital (OFC), ventromedial (vmPFC-subgenual cingulate and medial OFC), and dorsal anterior cingulate (dACC) cortical-basal ganglia circuits. OCD-linked patterns of activity in these PFC regions are accentuated during provocation of symptoms and can predict treatment response; they tend to normalize following successful treatment (Greenberg et al, 2010). Moreover, neurosurgical interventions (lesions or deep brain stimulation-DBS) within the ventral internal capsule (VC), ventral striatum (VS), or dACC (treatments for intractable OCD) all act on subcomponents of the vmPFC/OFC/dACC-basal ganglia network (Greenberg et al, 2010). Indeed, DBS interventions specifically affect vmPFC, OFC, and possibly dACC connections with striatum, thalamus, and/or brainstem (Figure 1) (Lehman et al, 2011). The efficacy of VC/VS DBS (or lesions) for OCD likely requires modulating the OFC/vmPFC/dACC-basal ganglia circuit. Interestingly, high frequency stimulation (HFS) in a rat homolog of the VC/VS DBS target reduces OFC activity, enhances local field potential delta band activity in OFC, and enhances synchrony between specific regions within this prefrontal network (McCracken and Grace, 2009). Thus, OCD pathophysiology likely represents dysfunctional network interactions rather than only disruption within specific structures.
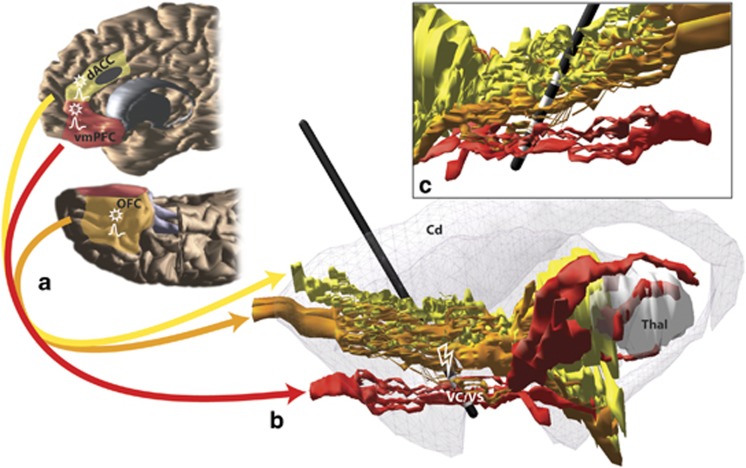
Schematic illustrating key cortical areas involved in obsessive-compulsive disorder (OCD) and their pathways through the internal capsule. (a) Red, orange, and yellow fibers originate in ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), and dorsal anterior cingulate (dACC), respectively. The approximate cingulotomy site is depicted as a dark gray oval. (b) Medial view of a sagittal section with vmPFC, OFC, and dACC fibers passing through the internal capsule. The striatum is indicated in gray. A model of the electrode with its four contacts is placed at ventral internal capsule (VC)/ventral striatum (VS) deep brain stimulation (DBS) site. (c) Lateral view of a sagittal section shows the four electrode contact points in the VC/VS. VmPFC and OFC fibers pass through the ventral contacts, and OFC and dACC fibers pass through the dorsal contact points (Lehman et al, 2011). Action potential symbols indicate possible regions with changes in firing rates and/or local field potentials following HFS in the VS (McCracken and Grace, 2009); stars indicate regions with possible changes in plasticity following HFS to VS (Rodriguez-Romaguera et al, 2012).
OCD is often characterized by abnormal risk assessment and unrealistic fears leading to excessive avoidance. Changes in vmPFC/OFC/dACC activity have been linked to fear conditioning and recall in normal subjects (Milad et al, 2007), with vmPFC activity and structure particularly relevant for fear extinction recall. Overlap in the circuits associated with fear conditioning and OCD dysfunction suggests that these patients may be less flexible in adjusting adverse responses based on new information. Indeed, using a rat model of DBS, with the HFS targeting the VS, a recent study showed that stimulation strengthened fear extinction and retention (Rodriguez-Romaguera et al, 2012), while enhancing plasticity in the infralimbic, orbitofrontal, and prelimbic cortices (probable homologs of the vmPFC, OFC, and, perhaps, dACC). Taken together, dysfunction of the vmPFC/OFC/dACC network may lead to an increase in incentive-based fear learning and habit formation.
However, PFC regions associated with OCD pathology are not only involved in aversive behaviors and avoidance; they also mediate reward processing. Indeed, OCD patients are also impaired on tasks using rewarding outcomes (Gillan et al, 2011). They underperform when required to flexibly adjust responses based on new or changing reward feedback. These tendencies suggest impairment in goal-directed behaviors and may lead patients to rely too heavily on habit-based responding, even in the positive-incentive domain (Gillan et al, 2011). Therefore, rather than being specific to aversive vs reward processing, the vmPFC, OFC, and dACC cortices are involved in value representation, stimulus-outcome associations, and action-outcome associations, regardless of valence. Thus, a heuristic approach could posit that OCD symptoms may not be specific to fear learning and habits, but are related to interference in the normal balance between negative and positive-incentive learning based on values attributed to particular stimuli or actions. Probing potential abnormalities in incentive learning strategies and linking them with functional neurocircuitry can be used both as a research tool and to help design innovative therapeutic approaches.
Notes
Dr Haber has received consultation fees from Medtronic, Inc and Pfizer, Inc. Dr Heilbronner declares no conflict of interest.
References
- Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. [Europe PMC free article] [Abstract] [Google Scholar]
- Greenberg BD, Rauch SL, Haber SN. Invasive Circuitry-Based Neurotherapeutics: Stereotactic Ablation and Deep Brain Stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. [Europe PMC free article] [Abstract] [Google Scholar]
- Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392–10402. [Europe PMC free article] [Abstract] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. [Europe PMC free article] [Abstract] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. [Abstract] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci USA. 2012;109:8764–8769. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Neuropsychopharmacology are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/npp.2012.182
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3521984?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/npp.2012.182
Article citations
Deep Brain Stimulation (DBS) in Treatment-Resistant Depression (TRD): Hope and Concern.
Adv Exp Med Biol, 1456:161-186, 01 Jan 2024
Cited by: 0 articles | PMID: 39261429
Review
Unraveling the mechanisms of deep-brain stimulation of the internal capsule in a mouse model.
Nat Commun, 14(1):5385, 04 Sep 2023
Cited by: 4 articles | PMID: 37666830 | PMCID: PMC10477328
Combined fractional anisotropy and subcortical volumetric deficits in patients with mild-to-moderate depression: Evidence from the treatment of antidepressant traditional Chinese medicine.
Front Neurosci, 16:959960, 23 Aug 2022
Cited by: 1 article | PMID: 36081664 | PMCID: PMC9448251
Deep Brain Stimulation for Depression.
Neurotherapeutics, 19(4):1229-1245, 11 Jul 2022
Cited by: 32 articles | PMID: 35817944 | PMCID: PMC9587188
Review Free full text in Europe PMC
Developmental impact of glutamate transporter overexpression on dopaminergic neuron activity and stereotypic behavior.
Mol Psychiatry, 27(3):1515-1526, 20 Jan 2022
Cited by: 6 articles | PMID: 35058566 | PMCID: PMC9106836
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Toward a neuroanatomy of obsessive-compulsive disorder revisited.
Biol Psychiatry, 73(4):298-299, 01 Feb 2013
Cited by: 9 articles | PMID: 23351888
Abnormal topological organization in white matter structural networks revealed by diffusion tensor tractography in unmedicated patients with obsessive-compulsive disorder.
Prog Neuropsychopharmacol Biol Psychiatry, 51:39-50, 16 Jan 2014
Cited by: 17 articles | PMID: 24440373
Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder.
Biol Psychiatry, 73(4):321-328, 28 Nov 2012
Cited by: 121 articles | PMID: 23200527
The neurocircuitry of obsessive-compulsive disorder and disgust.
Prog Neuropsychopharmacol Biol Psychiatry, 30(3):389-399, 27 Jan 2006
Cited by: 46 articles | PMID: 16443315
Review
Funding
Funders who supported this work.
NIMH NIH HHS (5)
Grant ID: MH 086400
Grant ID: MH7311
Grant ID: P50 MH086400
Grant ID: MH045573
Grant ID: R01 MH045573




