Abstract
Free full text

H+-coupled divalent metal-ion transporter-1: Functional properties, physiological roles and therapeutics
Abstract
Divalent metal-ion transporter-1 (DMT1) is a widely-expressed, iron-preferring membrane transport protein. Animal models establish that DMT1 plays indispensable roles in intestinal nonheme iron absorption and iron acquisition by erythroid precursor cells. Rare mutations in human DMT1 result in severe microcytic–hypochromic anemia. When we express DMT1 in RNA-injected Xenopus oocytes, we observe rheogenic Fe2+ transport that is driven by the proton electrochemical potential gradient. In that same preparation, DMT1 also transports cadmium and manganese but not copper. Whether manganese metabolism relies upon DMT1 remains unclear but DMT1 contributes to the effects of overexposure to cadmium and manganese in some tissues. There exist at least four DMT1 isoforms that arise from variant transcription of the SLC11A2 gene. Whereas these isoforms display identical functional properties, N- and C-terminal variations contain cues that direct the cell-specific targeting of DMT1 isoforms to discrete subcellular compartments (plasma membrane, endosomes, and lysosomes). An iron-responsive element in the mRNA 3′-untranslated region permits the regulation of some isoforms by iron status, and additional mechanisms by which DMT1 is regulated are emerging. Natural resistance-associated macrophage protein-1 (NRAMP1)—the only other member of the mammalian SLC11 gene family—contributes to antimicrobial function by extruding from the phagolysosome divalent metal ions (e.g. Mn2+) that may be essential cofactors for bacteria-derived enzymes or required for bacterial growth. The principal or only intestinal nonheme-iron transporter, DMT1 is a validated therapeutic target in hereditary hemochromatosis and other iron-overload disorders.
I. Introduction and History
DMT1 (NRAMP2, DCT1) was cloned by Hediger’s group in 1997 by the functional screening of a complementary DNA library prepared with duodenal mRNA isolated from rats fed a low-iron diet (Gunshin et al., 1997). We found that expression of the rat DMT1 in RNA-injected Xenopus oocytes stimulated 55Fe2+ uptake and Fe2+-evoked currents. We also found that this widely expressed transporter was reactive with several other divalent metals ions. Andrews’ group around the same time had used a positional cloning strategy to identify the defective gene responsible for the microcytic anemia phenotype of the mk inbred mouse strain (Fleming et al., 1997). These two findings established DMT1 as a divalent metal-ion transporter with a central role in iron metabolism.
During the intervening 15 years, DMT1 has been the focus of intense research effort—among the reasons for which, DMT1 plays nonredundant roles in iron homeostasis. Its widespread expression—present in at least some cell types in every organ tested (Gunshin et al., 1997; Hubert & Hentze, 2002; Wang et al., 2012a)—places DMT1 at the nexus of multiple pathways of epithelial iron transport and cellular iron acquisition in health and disease, and the transport of other metals (e.g. cadmium, manganese) particularly in chronic exposure. DMT1 is a mechanistically complex transporter with multiple isoforms that function in varied environments. This article is an attempt to review just some of this breadth and complexity. We will review lessons learned from animal models and human mutants, studies of the functional properties, structure–function, regulation, and tissue-specific roles of DMT1, and the development of new therapeutic strategies.
II. Animal Models
The Belgrade (b) rat and microcytic (mk) mouse inbred strains have long been studied by investigators interested in iron metabolism (Bannerman, 1976; Wang et al., 2012a). Both strains harbor a mutation that causes a Gly185→Arg substitution in the putative fourth transmembrane region (TM4) of DMT1 (Fig. 1) and exhibit a severe hypochromic–microcytic anemia (Fleming et al., 1997; Fleming et al., 1998). Tissue or vesicle preparations from the b rat and mk mouse exhibited deficiencies in intestinal iron transport (Edwards & Hoke, 1972; Knöpfel et al., 2005b). Bypassing the intestine by giving parenteral iron results in some improvement but does not cure the anemia, revealing some other role in red blood cell production. A similar phenotype was observed in the ‘chardonnay’ zebrafish mutant in which ethylnitrosourea mutagenesis introduced an early stop codon in place of Lys-264 of DMT1 (Donovan et al., 2002).

Predicted topology model of human DMT1, 1B/IRE(+) isoform (based on the hydrophobicity plot generated by using the Kyte–Doolittle algorithm at http://web.expasy.org/protscale/ and default values for predicting transmembrane regions) showing sites of the human mutations reported so far. Also labeled are amino acid residues corresponding to those mutated in rat and mouse DMT1 (including the G185R mutation found in the b and mk rodent models).
The chardonnay mutant, when expressed in HEK293T cells, exhibited around 10% the iron-transport activity observed for wildtype zebrafish DMT1 (Donovan et al., 2002). Meanwhile, expression of the mouse G185R in several transfected cell lines (HEK293T, LLC-PK1, and CHO) has revealed that the mutant protein is correctly targeted to the plasma membrane and endosomes; however, G185R-DMT1 protein levels at the plasma membrane are lower than those of wildtype (Su et al., 1998; Touret et al., 2004). In one of the studies just cited, metal-ion transport activity for the G185R mutant was only modestly reduced (Touret et al., 2004) whereas, in the other study, it was substantially reduced (Su et al., 1998). Mn2+ transport activity in brush-border membrane vesicles (BBMV) isolated from homozygous Belgrade rats (b/b) was <10% that of the heterozygote (+/b) despite the much higher DMT1 protein levels detected in b/b intestine (Knöpfel et al., 2005b). Expression of the G185R mutant protein in mk/mk mouse duodenum is also dramatically increased compared with the heterozygote (+/mk) but is not correctly targeted to the brush-border membrane (Canonne-Hergaux et al., 2000).
Although the species and preparations used appear to differ with regard to the activity of the G185R-DMT1 protein, it is apparent that this mutant retains a fraction of wildtype metal-ion transport activity. Coupled with the expected upregulation of the protein due to iron deficiency, this residual activity may help to explain why the b and mk animals survive. Whereas homozygous mk mice generally survived to adulthood, mice in which the SLC11A2 gene coding for DMT1 was globally inactivated (SLC11A2−/−) did not survive more than 7 days (Gunshin et al., 2005a). The SLC11A2−/− neonates exhibited a severe hypochromic–microcytic anemia. Total body iron was normal, indicating that maternofetal iron transfer was sufficient; however, liver iron stores were elevated, consistent with impaired erythroid iron utilization. Strengthening the conclusion of a critical defect in erythroid iron utilization, transfusion of red blood cells but not parenteral iron injections improved survival of these animals. In that same study, investigators also transplanted hematopoietic stem cells from SLC11A2−/− mice into lethal-dose-irradiated wildtype mice and found that the recipients exhibited defective erythropoiesis, consistent with iron deficiency (Gunshin et al., 2005a).
To assess the role of DMT1 in the intestine, the same investigators generated a conditional intestine-specific knockout (SLC11A2int/int) by crossing floxed DMT1 and villin-Cre transgenic lines (Gunshin et al., 2005a). Although born healthy, the SLC11A2int/int mice soon developed progressive anemia and exhibited lower tissue-iron levels than did wildtype mice (Gunshin et al., 2005a; Shawki et al., 2012). Parenteral iron injections rescued the SLC11A2int/int anemia phenotype, confirming a primary defect in intestinal iron absorption (Gunshin et al., 2005a; Shawki et al., 2012).
These studies in animal models collectively demonstrate the importance of DMT1 in intestinal iron absorption and erythroid iron utilization. Whereas ablation of the SLC11A2 gene may provide superior models in which to test the essential roles of DMT1, the b and mk rodent models are likely to be of continued utility in studies for which a less severe phenotype is desirable. Meanwhile, novel transgenic mouse models in which intestinal DMT1 is overexpressed may prove especially useful in expanding our understanding of how DMT1 is regulated (Barrientos De Renshaw et al., 2011).
III. Mutations in Human DMT1
Mutations in human DMT1 have been reported now in five unrelated cases of hypochromic–microcytic anemia, characterized by lowered values for hematocrit, blood hemoglobin concentration, mean corpuscular volume, and mean corpuscular hemoglobin content (Table 1). The first identified was a Czech proband homozygous for a G→C substitution in the last nucleotide of exon 12, with the following two consequences: (1) expression of a full-length DMT1 protein containing a conservative E399D substitution, and (2) increased frequency of skipping exon 12 from the normal 10% to 90% in the proband (Mims et al., 2005; Priwitzerova et al., 2004). Exon-12 deletion is predicted to result in a protein lacking putative TM8 and the cytosolic loop between TM8 and TM9 (Fig. 1). In experiments in which these constructs were expressed in mammalian cell lines or Xenopus oocytes, E399D-DMT1 functioned normally, whereas the exon-12 deletion product showed defects in expression, trafficking and transport activity (Gunshin et al., 2005b; Lam-Yuk-Tseung et al., 2005; Priwitzerova et al., 2005; Shawki et al., 2006).
Table 1.
Hematological variables shown were obtained at birth or *at diagnosis (age shown), and are compared with reference values in the final row.
| Proband | Mutation | [Hb] (g/dL) | Mean corpuscular volume (fL) | Mean corpuscular hemoglobin (pg/red cell) | Haematocrit (%) | Urinary hepcidin (− or +) | Liver iron overload − or + (values in μg Fe/g tissue) | References and description of the impact of the mutation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Peptide | Description | ||||||||
| Czech | G1285C | E399D | Homozygous; conservative; increased skipping of exon 12 from normal 10% to 90% | 7.4* | 54* | 15* | 25* | – | +* (16,286; 19 years) | E399D does not affect expression, stability, or maturation unlike Exon 12 (Gunshin et al., 2005; Mims et al., 2005; Priwitzerova et al., 2004; Priwitzerova et al., 2005); E399D but not Exon12 del DMT1 transports radiotracer iron (Priwitzerova et al., 2005; Shawki et al., 2006) |
| 3 months | ||||||||||
| Italian | C1246T; CTT del | R416C | Compound heterozygous; 3-bp del in intron 4 leading to 30–35% skipping of exon 5 | 4.0 | 71 | 13 | 25* | – | + (2536) | (Iolascon et al., 2006); Defective processing—not fully glycosylated and retained in the ER and cell-surface expression severely reduced (Lam-Yuk-Tseung et al., 2006); Exon5del leads to loss of iron-transport activity (Shawki et al., 2006) |
| 2 months | ||||||||||
| French [Paris] | G723T, G428T429G430 del | G212V, V114 del | Compound heterozygous; del leads to in-frame shift | 8.3 | 64 | 15 | 26* | – | +* (250; 5–6 years) | G212V but not V114del DMT1 transports radiotracer iron (Shawki et al., 2008) |
| 5–6 years | ||||||||||
| Ecuadorian | G311A | G75R | Homozygous | 5.1 | 54* | 22* | 27 | – | −* (4 years) | G75R postulated to be fully defective (Blanco et al., 2009) |
| 4 years | ||||||||||
| French [Rennes] | G723T, A1560G | G212V, N491S | Compound heterozygous | 8.6* | 58* | 18* | N/A | N/A | +* (300; 17 years) | G212V as before; N491S leads to a truncated protein (Bardou-Jacquet et al., 2011) |
| 13 years | ||||||||||
| Reference values | 11–18 9.0–20.5* | 70–126 70–126* | 25–31 23–40* | 35–45 28–67* | 10–200 | <36 | (Bardou-Jacquet et al., 2011) *(Children’s Hospitals and Clinics) | |||
Three other probands are compound heterozygotes. Among them, there are five discrete mutations—one of these, a missense mutation leading to a Gly212→Val substitution in putative TM5 (Fig. 1), appears in two of the probands (Table 1) (Bardou-Jacquet et al., 2011; Beaumont et al., 2006; Iolascon et al., 2006). The parents (and siblings) of each of the compound heterozygous probands are healthy, indicating that mutations at both alleles contribute to the anemia phenotype. The mutant proteins are deficient in their transport activity or targeting to the endosomal or plasma membranes (summarized in Table 1).
Not only were these four probands anemic, all showed signs of hepatic iron overload—a finding which, at first, did not appear consistent with DMT1’s perceived role in iron absorption. The first possible explanation offered was that intestinal heme-iron absorption could be upregulated, independently of DMT1 (Priwitzerova et al., 2004). In the study just cited, the authors suggested that iron overload was not apparent in the b and mk rodent models because rodents have poorly developed heme-absorptive pathways and heme iron is not typically a part of rodent chow. However, the b rat—in which a defect in intestinal metal-ion uptake has been established directly (Knöpfel et al., 2005b)—also displays hepatic iron loading (Thompson et al., 2006).
It is plausible that the mutations affect the function of various DMT1 isoforms (section V.D.) differently, or that the severity of the mutation is more pronounced in the environment of the erythroid precursor cell endosome than it is at the intestinal brush border. A more straightforward explanation may be that the human mutations—like the rodent G185R mutation—possess some residual activity which, coupled with upregulation of the intestinal DMT1 isoform 1A/IRE(+) (section V.D.) by an erythropoietic factor, permits some iron absorption to proceed in these probands. Because the absorbed iron is poorly utilized by erythroid precursors, much is diverted to the liver stores. Supporting this notion, urinary levels of the iron-regulatory hormone hepcidin, whose actions lead to a downregulation of iron absorption (Ganz & Nemeth, 2006), are low or markedly suppressed in these probands (Table 1).
In contrast, a fifth anemic proband, homozygous for a missense mutation leading to a G75R substitution in putative TM1 (Fig. 1), displayed no hepatic iron loading (Blanco et al., 2009). Not discounting the possibility that iron loading may progress later in life, the authors in the study just cited postulated that the G75R mutant should be completely deficient in DMT1 functional activity and would more fully ablate iron absorption. To begin to test this hypothesis, we expressed wildtype DMT1 and the G75R mutant in RNA-injected Xenopus oocytes and measured 55Fe2+ transport and currents.
In oocytes expressing DMT1–enhanced green fluorescent protein (EGFP), we observed strong EGFP fluorescence over the entire cell perimeter, whereas we detected only very faint fluorescence in oocytes expressing G75R–EGFP (Fig. 2A). We found no detectable iron-transport activity in the mutant (Fig. 2B). In oocytes expressing wildtype DMT1 superfused first with pH-7.5 medium, then pH 5.5, we observed a small inward current (Fig. 2C) that we have previously demonstrated to be a H+ ‘leak’ pathway (Gunshin et al., 1997; Mackenzie et al., 2006), and a large inward current upon the addition of 50 μM Fe2+. In contrast, G75R mediated a very large inward H+ leak current that was inhibited partly by the addition of Fe2+. Therefore, although the G75R mutant was not processed to the oocyte plasma membrane to the same extent as was wildtype DMT1, G75R exhibited a partial reaction (H+ transport) and was completely deficient in iron-transport activity.
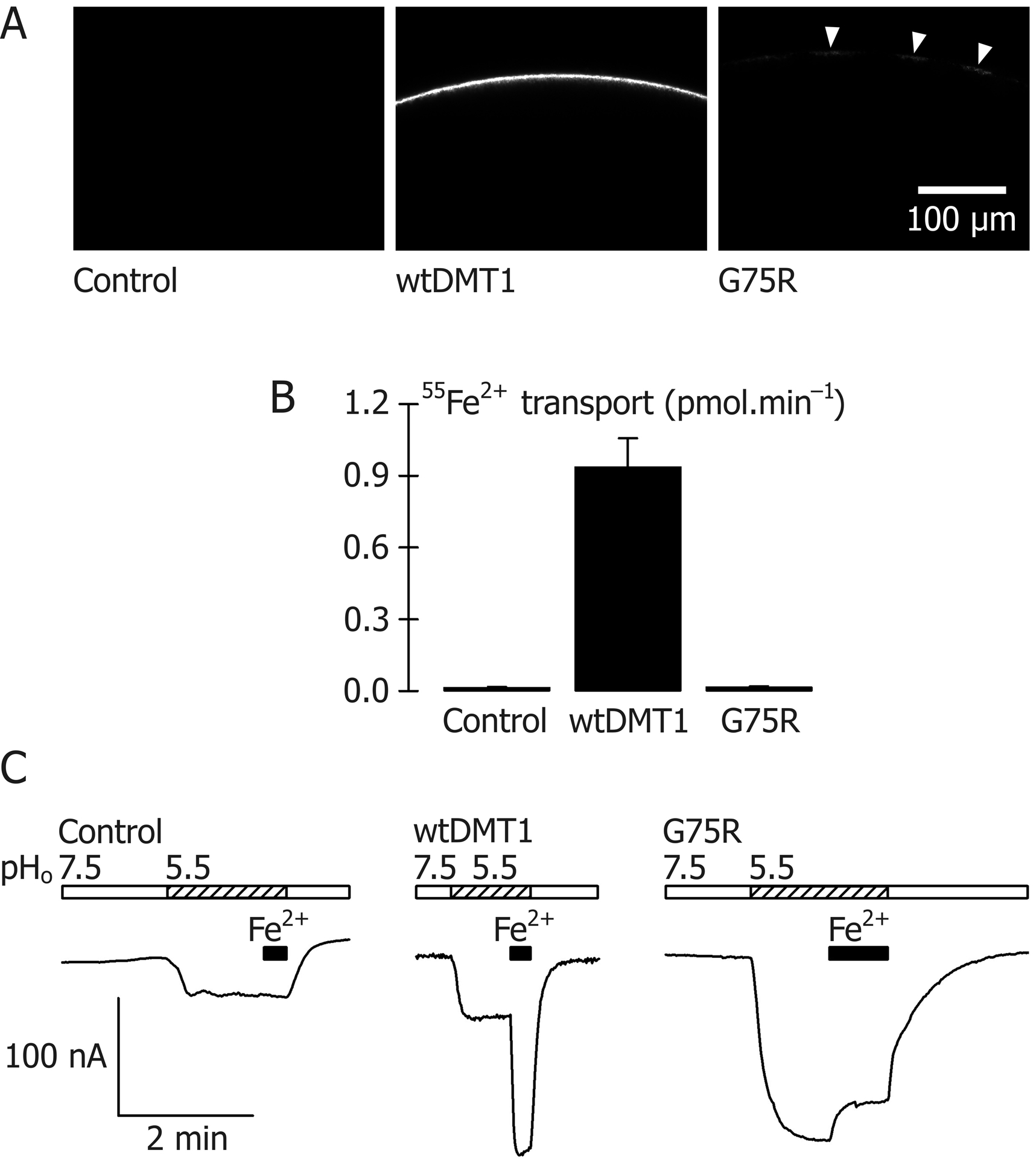
Impact of the G75R mutation on expression and activity of human DMT1 in RNA-injected Xenopus oocytes. A, Imaging of control oocytes, and wildtype DMT1 (wtDMT1)–enhanced green fluorescent protein (EGFP) or G75R–EGFP expression in RNA-injected Xenopus oocytes as described (Pinilla-Tenas et al., 2011) using the Zeiss LSM 7 DUO confocal laser-scanning microscope (excitation at 488 nm) fitted with the LD C-Apochromat 40×/1.1 W Korr objective to measure emission in the band 500–531 nm at a pinhole setting of 36 μm. Representative images are presented in which the optical slice (0.6 μm depth) approximately bisects the oocyte. Faint fluorescence at the cell perimeter of oocytes expressing G75R is marked by the white arrowheads. B, Transport of 2 μM 55Fe2+ was measured in control oocytes and oocytes expressing wildtype human DMT1 (wtDMT1) or G75R for 10 min at pH 5.5 in the presence of 1 mM L-ascorbic acid as described (Shawki & Mackenzie, 2010). Data are mean and S.D. for 10–11 oocytes per group. Analysis of variance (P < 0.001) followed by Holm–Šidák pairwise comparisons revealed that G75R did not differ from control (adjusted P = 0.94). C, Continuous currents recordings in a control oocyte and oocytes expressing wildtype human DMT1 or G75R and voltage clamped at −70 mV as described (Shawki & Mackenzie, 2010). Oocytes were continuously superfused for the periods shown by the bars with pH 7.5 medium (empty bars) or pH 5.5 medium (hatched bars) and 50 μM Fe2+ (black bars) in the presence of 1 mM L-ascorbic acid.
For many years, investigators have known of human pedigrees containing multiple members displaying a severe microcytic-anemia phenotype, characterized by a deficiency in intestinal iron absorption that was only partially corrected with parenteral iron administration (Buchanan & Sheehan, 1981; Hartman & Barker, 1996). Whether or not such pedigrees harbor mutations in DMT1 is not known, but their phenotypes are strikingly similar to those described for the DMT1 mutants just described. In any event, mutations in human DMT1 are extremely rare—presumably a reflection of both its critical, nonredundant functional roles and its long phylogenetic ancestry (Chaloupka et al., 2005), such that mutations in DMT1 are not generally tolerated.
IV. Functional Properties of DMT1
A. Molecular Mechanisms of DMT1-Mediated Iron Transport
The proton electrochemical potential gradient provides the thermodynamic driving force for concentrative iron transport from the cell exterior or endosome into the cytoplasm, placing DMT1 among an important class of proteins we call cotransporters (symporters). When we expressed rat or human DMT1 in RNA-injected oocytes under voltage-clamp conditions, we found that Fe2+ evoked large inward currents (Gunshin et al., 1997; Illing et al., 2012; Mackenzie et al., 2006; Mackenzie et al., 2007; Shawki & Mackenzie, 2010). Such rheogenicity could conceivably arise from influx of Fe2+ alone; however, the observation of presteady-state currents in the absence of metal-ion substrates pointed to the involvement of a second ligand. Presteady-state currents, which decayed with time constants of 15–40 ms, were observed following step-changes in membrane potential (Cohen et al., 2003; Gunshin et al., 1997; Mackenzie et al., 2006; Mackenzie et al., 2007). That these currents were pH-dependent was our first indication that DMT1 might be H+-coupled. Presteady-state currents have been observed for other ion-coupled transporters, e.g. the H+/peptide cotransporter PEPT1 (Mackenzie et al., 1996a), the Na+/glucose cotransporters SGLT1 and SGLT3 (Loo et al., 1993; Mackenzie et al., 1996b), and the Na+/ascorbic acid cotransporter SVCT1 (Mackenzie et al., 2008). In the studies just cited, computer simulation of transport models have allowed us to attribute presteady-state currents in cotransporters to two steps in the catalytic cycle, namely (1) reorientation of the empty, charged carrier within the membrane electric field, and (2) binding/dissociation of the driving ion partway within the membrane electric field (i.e., an “ion-well” effect). The magnitude of the presteady-state currents correlates with plasma-membrane expression of the transporter and provides an estimate of the number of functional units (Mackenzie et al., 2007; Zampighi et al., 1995). When we expressed in oocytes a human DMT1 mutation (R416C) associated with disease, we observed altered kinetics of the presteady-state currents (compared with wildtype) consistent with the mutation having slowed the return of the empty carrier to the outward-facing state (Shawki et al., 2006).
55Fe2+ uptake and Fe2+-evoked currents in oocytes expressing DMT1 were stimulated at low pH (at least to lower limits tested, pH 5.2–5.5) compared with pH 7.2–7.5 (Gunshin et al., 1997; Illing et al., 2012; Mackenzie et al., 2006; Mackenzie et al., 2007). Such pH dependence of metal-ion transport was observed also in other preparations expressing DMT1 (Elisma & Jumarie, 2001; Forbes & Gros, 2003; Garrick et al., 2006; Lam-Yuk-Tseung et al., 2003; Linder et al., 2006; Picard et al., 2000; Tandy et al., 2000; Xu et al., 2004; Zhang et al., 2008) with one exception (Worthington et al., 2000), which we have discussed elsewhere (Mackenzie et al., 2006). Kinetic analyses in DMT1-expressing oocytes indicated that low extracellular pH exerted the following two effects: (1) to increase the apparent affinity for Fe2+, and (2) to provide the thermodynamic driving force for Fe2+ transport (Mackenzie et al., 2006). Conversely, we also found that Fe2+ transport was associated with a substantial H+ influx (Gunshin et al., 1997; Mackenzie et al., 2006). Collectively, these experimental observations indicate that DMT1-mediated Fe2+ and H+ fluxes are functionally coupled, i.e. DMT1 is a H+/Fe2+ cotransporter (symporter). Whereas H+ can substitute for Na+ as the driving ion in the Na+/glucose cotransporter SGLT1 (Hirayama et al., 1994), Na+ cannot substitute for H+ as the driving ion in DMT1 (Mackenzie et al., 2007).
Conventional cotransporter models are deterministic—they comprise a series of ligand-induced conformational changes that result in alternating access to a substrate-binding region, and ion-coupling ratios are fixed. Experimental data for DMT1, however, are difficult to reconcile with such a model. We and others have expressed wildtype mammalian DMT1 or mutant proteins in Xenopus oocytes and observed significant uncoupled fluxes (‘slippage’) both of H+ and Fe2+, and variable H+: Fe2+ coupling ratio (Mackenzie et al., 2006; Nevo & Nelson, 2004; Shawki et al., 2008). Development of models that can account for such slippage should direct experimental investigation into the molecular mechanisms of DMT1.
B. Metal-Ion Substrate Profile and Selectivity of DMT1
Which metal ions are transported by DMT1? That the question has been addressed in nearly as many reviews as there have been original research articles on the topic, we suspect, reflects both the high level of interest in the answer and the lack of complete consensus. A broad range of metal ions evoked currents in voltage-clamped Xenopus oocytes expressing rat or human DMT1 (Gunshin et al., 1997; Mackenzie et al., 2007). Many of these metal ions have been shown to inhibit radiotracer iron uptake in mammalian cell preparations in which DMT1 is constitutively or heterologously expressed (Bannon et al., 2003; Conrad et al., 2000; Garrick & Dolan, 2002; Linder et al., 2006; Picard et al., 2000; Zhang et al., 2008). Nevertheless, currents and inhibition only demonstrate reactivity with the transporter, but do not demonstrate per se that the metal ion is transported by DMT1.
We performed a comprehensive substrate-profile analysis of DMT1 by using two methods that would directly test for metal-ion transport in the oocyte system—radiotracer assay and a fluorescence-based assay using the metal-sensitive fluorophore PhenGreen SK (Illing et al., 2012). We found that Cd2+, Co2+, Fe2+, Mn2+, Ni2+, VO2+, and Zn2+ are transported substrates of DMT1; however, we found no evidence of DMT1-mediated transport of Ca2+, Cr2+, Cr3+, Cu1+, Cu2+, Fe3+, Ga3+, Hg2+, or VO1+ (Illing et al., 2012; Shawki & Mackenzie, 2010). Our findings extended the catalogue of DMT1 substrates and nonsubstrates and, to the extent that a few of these metals had been tested previously (see (Illing et al., 2012) for references), were consistent with previous studies with the notable exception of copper. Other investigators have measured copper transport in the human intestinal cell model Caco-2 cells and concluded that DMT1 can transport cuprous ion (Cu1+) (Arredondo et al., 2003; Espinoza et al., 2012), or have measured copper uptake in intestinal BBMV and concluded that DMT1 can transport cupric ion (Cu2+) (Knöpfel et al., 2005a). We found instead that DMT1 did not transport Cu1+ or Cu2+ in the oocyte preparation. One advantage of the oocyte system is its superior sensitivity—expression of DMT1 stimulated iron transport more than 700-fold over control (Illing et al., 2012)—whereas assigning transport activity to DMT1 is less clear in the mammalian systems. Still, it is possible that a subunit normally expressed in mammalian cells and required for copper transport is not expressed in the Xenopus oocyte, or that an isoform other than the 1A/IRE(+) expressed in the study just cited is capable of transporting copper. Clearly, further studies are needed if we are to conclude that copper is a transported substrate of DMT1 and, if so, to determine the valence of the transported species.
Assessing whether DMT1 plays important roles in the absorption or cellular transport of each of its substrates requires that we first take into account the relative selectivity with which DMT1 reacts with and transports each of its substrates. We found that DMT1 exhibited a strong preference (selectivity) for Fe2+ (
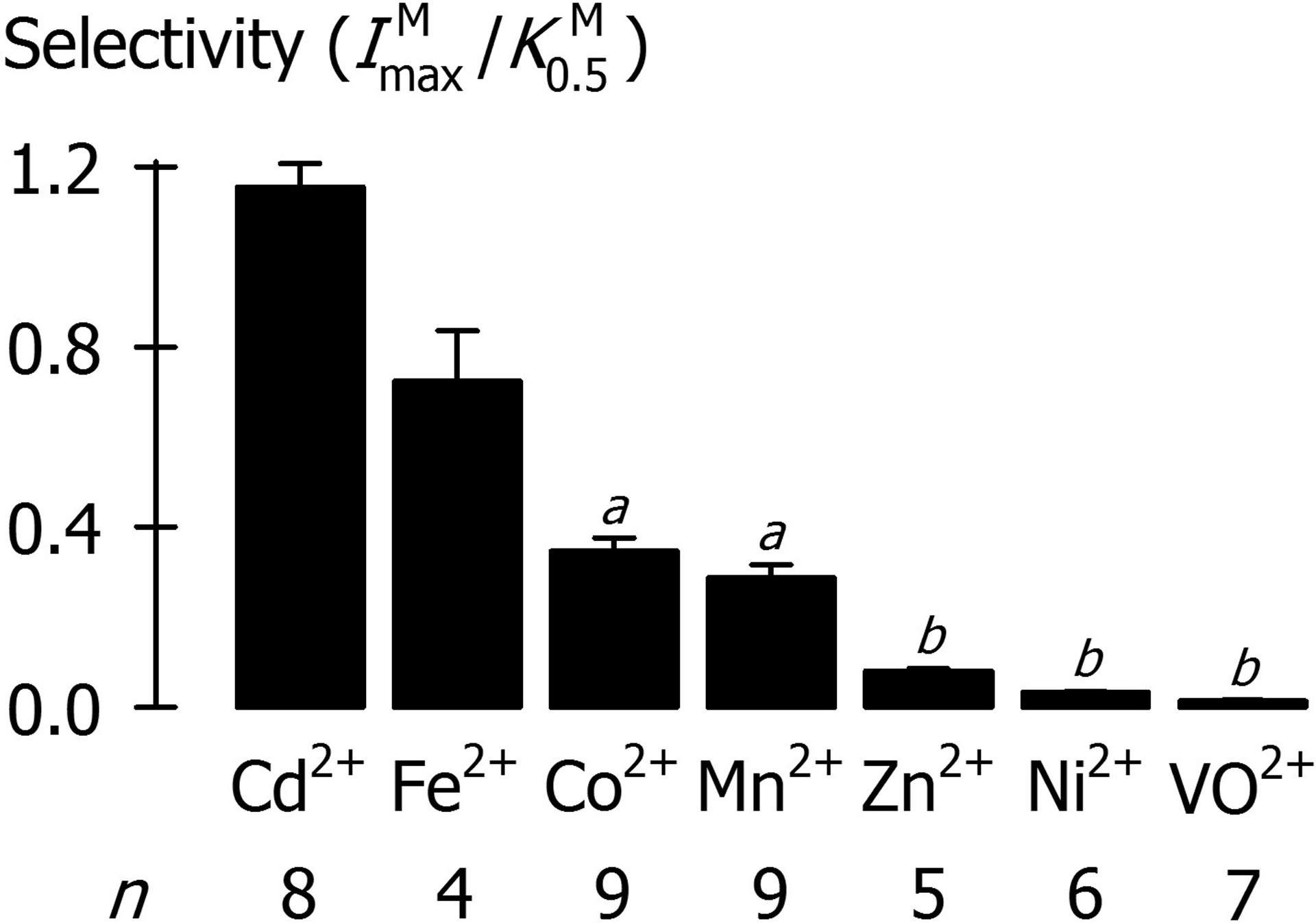
Metal-ion substrate selectivity of DMT1, expressed as the ratio
C. Structure–Function Analysis of DMT1 and its Homolog NRAMP1
Human DMT1 shares 61% identity at the amino-acid sequence level with NRAMP1, the only other member of the SLC11 gene family in mammals. DMT1 and NRAMP1 belong to an NRAMP superfamily in animals, plants and fungi, and extending to prokaryotes (Cellier et al., 2001; Chaloupka et al., 2005). Since there exists no crystal structure of mammalian DMT1, NRAMP1, or their orthologues, investigators have relied on the following approaches to study the structure–function relationships of SLC11 proteins: (1) predicting and testing membrane topology models, (2) site-directed mutagenesis and functional analysis, (3) structural analysis of short synthetic model peptides, and (4) modeling on known crystal structures of homologous proteins.
Predicting and testing membrane topology models—
DMT1 is an integral membrane protein that is observed in vitro to undergo glycosylation (Gruenheid et al., 1999; Mackenzie et al., 2007). Kyte–Doolittle hydrophobicity analysis predicted 12 transmembrane (TM) regions for rat (Gunshin et al., 1997), mouse (Czachorowski et al., 2009; Lam-Yuk-Tseung et al., 2003) and human DMT1 (Fig. 1). Two consensus sites of N-linked glycosylation (at residues Asn-336, Asn-349 according to numbering for the human 1B isoforms) reside in the fourth extracellular loop between TM7–8 (Mackenzie et al., 2007). To test the predicted membrane topology, hemagglutinin (HA) epitope tags were inserted into nonconserved regions of the protein, and the constructs expressed in Chinese hamster ovary (CHO) or pig kidney epithelial (LLC-PK1) cells (Czachorowski et al., 2009; Picard et al., 2000). Tags inserted in the loops between TM5–6, TM7–8, and TM11–12 were accessible to an anti-HA antibody in nonpermeabilized cells, confirming that these loops are exofacial (cell exterior or endosomal lumen). Tags inserted into the N- or C-terminal regions, or between TM4–5, TM6–7, and TM10–11, were accessible only in permeabilized cells, confirming their intracytoplasmic location. Introduction of the HA epitope tag in other regions disrupted DMT1 transport activity in whole or in part, so the orientation of the loops between TM1–2, 2–3, 3–4, 8–9, and 9–10 could not be definitively assigned. The DMT1 homologue NRAMP1 is a phosphoglycoprotein (Vidal et al., 1996) whose predicted membrane topology is very similar to that of DMT1. Consistent with that prediction, detection of HA epitope tags—used to drive the plasma-membrane expression of NRAMP1—in intact or permeabilized cells indicated that the loop between TM7–8 was extracellular and the N-terminus cytoplasmic (Forbes & Gros, 2003). Other investigators have proposed, however, that NRAMP1 be oriented oppositely (the C-terminus directed into the phagosomal lumen and the TM7–8 loop cytoplasmic) (Kuhn et al., 2001) and that NRAMP1 transport metal ions into the phagosome (or out of the cell if NRAMP1 was expressed on the plasma membrane) (Goswami et al., 2001; Kuhn et al., 2001; Techau et al., 2007).
Site-directed mutagenesis and functional analysis—
We and other investigators have used site-directed mutagenesis in an effort to probe the structure–function of DMT1 and identify critical elements in the structure of DMT1. Strategies for targeting amino-acid residues have included (1) identifying conserved residues, (2) predicting critical residues based on functional homology or structural modeling, and (3) identifying mutations associated with disease phenotypes in human patients or animal models. Around 30 amino acid residues are highly conserved among 145 NRAMP homologues and include a highly-conserved DPGN motif (Chaloupka et al., 2005). Disruption of the DPGN motif in a distant NRAMP homologue, the Escherichia coli manganese transporter MntH, ablated metal-dependent H+ transport in transfected E. coli cells (Chaloupka et al., 2005; Courville et al., 2008). In human DMT1, the corresponding D86PGN motif is predicted to reside in putative TM1 (Fig. 1); however, in one study this motif was assigned to the first external loop between TM1−2 (Cohen et al., 2003). Mutations at Asp-86 or Gly-88 of mouse or rat DMT1 (numbered according to the 1B isoforms) abolished metal-ion uptake in RNA-injected Xenopus oocytes or transfected CHO cells (Cohen et al., 2003; Lam-Yuk-Tseung et al., 2003). Additional residues in that region have been proposed to participate in metal binding; however, metal-ion coordination by DMT1 may be a function of several discrete elements in the protein.
We targeted a pair of histidine residues in putative TM6 of rat DMT1 because histidine residues are known to participate in metal binding in other proteins, and contribute to regulating transport activity in other transporters. Whereas the His267→Ala substitution had little impact on DMT1 transport activity, a His272→Ala substitution uncoupled the H+ and Fe2+ fluxes—a conclusion based on the observations that H272A supported a large H+ leak that was inhibited by Fe2+ and conversely mediated 55Fe2+ transport that was independent of pH (Mackenzie et al., 2006). Mutation of either His-267 or His-272 in mouse DMT1 affected the pH sensitivity of metal-ion transport in transfected CHO cells (Lam-Yuk-Tseung et al., 2003), leading these investigators to speculate that these two residues may form elements of the H+-conductive pathway through DMT1. Other residues can also participate in H+ transport or H+-coupling; for example, a Phe194→Ile substitution in rat DMT1 and the a Gly212→Val human DMT1 mutation associated with a disease phenotype appear to reduce the proton slippage observed for wildtype DMT1 (Nevo & Nelson, 2004; Shawki et al., 2008). Most other human mutations (section IV) and the G185R mutation found in the b rat and mk mouse appear to result in defects in protein processing or trafficking but, like G212V, some may also retain partial reactions. The mouse G185R mutant, however, resulted in an unexpected gain of function, involving a Ca2+ conductance not present in wildtype DMT1 (Xu et al., 2004).
Structural analysis of short synthetic model peptides—
To gain clues about DMT1 tertiary and quaternary structure, investigators have generated short synthetic model peptides, incorporated these into membrane-mimetic environments (solvents or micelles), and studied their structure by using circular dichroism spectropolarimetry and NMR spectroscopy. A peptide corresponding to TM4 of rat DMT1 assumed a central α-helical structure, randomly coiled N-terminal region, and (in SDS micelles at physiological pH 6.0–7.4) a disordered C-terminus (Li et al., 2003; Li et al., 2004). Despite their being embedded in the SDS interior, the side chains of some hydrophilic residues were water-accessible and may therefore form part of a water-filled channel. The observation that the TM4 peptide self-assembled into trimers suggested to these investigators that TM4 of DMT1 may assemble with other TM regions or several DMT1 monomers to form part of a translocation pore that is lined by hydrophilic residues in TM4 (Thr-189, Asp-192, Thr-193, and Asp-200) (Li et al., 2008). An identical peptide, except for the introduction of the G185R mutation, assumed the same structure as the wildtype TM4 peptide but self-assembled differently from the wildtype peptide, largely forming hexamers instead of trimers (Li et al., 2005). This observation led investigators to speculate that the introduction of the bulky, charged Arg residue in place of Gly at residue 185 should disrupt the quaternary structure of DMT1 or its multimeric assembly.
Synthetic peptides corresponding to TM1 and TM6 of rat DMT1 each formed discontinuous helical structures (i.e., α-helix–extended peptide–α-helix) (Wang et al., 2011b; Xiao et al., 2010; Xiao et al., 2011). Amino-acid substitutions in TM1 disrupted metal binding in TM1. The DPGN motif in TM1 is thought to comprise a flexible linker region (extended peptide) between the two α-helices, and pH-dependent effects upon this region could regulate the relative orientation of the α-helices and thus affect metal binding. The flexible linker in TM6, like that in TM1, is predicted to undergo rapid, subtle H+-induced conformational changes. Substitution of His-272 with Ala—which uncoupled the H+ and Fe2+ fluxes through rat DMT1 expressed in Xenopus oocytes (Mackenzie et al., 2006)—resulted in an unfolding of the N-terminal α-helix in TM6 and decreased intermolecular interactions (Xiao et al., 2010; Xiao et al., 2011). These observations confirm a critical role for His-272 in DMT1, and are consistent with the notion that this residue forms part of the mechanism by which Fe2+ transport is coupled to H+ translocation.
Modeling on known crystal structures of homologous proteins—
While no crystal structure is available for DMT1, investigators have used a threading approach to predict structural features of DMT1 by using the primary sequences of DMT1 or SLC11 homologues to query known crystal structures of homologous proteins (Courville et al., 2008; Czachorowski et al., 2009). Despite very little similarity at the amino acid level, SLC11 transporters could be superimposed on the structures of the bacterial Na+/Cl−-dependent leucine transporter (LeuT) of the SLC6 family (Yamashita et al., 2005) and the Na+-coupled galactose transporter (vSGLT) of the SLC5 family (Faham et al., 2008). Modeling SLC11 homologs on the LeuT structure suggested an internal symmetry in which TM1–5 and TM6–10 fold similarly but are oriented oppositely (Courville et al., 2008). The LeuT fold also predicts (Courville et al., 2008) the discontinuous helical structures of TM1 and TM6 observed for the short synthetic model peptides (Wang et al., 2011b; Xiao et al., 2010; Xiao et al., 2011) and places the D86PG and (inverted) H267PM motifs in the extended peptide regions of TM1 and TM6 respectively (Courville et al., 2008).
Thus, experimental results and model predictions provide evidence that TM1, TM4 and TM6 in particular contain structural elements that are critical for the catalytic activity of DMT1-mediated H+-coupled metal-ion transport. That mutations or the introduction of HA epitope tags elsewhere in DMT1 disrupt DMT1 catalytic activity or the correct processing of the protein would suggest that additional critical elements await their discovery.
D. Multiple Isoforms of DMT1: Tissue Distribution, Subcellular Targeting, and Regulation
There exist at least four isoforms of the human DMT1 protein. The bulk of the protein (531 or more amino acid residues) is conserved among all isoforms; however, they differ in both their N- and C-termini as a result of variant transcription of the human SLC11A2 gene (Table 2) at locus 12q13. Transcript variants arise from use of at least two, possibly three, discrete transcription initiation sites. Initiation from exon 1A, upstream of a second initiation site in exon 1B, adds 29 N-terminal amino acids not present in the 1B isoform (Hubert & Hentze, 2002). Both variants are spliced to exon 2 and, because exon 1B contains no start codon, translation of the 1B isoform begins with a start codon in exon 2. Orthologous transcript variants were also found in rat and mouse (Hubert & Hentze, 2002). Whereas the 1A isoform is found predominantly in intestine and kidney, 1B isoforms are widely expressed (Hubert & Hentze, 2002). In the NCBI Reference Sequence record for the human SLC11A2 gene (NG_021139.1, version 27 June 2012), these exons—originally described as 1A, 1B, and 2—are annotated exons 1, 2A, and 4, respectively. Initiation of transcription from exon 5 codes a putative, shorter isoform with a distinct N-terminus (MSTVDYL). All three 5′ variants are spliced to exon 6A, and transcription proceeds in common for most of the remainder of the coding region.
Table 2.
The SLC11A2 gene coding for DMT1 is described at NCBI Reference Sequence NG_021139.1 (accessed on June 27, 2012). The 1A/IRE(−) isoform is presumptive since a full-length transcript variant that would encode this isoform has not been recorded so far.
| Transcript variant | Protein isoform | ||||||
|---|---|---|---|---|---|---|---|
| Transcript number | NCBI reference sequence | Isoform number | Name | NCBI reference sequence | Peptide length | N- and C- terminal sequences | Predominant localization |
| 1 | NM_001174125.1 | 1 | 1A/IRE(+) | NP_001167596.1 | 590 | N– MRKKQLKTEAAPHCELKSYSKNSATQVSTMVLGPEQKMSDD… / …VSISKGLLTEEATRGYVK–C | Apical membrane, polarized epithelia |
| 2 | NM_001174126.1 | 2 | 1B/IRE(−) | NP_001167597.1 | 568 | N–MVLGPEQKMSDD… / …CHLGLTAQPELYLLNTMDADSLVSR–C | Early (recycling) endosomes, erythroid precursors |
| 3 | NM_001174127.1 | NP_001167598.1 | |||||
| 4 | NM_000617.2 | 3 | 1B/IRE(+) | NP_000608.1 | 561 | N–MVLGPEQKMSDD… / …VSISKGLLTEEATRGYVK–C | Late endosomes and lysosomes, widespread cellular distribution |
| 5 | NM_001174128.1 | NP_001167599.1 | |||||
| 6 | NM_001174129.1 | NP_001167600.1 | |||||
| 7 | NM_001174130.1 | 4 | Short-form IRE(+) | NP_001167601.1 | 557 | N–MSTVDYL… / …VSISKGLLTEEATRGYVK–C | |
| 8 | NR_033421.1 | Noncoding | |||||
| 9 | NR_033422.1 | Noncoding | |||||
| 1A/IRE(−) | 597 | N–MRKKQLKTEAAPHCELKSYSKNSATQVSTMVLGPEQKMSDD … / …CHLGLTAQPELYLLNTMDADSLVSR–C | Recycling endosomes* | ||||
Alternative splicing at the 3′ end of the SLC11A2 gene results in mRNA transcripts that differ in both their 3′-translated and 3′-untranslated regions (Canonne-Hergaux et al., 1999; Lee et al., 1998). One variant, IRE(+), contains in its 3′-untranslated region (3′-UTR) a stem-loop structure featuring a consensus iron-responsive element (IRE) that is important for post-transcriptional mRNA stabilization according to cellular iron status (section VI). Thus, the IRE(+) and IRE(−) isoforms are expected to differ mainly in their regulation by iron (Hubert & Hentze, 2002). The IRE(+) and IRE(−) transcripts also give rise to variability in the C-terminal amino acid sequences (Table 2). Investigators have found a total of 7 ‘coding’ transcript variants for human DMT1, and these code for four isoforms (Table 2). A fifth isoform, 1A/IRE(−), is presumptive because a corresponding human mRNA that would code for it has not been reported in the database. No mouse tissue tested expressed exclusively 1A and IRE(−) transcripts (Hubert & Hentze, 2002), although a 1A-IRE(−) cDNA has been generated from rat kidney cortex RNA (Abouhamed et al., 2006). An engineered 1A/IRE(−) human construct has been expressed in RNA-injected Xenopus oocytes, and the expressed protein was functional (Mackenzie et al., 2007).
The DMT1 isoforms differ also in their tissue distribution and subcellular targeting (Table 2). Epithelial cell lines predominantly express the IRE(+) isoforms whereas blood cell lines, the IRE(−) isoforms (Tabuchi et al., 2002). The N- and C-termini of the DMT1 isoforms may contain signal sequences that direct the subcellular targeting of the protein. Mutational analyses in transfected epithelial cell lines (HEp-2 and MDCK) have revealed that disruption of the N-terminal region H13CELKS (critically, Leu-16) prevents the insertion of the 1A isoform into the plasma membrane (Yanatori et al., 2010), and that the Y555XLXX region in the C-terminal cytoplasmic tail of the IRE(−) isoform is required for its targeting to early endosomes (Tabuchi et al., 2002). In contrast, IRE(+) was localized to the apical plasma membrane, late endosomes, and lysosomes of polarized cells, and its apical-membrane insertion was dependent upon N-glycosylation of DMT1 (Tabuchi et al., 2002).
The N- and C-terminal sequence variations between the isoforms do not however alter the functional properties of the isoforms. We have expressed 1A/IRE(+), 1A/IRE(−), 1B/IRE(+), and 1B/IRE(−) isoforms of human DMT1 in Xenopus oocytes and found (1) that the cellular uptake of 55Fe2+ correlated with the levels of expression at the plasma membrane, and (2) that the transport cycle turnover rate did not differ among the four isoforms, i.e., all four isoforms transport Fe2+ with equal efficiency (Mackenzie et al., 2007). We also found, after normalization for expression levels, that the 1A/IRE(+) and 1B/IRE(+) isoforms did not differ in their apparent affinity for Fe2+, pH dependence, or relative ability to transport a range of metal ions (Mackenzie et al., 2007; Mackenzie et al., 2010). Likewise, doxycycline-induced expression of the rodent 1A/IRE(+) and 1B/IRE(+) isoforms in stably transfected human embryonic kidney HEK293 cells revealed no differences in their ability to transport Fe2+ or Mn2+ (Garrick et al., 2006; Mackenzie et al., 2010).
V. Regulation of the Cellular Expression of DMT1
The dominant mechanism by which DMT1 is regulated at the cellular level is thought to be via post-transcriptional mRNA stabilization of the IRE(+) isoform in response to cellular iron levels. Binding of the 3′-UTR mRNA iron-responsive element (IRE) by iron-responsive binding protein-1 (IRP1) confers RNA stability under conditions of low iron (Gunshin et al., 2001). In contrast, the IRE(−) form is not subject to iron-dependent regulation (Rolfs et al., 2002; Zoller et al., 2001). Less is known about IRE/IRP interactions in DMT1 than for other IRE-containing transcripts, but control of expression of the DMT1 IRE(+) isoforms appears analogous to that of the transferrin receptor (TfR) (Pantopoulos, 2004; Wallander et al., 2006). Other metals, such as Mn2+, are also bound by iron-responsive binding proteins (Kwik-Uribe et al., 2003) and may exert some control over DMT1 expression.
The liver hormone hepcidin regulates iron metabolism predominantly via its interaction with the iron-export protein ferroportin (Ganz & Nemeth, 2006). Ferroportin binds hepcidin, triggering the internalization and degradation of this transporter. The resulting transient increase in intracellular iron is thought to act via the IRE/IRP system to downregulate expression of DMT1 in enterocytes. Nevertheless, novel mechanisms are emerging by which intestinal DMT1 may be regulated independently of ferroportin or independently of hepcidin. Two studies have proposed that hepcidin directly controls DMT1 expression. Hepcidin inhibited apical 55Fe uptake in both Caco-2 cells and rat duodenal segments apparently by decreasing DMT1 transcription with no change in ferroportin mRNA or protein (Mena et al., 2008). Other investigators using similar preparations also observed decreased DMT1 mRNA but attributed the loss of DMT1 transport activity mainly to its ubiquitin-dependent proteasomal degradation (Brasse-Lagnel et al., 2011). Such degradation may be facilitated by the adapter proteins Ndfip1 and Ndfip2 (Foot et al., 2008). Indeed, DMT1 levels in enterocytes were elevated in Ndfip1-null mice fed a low-iron diet more strongly than in wildtype mice (Foot et al., 2011). Ubiquitination and proteasomal degradation of DMT1 is reviewed elsewhere (Garrick et al., 2012), and data provided there indicate that ubiquitination by Parkin is specific to the 1B/IRE(−) isoform and not 1A/IRE(+).
Mutations in the hemochromatosis gene HFE are responsible for the majority of clinical cases of iron overload. Much emphasis has been placed on its controlling iron absorption by modulating the expression of hepcidin in the liver, but there is evidence also of a role for HFE in the enterocyte (reviewed, (Fleming & Britton, 2006)). Some colocalization of HFE with DMT1 is observed in rat enterocytes (West et al., 2006). Ectopic expression of HFE in Caco-2 cells increased the apical distribution of an HFE–β2 microglobulin complex that coprecipitated with DMT1, and direct interaction of DMT1 with HFE–β2 microglobulin was proposed to explain the decreased apical iron-uptake activity (Arredondo et al., 2006).
Transcript variants lacking the IRE are not subject to regulation by interactions of IRP at the translational level; however, a microRNA, miR-Let-7d, appears capable of inhibiting translation of the DMT1 IRE(−) isoform in erythroid cell lines CD34(+), K562, and HEL (Andolfo et al., 2010), suggesting that miR-Let-7d could regulate iron acquisition as erythroid cells differentiate. Endocytosis of DMT1 protein (corresponding to the IRE(+) isoform) from the brush-border membrane of enterocytes has been observed following iron feeding, a response that appears to be directed by peripheral benzodiazepine receptor-associated protein 7 (PAP7) based on the observation that DMT1 and PAP7 could be copurified from BBMV and were internalized together (Okazaki et al., 2012). In addition, siRNA inhibition of PAP7 in K562 cells caused a reduction in the plasma-membrane expression of DMT1 protein with no change in DMT1 mRNA (Okazaki et al., 2012). PAP7 expression is widespread (Okazaki et al., 2012) and appears to interact with DMT1 also in neurons (Cheah et al., 2006).
DMT1 is also regulated at the transcriptional level by hypoxia and stress-related signaling. Exposure of P19 embryonic carcinoma cells to nitric oxide decreased expression of the 1B but not 1A isoform (representing both IRE(+) and IRE(−) isoforms). This effect appears to have resulted from decreased binding of the nuclear factor NF-κB (p65 subunit) to the 1B promoter region of DMT1 (Paradkar & Roth, 2006). In the intestine, hypoxia regulates the expression of DMT1 via stabilization of the hypoxia-inducible transcription factor HIF2α, which can bind the 1A promoter region of DMT1, and intestine-specific ablation of HIF2α blunted the increased expression of DMT1 in response to iron deficiency (Mastrogiannaki et al., 2009; Shah et al., 2009).
VI. Tissue-Specific Roles of DMT1 in Physiology and Pathophysiology
A. Erythroid Precursor Cells
Lethal-dose-irradiated wildtype mice into which hematopoietic stem cells were transplanted from SLC11A2−/− mice exhibited defective erythropoiesis, whereas those injected with hematopoietic stem cells of wildtype origin were normal (Gunshin et al., 2005a), revealing a critical role for DMT1 in erythroid cells. The predominant DMT1 isoform in erythroid precursor cells, 1B/IRE(−), is localized to early (recycling) endosomes in which it participates in transferrin (Tf)-dependent iron acquisition (Canonne-Hergaux et al., 2001; Gruenheid et al., 1999; Touret et al., 2003). The transferrin cycle and role of DMT1 is illustrated in Fig. 4. DMT1 transports free Fe2+ from the endosome to the cytoplasm, a step that is thought to be energized by the H+ electrochemical potential gradient established by the vacuolar H+-ATPase (V-ATPase).
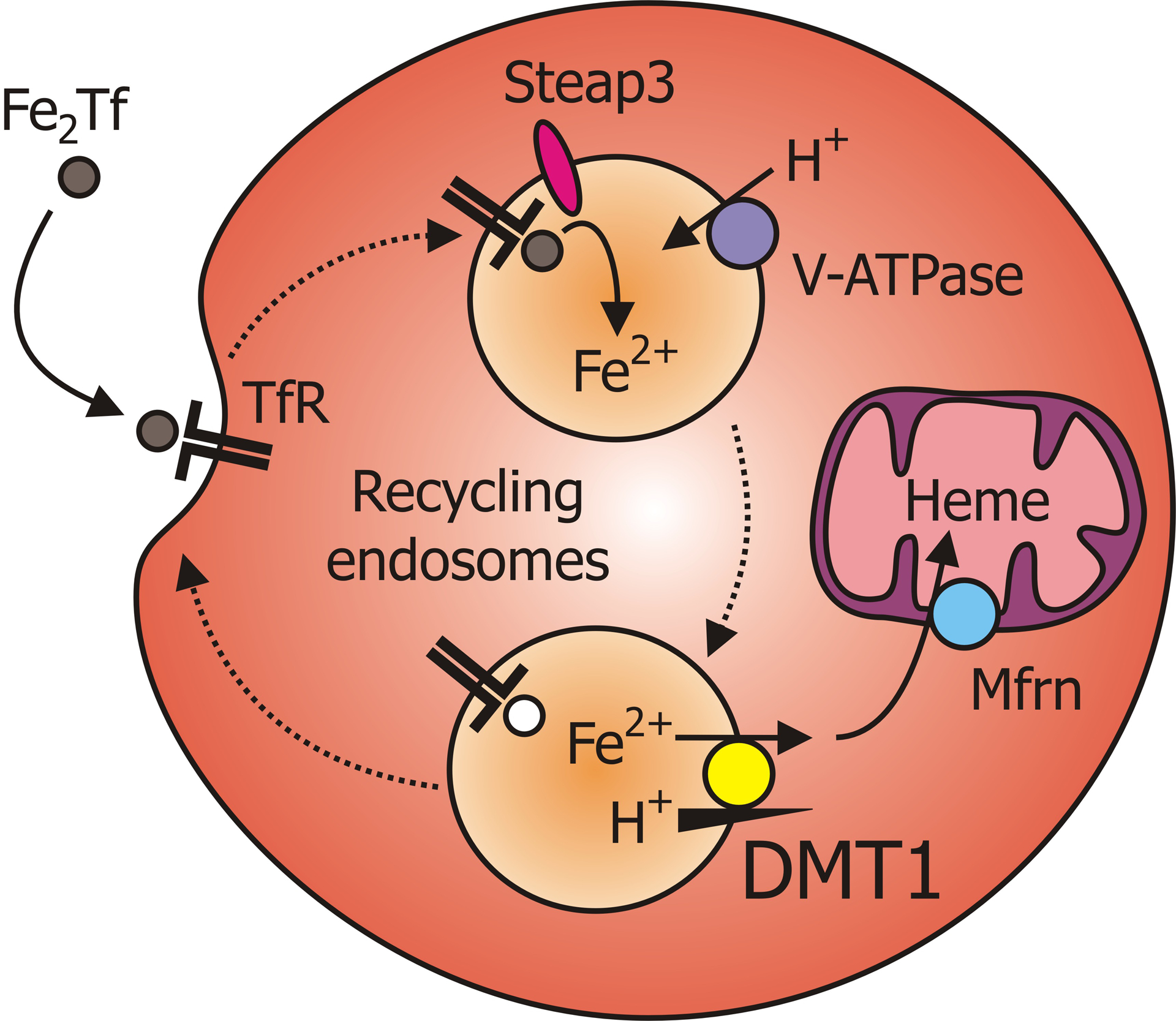
Model of iron uptake in erythroid precursor cells. Iron acquisition in erythroid precursor cells begins with the binding of holotransferrin (FeTf or Fe2Tf) by transferrin receptor-1 (TfR) and their endocytosis. Acidification of the endosome by the vacuolar H+-ATPase (V-ATPase) (Saroussi & Nelson, 2009) permits the dissociation of Fe3+ from the Tf–TfR complex and provides the proton-motive force to drive DMT1-mediated transport of iron, after its reduction by the ferrireductase six-transmembrane epithelial antigen of the prostate-3 (Steap3) (Knutson, 2007; Ohgami et al., 2005), from the endosome to the cytoplasm. Alternatively, Steap3 may interact directly with the Fe–Tf–TfR complex, and this may promote the dissociation of the reduced iron (Dhungana et al., 2004). The apotransferrin–TfR complex is returned to the plasma membrane, and cytoplasmic iron meanwhile is transported via mitoferrin (Shaw et al., 2006) into the mitochondrion for the production of heme. Redrawn and modified from Mackenzie & Hediger, 2004; © Springer Science+Business Media B.V., used with permission.
Some DMT1 is also detected on the plasma membrane of erythroid cells. Following endocytosis, the retromer cargo-recognition complex in maturing endosomes directs DMT1 to the recycling pathway (Tabuchi et al., 2010) and, subsequently, the return of DMT1 to the plasmalemma is dependent on phosphatidylinositol 3-kinase activity, but not lipid rafts or caveolae (Touret et al., 2003). Although DMT1 retains some activity at pH 7.4 (Garrick et al., 2006; Mackenzie et al., 2006), plasmalemmal DMT1 is not expected to contribute significantly to iron acquisition in erythroid precursors because (1) most blood iron is associated with transferrin under normal conditions and (2) the endosomal pH (≈ 6.0) is more optimal for DMT1-mediated Fe2+ transport than is plasma pH (≈ 7.4) (Mackenzie et al., 2006).
Expression of DMT1 is detected in at least some cell types in every organ tested (Gunshin et al., 1997; Hubert & Hentze, 2002; Wang et al., 2012b). We will see that, in most other cell types, DMT1 participates in Tf-dependent iron acquisition akin to that in the erythroid precursor except that, in many nonerythroid cells, this function may be served by the 1B/IRE(+) isoform predominantly observed in late endosomes and lysosomes (Table 2).
B. Epithelia
Intestinal DMT1 is critical for iron homeostasis in the mouse (Gunshin et al., 2005a; Shawki et al., 2012), although defective intestinal iron transport has not yet been demonstrated in the DMT1int/int model as it has for the mk mouse (Edwards & Hoke, 1972) and b rat (Knöpfel et al., 2005b). Haploinsufficiency of intestinal absorption is suggested by the lower iron stores in the SLC11A2+/− heterozygote (Gunshin et al., 2005a), but this possibility will be better tested in heterozygotes (DMT1+/int) of the conditional intestinal knockout model. The 1A/IRE(+) isoform predominates in the intestine, and its expression is exquisitely upregulated by low iron status (Canonne-Hergaux et al., 1999; Gunshin et al., 1997; Hubert & Hentze, 2002; Tabuchi et al., 2002) (see Table 2). Intestinal DMT1 is also, inappropriately, upregulated in hereditary hemochromatosis and is expected to contribute to the hyperabsorption of iron observed in this and other iron-overload disorders (section VIII.B.). DMT1 is expressed at the brush-border membrane of human and mouse duodenal enterocytes, and Caco-2 cells (Canonne-Hergaux et al., 1999; Griffiths et al., 2000; Tandy et al., 2000). DMT1 mediates the apical uptake of iron (reviewed, (Mackenzie & Garrick, 2005)) after its reduction by luminal ascorbic acid or surface ferrireductases, which may include DcytB (Cybrd1) and Steap2 (reviewed, (Knutson, 2007; McKie, 2008)).
The acidic microclimate at the intestinal brush border, generated by the intestinal Na+/H+ exchangers, is expected to provide the H+ to drive DMT1-mediated iron uptake (Mackenzie et al., 2011). Na+/H+ exchanger expression and activity is switched on towards weaning in the rat (Collins et al., 1997) and iron absorption is not impaired in suckling Belgrade rat pups (Thompson et al., 2007a), suggesting that a transporter other than DMT1 serves iron absorption prior to weaning. DMT1 immunoreactivity (possibly the 1B/IRE(+) isoform) has also been identified on the basolateral membrane of enterocytes, and iron-transport activity in intestinal basolateral membrane vesicles or basolateral membranes of Caco-2 cells has been ascribed to DMT1 (Knöpfel et al., 2005b; Núñez et al., 2010). What roles DMT1 may serve on enterocyte basolateral membranes are not yet clear, but may include supplying iron to enterocytes during dietary iron deficiency or participating in the regulation of iron absorption.
DMT1 efficiently transports cadmium (Fig. 3) (Illing et al., 2012). Iron deficiency is a risk factor for cadmium intoxication (see the work just cited for references), suggesting that cadmium and iron share a common absorptive pathway. Indeed, investigators have ascribed to DMT1 the Cd2+ transport observed in Caco-2 cells (Bannon et al., 2003). Iron restriction increased both intestinal DMT1 mRNA and cadmium accumulation in rats and mice (Park et al., 2002; Suzuki et al., 2008), implicating DMT1 in cadmium absorption. Clouding that conclusion, in one of the studies just cited (Suzuki et al., 2008), mk mice accumulated cadmium to the same levels as did wildtype mice; however, direct measurement of rates of absorption may be needed to test the role of DMT1 in cadmium absorption and intoxication.
DMT1 is abundantly expressed in the kidney (Hubert & Hentze, 2002; Wang et al., 2012b). One group has found that DMT1-IRE(+) immunoreactivity in rat kidney was strongest at the apical membranes of the collecting duct and the thick ascending limb of Henle’s loop (Ferguson et al., 2001), roughly consistent with the major sites of absorption of a 55FeCl3 dose delivered by intratubular microinjection (Wareing et al., 2000). Strong DMT1 immunoreactivity was also observed in the proximal tubule but confined to an intracellular location (Ferguson et al., 2001; Ferguson et al., 2003). Because no uptake of a 55FeCl3 dose was observed in proximal tubule, we might surmise that an endosomal DMT1 isoform serve instead Tf-dependent iron uptake in the proximal tubule. Evidence for the presence of Tf in the glomerular filtrate is reviewed elsewhere (Smith & Thévenod, 2009), and internalization of Tf by megalin-dependent, cubilin-mediated endocytosis has been described for mammalian kidney proximal tubules (Kozyraki et al., 2001). A second group studying DMT1 localization—this time in mouse kidney—has observed strong DMT1-IRE(+) immunoreactivity in the proximal tubule and concluded that DMT1 is expressed at the apical membrane (Canonne-Hergaux & Gros, 2002). It is possible that the discrepancy in the subcellular localization of mouse and rat DMT1 in these studies is explained by a species difference, detection of different isoforms (the antibodies used were against the same region of the protein but not identical), or technical difficulties; however, Smith & Thévenod (2009) observed sub-apical DMT1 immunoreactivity also in the mouse and attributed this to expression of DMT1 in late endosomes and lysosomes. Immunogold electron microscopy of rat kidney cortex also places DMT1 in late endosomes and lysosomes, and DMT1 colocalized with the late endosomal/lysosomal markers LAMP1 in the WKPT-0293 Cl.2 cell line derived from rat proximal tubule (Abouhamed et al., 2006). Nevertheless, given the functional homology between the small intestine and renal proximal tubule, and the observation (Hubert & Hentze, 2002) that expression of the 1A/IRE(+) isoform is essentially limited to those tissues, the possibility should not be discounted that DMT1 appear on the apical membrane of proximal tubular cells at least in response to certain stimuli or regulators.
The relative importance of DMT1 in the renal reabsorption of iron (a topic that, historically, has not received much attention) is yet to be fully explored. Meanwhile, the literature supports an involvement of DMT1 in renal pathophysiology. Nephrotic syndromes may be associated with iron-induced damage resulting from increased glomerular permeability, increased transferrin uptake in the proximal tubule, and increased DMT1-mediated iron transport into the cytosol (reviewed, Smith & Thévenod 2009). DMT1 is thought also to contribute to cadmium-induced nephrotoxicity (reviewed, (Vesey, 2010)) by transporting cadmium from the late endosome/lysosome to the cytosol following its liberation from metallothionein. Cadmium–metallothionein-1 internalization by WKPT-0293 Cl.2 proximal tubular cells, via the same megalin-dependent receptor-mediated endocytic pathway used by Tf, leads to cytotoxicity (Wolff et al., 2006) that can be prevented by RNAi knockdown of late endosomal/lysosomal DMT1 (Abouhamed et al., 2007).
DMT1 is expressed in human term placenta (Georgieff et al., 2000; Li et al., 2012) and mouse placenta at gestational day 18 (Balesaria et al., 2012). Those studies differ with regard to the precise localization of DMT1 in placenta, but endosomal DMT1 would appear to participate in transferrin-dependent uptake from the maternal side. Transcript levels of an IRE(+) isoform are responsive to iron (Gambling et al., 2001; Li et al., 2012). Total body iron was normal in SLC11A2 global knockout mouse neonates, suggesting that fetal DMT1 is not critical for sufficient maternofetal iron transfer (Gunshin et al., 2005a); however, a placenta-specific DMT1 knockout may be needed to assess precisely the contribution of DMT1 to placental iron transfer. Clearly, there is some redundancy among placental iron-transfer pathways, which may include Zrt-/Irt-like transporter-8 (ZIP8, SLC39A8) (Jenkitkasemwong et al., 2012; Wang et al., 2011a).
Nasal and pulmonary epithelia express DMT1 that may provide additional routes of absorption of excess iron and of toxic heavy metals (e.g., cadmium, manganese) following occupational exposure (Ghio et al., 2005; Heilig et al., 2005; Heilig et al., 2006; Ruvin Kumara & Wessling-Resnick, 2012; Thompson et al., 2007b), although DMT1-mediated clearance of metals from the lung may serve to minimize metal-related injury in that tissue (Ghio et al., 2005; Wang et al., 2002b).
C. Phagocytes—DMT1 and NRAMP1 in Iron Recycling and Host Defense
DMT1 has been observed to colocalize with Tf in early endosomes in RAW264.7 murine macrophages transfected with a c-Myc-tagged DMT1 construct (Gruenheid et al., 1999); however, following phagocytosis of opsonized red blood cells, the tag was also observed in erythrocyte-containing phagosomes (Jabado et al., 2002). In the latter study, investigators also observed that endogenous DMT1 in TM4 and 15P-1 Sertoli cell lines was recruited to phagosomes containing latex beads or spermatozoids. It was therefore proposed that DMT1 participate in the recovery of iron from effete red blood cells in macrophages, and serve an analogous role in the recovery of iron (or other metals) from senescent sperm in Sertoli cells. If DMT1 plays a role in iron recycling, then it would appear to be shared with NRAMP1 because deficiencies in either protein reduced the efficiency of iron recycling in RAW264.7 macrophages (Soe-Lin et al., 2010). Supporting such a role for NRAMP1, investigators have observed (1) that iron recycling was accelerated in macrophages overexpressing NRAMP1 (Soe-Lin et al., 2008), and (2) that, in mice deficient in NRAMP1, iron accumulated in the liver and spleen during erythrophagocytosis and hemolytic anemia, whereas this iron was recycled to the marrow and erythrocytes in wildtype mice (Soe-Lin et al., 2009).
A more recent study has relied on endogenous expression of SLC11 proteins in bone marrow-derived macrophages (BMDM) presented with artificially aged erythrocytes. This model may be superior to transfection of DMT1 and NRAMP1 in RAW264.7 macrophages, and more physiological than the use of opsonized red blood cells for phagocytosis. Whereas DMT1 protein was detected in both quiescent and activated BMDMs, it largely colocalized with TfR1 in recycling endosomes and was not recruited to the erythrophagosome (Delaby et al., 2012). NRAMP1 on the other hand was recruited to the erythrophagosome in BMDMs. Importantly, the observation that the putative heme transporter (heme-responsive gene-1, HRG1)—but neither heme oxygenase protein HO-1 or HO-2—is recruited to the erythrophagosome (Delaby et al., 2012) prompts us to consider that heme may exit the erythrophagosome intact, and to question whether either NRAMP1 or DMT1 plays any role in iron exit from the phagosome. Whether they could serve that role in macrophages at other loci or participate in iron recycling at some other step, and whether NRAMP1 could transport metals other than Fe2+ out of the erythrophagosome, remain to be tested.
NRAMP1 was discovered in 1993 by positional cloning of a mouse gene affecting the capacity with which macrophages can fight bacterial pathogens (Vidal et al., 1993). Polymorphisms in the SLC11A1 gene coding for human NRAMP1 have been strongly associated with susceptibility to mycobacterial infections (tuberculosis, leprosy), and also associated with certain other infections, immune disorders and autoimmune disease (reviewed, (Cellier et al., 2007; Mackenzie & Hediger, 2004)). NRAMP1 expression is confined to phagocytes (neutrophils and macrophages) (Canonne-Hergaux et al., 2002; Govoni et al., 1997). A conserved N-terminal tyrosine-based motif (YGSI) directs NRAMP1 protein to the lysosomal membrane (Lam-Yuk-Tseung et al., 2006b). Upregulated in professional phagocytes, NRAMP1 contributes to antimicrobial function by extruding from the phagolysosome divalent metal ions (e.g. Mn2+, Fe2+) that may be essential cofactors for bacteria-derived enzymes or required for bacterial growth (Atkinson & Barton, 1999; Cellier et al., 2007; Cellier, 2012; Forbes & Gros, 2003; Jabado et al., 2000).
D. Brain
Iron transport and metabolism in the brain (reviewed, (Burdo & Connor, 2002; Moos & Morgan, 2004a; Núñez et al., 2012; Rouault & Cooperman, 2006; Snyder & Connor, 2009)) warrant special attention because of the association of neurodegenerative disease and iron accumulation. The chief pathway by which iron enters the brain is via the brain capillary endothelial cells of the blood–brain barrier (BBB); the choroid plexus of the blood–cerebrospinal fluid barrier (BCB) presents a second pathway. At both sites, iron is taken up primarily via Tf-dependent endocytosis (Bradbury, 1997), and the identification of DMT1 in BBB and BCB (Burdo et al., 2001; Burdo et al., 2004; Gunshin et al., 1997; Moos & Morgan, 2004b; Rouault et al., 2009; Siddappa et al., 2003) implicates DMT1 in that pathway. Colocalization of DMT1 with early endosomal markers in neuronal and astrocyte cell lines (Lis et al., 2004; Lis et al., 2005) suggests that DMT1 also participates in Tf-dependent uptake in these cells; although, in one study, DMT1 colocalized with late endosomal markers in neurons (Pelizzoni et al., 2012). DMT1 is expressed in neurons throughout the brain, including cortex, cerebellum, and thalamus, anterior olfactory nucleus, substantia nigra, striatum, and hippocampal pyramidal and granule cells (Burdo et al., 2001; Gunshin et al., 1997; Knutson et al., 2004; Moos et al., 2000; Williams et al., 2000).
DMT1 protein is also observed on the plasma membrane of neurons (Lis et al., 2005; Roth et al., 2000) and astrocytes (Wang et al., 2002c), thereby prompting us to consider a role for DMT1 in uptake of nontransferrin-bound iron (NTBI). Although maximally stimulated at lower pH, DMT1 is still active at pH 7.4 (Garrick et al., 2006; Mackenzie et al., 2006). Brain interstitial fluid and cerebrospinal fluid contain substantial levels of nontransferrin-bound iron (NTBI) (Moos & Morgan, 1998), much of it as Fe2+ because of the high levels of ascorbate in the brain; thus, DMT1 expressed at the plasma membrane could take up NTBI without the need for surface ferrireductases. Such a role has recently been ascribed to voltage-operated Ca2+ channels largely from the observation that iron uptake was inhibited by Ca2+ (Pelizzoni et al., 2011); however, because Ca2+ also inhibits DMT1 (Shawki & Mackenzie, 2010), this transporter should not be discounted. Hypoxia increased the expression of DMT1 on neuronal plasma membranes, and we should also consider a role for DMT1 in the transport of manganese at that location (Lis et al., 2005).
Neuronal iron accumulation is at least associated with prevalent neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease. DMT1 is abundantly expressed in the substantia nigra pars compacta (SNpc) (Gunshin et al., 1997; Moos et al., 2000), a site of iron enrichment in healthy subjects. In Parkinson’s disease, substantial iron accumulation is observed in the SNpc, and this may be associated with the oxidative stress and neuronal damage characteristic of the disease (Snyder & Connor, 2009). Parkin, loss of which is characteristic of a familial form of Parkinson’s disease, directs the ubiquitination of DMT1 1B/IRE(−) and its proteasomal degradation (Garrick et al., 2012). There is no strong evidence with which to associate DMT1 mutations or single-nucleotide polymorphisms with the initiation of neurodegenerative diseases (Blasco et al., 2011; Jamieson et al., 2005); however, upregulation of DMT1 is implicated in their neuropathogenesis (Aguirre et al., 2012; Blasco et al., 2011; Huang et al., 2006; Núñez et al., 2012; Salazar et al., 2008; Song et al., 2007; Zheng et al., 2009) and, conversely, loss of DMT1 function in the b rat and mk mouse provides some protection against iron-mediated toxicity (Moos & Morgan, 2004b; Salazar et al., 2008).
Manganism—a syndrome resembling Parkinson’s disease—results from chronic exposure to manganese (e.g., via occupational exposure in miners and welders) (Rivera-Mancía et al., 2011; Roth, 2006). In addition to the typical routes by which iron may enter the brain, atmospheric manganese may gain access to the CNS via olfactory or trigeminal presynaptic nerve endings and retrograde axonal transport to the brain (Roth, 2006; Tjälve & Henriksson, 1999). Absorption of a nasally instilled manganese dose in olfactory epithelium required DMT1 and was increased by anemia (Thompson et al., 2007b). Within the brain, neuronal manganese uptake also appears to rely on DMT1, and this pathway may contribute to manganese-induced neurotoxicity (Au et al., 2008; Rivera-Mancía et al., 2011; Roth, 2006; Roth & Garrick, 2003).
E. Other Tissues
DMT1 is widely expressed in other tissues (Gunshin et al., 1997; Hubert & Hentze, 2002; Wang et al., 2012b) in which DMT1 is thought to participate in Tf-dependent uptake in a manner similar to that in erythroid cells (Fig. 4). Such a role for DMT1 in liver (reviewed, (Graham et al., 2007)) would appear redundant with that of Zrt-/Irt-like transporter-14 (ZIP14). ZIP14 is associated with TfR-containing endosomes in the hepatocellular carcinoma cell line HepG2, and knockdown of ZIP14 in those cells by siRNA reduced the assimilation of iron from 55Fe–Tf by nearly 50% (Zhao et al., 2010); however, the pH-dependence of ZIP14-mediated iron transport activity is much less suited to the acidic environment of the endosome than is DMT1 (Mackenzie et al., 2006; Pinilla-Tenas et al., 2011). DMT1 is considered nonessential in liver because the DMT1-null (SLC11A2−/−) mouse and Belgrade rat exhibit hepatic iron loading (Gunshin et al., 2005a; Thompson et al., 2006); however, we should bear in mind that this conclusion derives from animal models in which hepatic iron uptake may be primarily of NTBI (Thompson et al., 2006), and plasma NTBI is absent or very low under normal conditions. In contrast, Tf-dependent iron uptake in hepatocytes is tightly regulated by iron status (Trinder et al., 1990).
Nevertheless, DMT1 may also contribute to the cellular uptake of NTBI characteristic of iron-overload conditions (section VIII.B.) because (1) DMT1 was observed to relocate to the plasma membrane of hepatocytes in iron overload (Trinder et al., 2000), (2) hepatocytes and hepatoma (HLF) cells overexpressing DMT1 exhibit cell-membrane expression of DMT1 and increased NTBI uptake (Shindo et al., 2006), and (3) DMT1 retains iron-transport activity at plasma pH (Garrick et al., 2006; Mackenzie et al., 2006) so it is capable of functioning as an NTBI transporter at the cell membrane. The extent to which DMT1 contributes to NTBI uptake in those organs most affected by iron overload (e.g., heart, liver, pancreas) requires further investigation, although clearly there is overlap with other pathways that may include ZIP8, ZIP14, and calcium channels (Jenkitkasemwong et al., 2012; Kumfu et al., 2011; Liuzzi et al., 2006; Oudit et al., 2003; Pinilla-Tenas et al., 2011; Wang et al., 2012b).
VII. Therapeutics and Nutrition
A. Iron Deficiency
Iron deficiency remains the most prevalent micronutrient deficiency worldwide and results in iron-deficiency anemia, as well as neurological and developmental defects in children. Iron fortification of infant milk formulas and cereals, and better diet, has decreased the incidence of iron deficiency over recent decades; nevertheless, a more precise understanding of the iron-absorptive machinery will lead to new strategies for improved iron nutrition.
Dietary calcium is known to reduce iron bioavailability, but the molecular basis of this interaction has been poorly understood. Although Ca2+ is not a transported substrate of DMT1, we have found that Ca2+ inhibited 55Fe2+ transport activity and currents associated with DMT1 expression in Xenopus oocytes with inhibition constants (Ki) of 1–20 mM (Shawki & Mackenzie, 2010). Because intestinal luminal calcium concentrations periodically reach the millimolar range, dietary calcium (from sources such as milk) could substantially inhibit DMT1-mediated Fe2+ absorption. Calcium may also decrease intestinal brush-border iron uptake by stimulating the derecruitment of DMT1 from the apical membrane. Treatment of Caco-2 cells with 2.5 mM CaCl2 for 4 h was sufficient to cause an 80% reduction in DMT1 immunoreactivity of the membrane fraction (Thompson et al., 2010). Interactions of calcium with DMT1 therefore should be taken into consideration when developing strategies for improved iron nutrition, milk formulation, and iron fortification.
B. Iron-Overload Disorders
Since there exists no regulated mechanism for the excretion of iron, its homeostasis requires that absorption of the metal be tightly regulated. Excess iron (unless it is lost by cell desquamation or bleeding) is retained in the body. Accumulation in the liver, heart, and other vital organs can result in cirrhosis, hepatocellular carcinoma, cardiomyopathy, endocrine disorders, and arthritis. Hereditary hemochromatosis (HHC) is a disorder characterized by tissue iron overload secondary to excessive dietary-iron absorption, and generally results from a disruption of the hepcidin–ferroportin axis regulating iron metabolism (Darshan et al., 2010; Fleming & Ponka, 2012). The most common form, HHC type 1, is an autosomal recessive disorder resulting from mutations in HFE (Harrison & Bacon, 2003). Intestinal expression of DMT1 is elevated in human patients and animal models of HHC type 1 in spite of increased serum iron or ferritin levels (Byrnes et al., 2002; Fleming et al., 1999). Less commonly, HHC can result also from mutations in the genes coding for hemojuvelin (type 2A), hepcidin (type 2B), transferrin receptor-2 (type 3) or ferroportin (type 4); the last of these being autosomal dominant. The mainstay of treatment for HHC is phlebotomy; however, the effectiveness of this approach is limited by poor patient compliance and the fact that phlebotomy itself can lead to upregulation of DMT1 mRNA in HHC patients (Dostalikova-Cimburova et al., 2011; Kelleher et al., 2004), presumably as a result of signaling from increased erythropoiesis post-phlebotomy.
Targeting DMT1 in the intestine may lead to superior therapies for the prevention of iron overload in HHC patients. Validation of intestinal DMT1 as a therapeutic target derives from at least the following three observations: (1) intestinal expression of DMT1 is upregulated in HHC patients; (2) the severity of the anemia phenotype of human probands carrying mutations in DMT1, demonstrating the critical role of DMT1 in human iron metabolism; and (3) the severe anemia in the intestine-specific DMT1-null mouse, demonstrating that DMT1 is the principal or only transporter serving intestinal uptake of nonheme iron, at least in the mouse post-weaning.
In thalassemia, an autosomal recessive disorder characterized by defective hemoglobin production, iron overload results from blood transfusions given to treat the primary lesion. Iron-chelation therapy is the main approach used to treat iron overload in these patients. Notably, increased intestinal iron absorption (presumably driven by the ineffective erythropoiesis) is also evident in thalassemia (Darshan et al., 2010; Hershko, 2010), so blockade of intestinal DMT1 may be of benefit in treating this disorder.
Screening approaches have identified a number of compounds that can inhibit DMT1-mediated iron uptake in mammalian cells. It is likely that some of these do not act directly upon DMT1. For example, two unrelated antioxidants, ebselen and pyrrolidine dithiobarbamate, inhibited DMT1-mediated Fe2+ uptake apparently by altering intracellular redox status because (1) both agents increased intracellular glutathione, and (2) ebselen did not inhibit transport of Mn2+ (the free metal is not redox active in physiological environments) (Wetli et al., 2006). A screen identified 10 compounds that could inhibit cellular uptake of NTBI in nontransfected HeLa cells (Brown et al., 2004). Of these, six were cytotoxic and of low apparent affinity (i.e., the concentration at which inhibition was half-maximal, IC50 ≥ 20 μM), and may therefore be of limited utility. Two of the 10 also inhibited Tf-dependent iron uptake, so this group of compounds may serve as useful experimental tools with which to discriminate NTBI and Tf-dependent transport pathways. When one of these compounds, a polysulfonated dye (NSC306711), was tested in HEK293T cells stably transfected with DMT1, it reversibly and competitively inhibited DMT1-mediated iron uptake with Ki ≈ 7 μM (Buckett & Wessling-Resnick, 2009); however, the action of NSC306711 is complex because this same compound also induced the internalization and degradation of TfR (Horonchik & Wessling-Resnick, 2008).
High-throughput screening has identified the triazinone pyrazolone as an inhibitor of iron transport in CHO cells transfected with DMT1 (IC50 ≈ 3 μM) (Cadieux et al., 2012). Optimization efforts generated substituted pyrazoles with lower IC50 values (higher apparent affinity) but which did not completely inhibit iron transport. The same group has also identified a class of diaryl and tricyclic benzylisothiourea compounds that more potently inhibited iron transport with submicromolar IC50 values in CHO cells expressing DMT1 (Zhang et al., 2012). These compounds also boasted low cytotoxicity and low cell permeability—the latter property being significant in that it may be more desirable to block intestinal DMT1 using an agent that is not absorbed, thereby minimizing secondary effects. The mechanism of action of these blockers and the nature of inhibition has not been described; however, inhibition by the substituted pyrazoles did not appear to be mediated via redox effects or metal chelation (Cadieux et al., 2012).
The identification of DMT1 blockers offers both novel experimental tools that will help us more clearly define the roles of DMT1 in metal metabolism, and the prospect of improved therapies in iron-overload disorders.
Acknowledgments
We thank François Canonne-Hergaux (INSERM, Toulouse, France) for helpful discussions and Colin J. Mitchell (University of Cincinnati) for assistance in confocal microscopy in Xenopus oocytes. Research in the authors’ laboratory (including that reported in Fig. 2) is supported by PHS Grant R01 DK080047 (to B. Mackenzie) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). E. J. Niespodzany was supported by the University of Cincinnati Medical Student Summer Research Program, funded in part by PHS Grant DK060444 (Short-Term Medical Student Training Grant, NIDDK). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, or the National Institutes of Health.
References
- Abouhamed M, Gburek J, Liu W, Torchalski B, Wilhelm A, Wolff NA et al. (2006). Divalent metal transporter 1 in the kidney proximal tubule is expressed in late endosomes/lysosomal membranes: Implications for renal handling of protein-metal complexes. American Journal of Physiology Renal Physiology, 290, F1525–F1533. [Abstract] [Google Scholar]
- Abouhamed M, Wolff NA, Lee W-K, Smith CP, & Thévenod F (2007). Knockdown of endosomal/lysosomal divalent metal transporter 1 by RNA interference prevents cadmium-metallothionein-1 cytotoxicity in renal proximal tubule cells. American Journal of Physiology Renal Physiology, 293, F705–F712. [Abstract] [Google Scholar]
- Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura-Aguilar J et al. (2012). The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals, 25, 795–803. [Abstract] [Google Scholar]
- Andolfo I, De FL, Asci R, Russo R, Colucci S, Gorrese M et al. (2010). Regulation of divalent metal transporter 1 (DMT1) non-IRE isoform by the microRNA Let-7d in erythroid cells. Haematologica, 95, 1244–1252. [Europe PMC free article] [Abstract] [Google Scholar]
- Arredondo M, Tapia V, Rojas A, Aguirre P, Reyes F, Marzolo MP et al. (2006). Apical distribution of HFE-beta2-microglobulin is associated with inhibition of apical iron uptake in intestinal epithelia cells. Biometals, 19, 379–388. [Abstract] [Google Scholar]
- Arredondo M, Muñoz P, Mura CV, & Núñez MT (2003). DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. American Journal of Physiology Cell Physiology, 284, C1525–C1530. [Abstract] [Google Scholar]
- Atkinson PGP, & Barton CH (1999). High level expression of Nramp1G169 in RAW264.7 cell transfectants: Analysis of intracellular iron transport. Immunology, 96, 656–662. [Abstract] [Google Scholar]
- Au C, Benedetto A, & Aschner M (2008). Manganese transport in eukaryotes: The role of DMT1. Neurotoxicology, 29, 569–576. [Europe PMC free article] [Abstract] [Google Scholar]
- Balesaria S, Hanif R, Salama M, Raja K, Bayele HK, McArdle H et al. (2012). Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haematologica, 97, 661–669. [Europe PMC free article] [Abstract] [Google Scholar]
- Bannerman RM (1976). Genetic defects of iron transport. Federation Proceedings, 35, 2281–2285. [Abstract] [Google Scholar]
- Bannon DI, Abounader R, Lees PS, & Bressler JP (2003). Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. American Journal of Physiology Cell Physiology, 284, C44–C50. [Abstract] [Google Scholar]
- Bardou-Jacquet E, Island ML, Jouanolle AM, Détivaud L, Fatih N, Ropert M et al. (2011). A novel N491S mutation in the human SLC11A2 gene impairs protein trafficking and in association with the G212V mutation leads to microcytic anemia and liver iron overload. Blood Cells Molecules and Disease, 47, 243–248. [Abstract] [Google Scholar]
- Barrientos De Renshaw T, Bhutiani M, Montross LK, & Andrews NC (2011). Iron overload resulting from increased intestinal DMT1 expression. American Journal of Hematology, 86, E43 (abstract). [Google Scholar]
- Beaumont C, Delaunay J, Hetet G, Grandchamp B, de Montalembert M, & Tchernia G (2006). Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood, 107, 4168–4170. [Abstract] [Google Scholar]
- Blanco E, Kannengiesser C, Grandchamp B, Tasso M, & Beaumont C (2009). Not all DMT1 mutations lead to iron overload. Blood Cells Molecules and Disease, 43, 199–201. [Abstract] [Google Scholar]
- Blasco H, Vourc’h P, Nadjar Y, Ribourtout B, Gordon PH, Guettard YO et al. (2011). Association between divalent metal transport 1 encoding gene (SLC11A2) and disease duration in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 303, 124–127. [Abstract] [Google Scholar]
- Bradbury MW (1997). Transport of iron in the blood-brain-cerebrospinal fluid system. Journal of Neurochemistry, 69, 443–454. [Abstract] [Google Scholar]
- Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, & Beaumont C (2011). Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology, 140, 1261–1271. [Abstract] [Google Scholar]
- Brown JX, Buckett PD, & Wessling-Resnick M (2004). Identification of small molecule inhibitors that distinguish between non-transferrin bound iron uptake and transferrin-mediated iron transport. Chemistry and Biology, 11, 407–416. [Abstract] [Google Scholar]
- Buchanan GR, & Sheehan RG (1981). Malabsorption and defective utilization of iron in three siblings. Journal of Pediatrics, 98, 723–728. [Abstract] [Google Scholar]
- Buckett PD, & Wessling-Resnick M (2009). Small molecule inhibitors of divalent metal transporter-1. American Journal of Physiology Gastrointestinal and Liver Physiology, 296, G798–G804. [Europe PMC free article] [Abstract] [Google Scholar]
- Burdo JR, & Connor JR (2002). Iron transport in the central nervous system Molecular and Cellular Iron Transport, Templeton, D. M, Marcel-Dekker, New York, pp. 487–508. [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG et al. (2001). Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. Journal of Neuroscience Research, 66, 1198–1207. [Abstract] [Google Scholar]
- Burdo JR, Simpson IA, Menzies S, Beard J, & Connor JR (2004). Regulation of the profile of iron-management proteins in brain microvasculature. Journal of Cerebral Blood Flow and Metabolism, 24, 67–74. [Abstract] [Google Scholar]
- Byrnes V, Barrett S, Ryan E, Kelleher T, O’Keane C, Coughlan B et al. (2002). Increased duodenal DMT-1 expression and unchanged HFE mRNA levels in HFE-associated hereditary hemochromatosis and iron deficiency. Blood Cells Molecules and Disease, 29, 251–260. [Abstract] [Google Scholar]
- Cadieux JA, Zhang Z, Mattice M, Brownlie-Cutts A, Fu J, Ratkay LG et al. (2012). Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1). Bioorganic & Medicinal Chemistry Letters, 22, 90–95. [Abstract] [Google Scholar]
- Canonne-Hergaux F, Calafet J, Richer E, Cellier M, Grinstein S, Borregaard N et al. (2002). Expression and subcellular localization of NRAMP1 in human neutrophil granules. Blood, 100, 268–275. [Abstract] [Google Scholar]
- Canonne-Hergaux F, Fleming MD, Levy JE, Gauthier S, Ralph T, Picard V et al. (2000). The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood, 96, 3964–3970. [Abstract] [Google Scholar]
- Canonne-Hergaux F, & Gros P (2002). Expression of the iron transporter DMT1 in kidney from normal and anemic mk mice. Kidney International, 62, 147–156. [Abstract] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, & Gros P (1999). Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood, 93, 4406–4417. [Abstract] [Google Scholar]
- Canonne-Hergaux F, Zhang A-S, Ponka P, & Gros P (2001). Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood, 98, 3823–3830. [Abstract] [Google Scholar]
- Cellier MF, Courville P, & Campion C (2007). Nramp1 phagocyte intracellular metal withdrawal defense. Microbes and Infection, 9, 1662–1670. [Abstract] [Google Scholar]
- Cellier MFM (2012). Nutritional immunity: Homology modeling of Nramp metal import. Advances in Experimental Medicine and Biology, 946, 335–351. [Abstract] [Google Scholar]
- Cellier MFM, Bergevin I, Boyer E, & Richer E (2001). Polyphyletic origins of bacterial Nramp transporters. Trends in Genetics, 17, 365–370. [Abstract] [Google Scholar]
- Chaloupka R, Courville P, Veyrier F, Knudsen B, Tompkins TA, & Cellier MFM (2005). Identification of functional amino acids in the Nramp family by a combination of evolutionary analysis and biophysical studies of metal and proton cotransport in vivo. Biochemistry, 44, 726–733. [Abstract] [Google Scholar]
- Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE III, Papadopoulos V et al. (2006). NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron, 51, 431–440. [Europe PMC free article] [Abstract] [Google Scholar]
- Chua ACG, & Morgan EH (1997). Manganese metabolism is impaired in the Belgrade laboratory rat. Journal of Comparative Physiology [B], 167, 361–369. [Abstract] [Google Scholar]
- Cohen A, Nevo Y, & Nelson N (2003). The first external loop of the metal ion transporter DCT1 is involved in metal ion binding and specificity. Proceedings of the National Academy of Sciences of the United States of America, 100, 10694–10699. [Europe PMC free article] [Abstract] [Google Scholar]
- Collins JF, Xu H, Kiela PR, Zeng J, & Ghishan FK (1997). Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. American Journal of Physiology Cell Physiology, 273, C1937–C1946. [Abstract] [Google Scholar]
- Conrad ME, Umbreit JN, Moore EG, Hainsworth LN, Porubcin M, Simovich MJ et al. (2000). Separate pathways for cellular uptake of ferric and ferrous iron. American Journal of Physiology Gastrointestinal and Liver Physiology, 279, G767–G774. [Abstract] [Google Scholar]
- Courville P, Urbankova E, Rensing C, Chaloupka R, Quick M, & Cellier MFM (2008). Solute carrier 11 cation symport requires distinct residues in transmembrane helices 1 and 6. Journal of Biological Chemistry, 283, 9651–9658. [Abstract] [Google Scholar]
- Czachorowski M, Lam-Yuk-Tseung S, Cellier M, & Gros P (2009). Transmembrane topology of the mammalian Slc11a2 iron transporter. Biochemistry, 48, 8422–8434. [Europe PMC free article] [Abstract] [Google Scholar]
- Darshan D, Frazer DM, & Anderson GJ (2010). Molecular basis of iron-loading disorders. Expert Reviews in Molecular Medicine, 12, e36. [Abstract] [Google Scholar]
- Delaby C, Rondeau C, Pouzet C, Willemetz A, Pilard N, Desjardins M et al. (2012). Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis. PLoS One, 7, e42199. [Europe PMC free article] [Abstract] [Google Scholar]
- Dhungana S, Taboy CH, Zak O, Larvie M, Crumbliss AL, & Aisen P (2004). Redox properties of human transferrin bound to its receptor. Biochemistry, 43, 205–209. [Abstract] [Google Scholar]
- Donovan A, Brownlie A, Dorschner MO, Zhou Y, Pratt SJ, Paw BH et al. (2002). The zebrafish mutant gene chardonnay (cdy) encodes divalent metal transporter 1 (DMT1). Blood, 100, 4655–4659. [Abstract] [Google Scholar]
- Dostalikova-Cimburova M, Kratka K, Balusikova K, Chmelikova J, Hejda V, Hnanicek J et al. (2011). Duodenal expression of iron transport molecules in patients with hereditary hemochromatosis or iron deficiency. Journal of Cellular and Molecular Medicine, 16, 1816–1826. [Europe PMC free article] [Abstract] [Google Scholar]
- Edwards JA, & Hoke JE (1972). Defect of intestinal mucosal iron uptake in mice with hereditary microcytic anemia. Proceedings of the Society for Experimental Biology and Medicine, 141, 81–84. [Abstract] [Google Scholar]
- Elisma F, & Jumarie C (2001). Evidence for cadmium uptake through Nramp2: Metal speciation studies with Caco-2 cells. Biochemical and Biophysical Research Communications, 285, 662–668. [Abstract] [Google Scholar]
- Espinoza A, Le BS, Olivares M, Pizarro F, Ruz M, & Arredondo M (2012). Iron, copper, and zinc transport: Inhibition of divalent metal transporter 1 (DMT1) and human copper transporter 1 (hCTR1) by shRNA. Biological Trace Element Research, 146, 281–286. [Abstract] [Google Scholar]
- Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA et al. (2008). The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science, 321, 810–814. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferguson CJ, Wareing M, Delannoy M, Fenton R, McLarnon SJ, Ashton N et al. (2003). Iron handling and gene expression of the divalent metal transporter, DMT1, in the kidney of the anemic Belgrade (b) rat. Kidney International, 64, 1755–1764. [Abstract] [Google Scholar]
- Ferguson CJ, Wareing M, Ward DT, Green R, Smith CP, & Riccardi D (2001). Cellular localization of divalent metal transporter DMT-1 in rat kidney. American Journal of Physiology Renal Physiology, 280, F803–F814. [Abstract] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, & Andrews NC (1998). Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proceedings of the National Academy of Sciences of the United States of America, 95, 1148–1153. [Europe PMC free article] [Abstract] [Google Scholar]
- Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF et al. (1997). Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genetics, 16, 383–386. [Abstract] [Google Scholar]
- Fleming RE, & Britton RS (2006). Iron Imports. VI. HFE and regulation of intestinal iron absorption. American Journal of Physiology Gastrointestinal and Liver Physiology, 290, G590–G594. [Abstract] [Google Scholar]
- Fleming RE, Migas MC, Zhou X, Jiang J, Britton RS, Brunt EM et al. (1999). Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: Increased duodenal expression of the iron transporter DMT1. Proceedings of the National Academy of Sciences of the United States of America, 96, 3143–3148. [Europe PMC free article] [Abstract] [Google Scholar]
- Fleming RE, & Ponka P (2012). Iron overload in human disease. New England Journal of Medicine, 366, 348–359. [Abstract] [Google Scholar]
- Foot NJ, Dalton HE, Shearwin-Whyatt LM, Dorstyn L, Tan SS, Yang B et al. (2008). Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood, 112, 4268–4275. [Abstract] [Google Scholar]
- Foot NJ, Leong YA, Dorstyn LE, Dalton HE, Ho K, Zhao L et al. (2011). Ndfip1-deficient mice have impaired DMT1 regulation and iron homeostasis. Blood, 117, 638–646. [Abstract] [Google Scholar]
- Forbes JR, & Gros P (2003). Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood, 102, 1884–1892. [Abstract] [Google Scholar]
- Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N et al. (2001). Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochemical Journal, 356, 883–889. [Europe PMC free article] [Abstract] [Google Scholar]
- Ganz T, & Nemeth E (2006). Iron Imports. IV. Hepcidin and regulation of body iron metabolism. American Journal of Physiology Gastrointestinal and Liver Physiology, 290, G199–G203. [Abstract] [Google Scholar]
- Garrick MD, & Dolan KG (2002). An expression system for a transporter of iron and other metals. Methods in Molecular Biology, 196, 147–154. [Abstract] [Google Scholar]
- Garrick MD, Kuo HC, Vargas F, Singleton S, Zhao L, Smith JJ et al. (2006). Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochemical Journal, 398, 539–546. [Europe PMC free article] [Abstract] [Google Scholar]
- Garrick MD, Zhao L, Roth JA, Jiang H, Feng J, Foot NJ et al. (2012). Isoform specific regulation of divalent metal (ion) transporter (DMT1) by proteasomal degradation. Biometals, 25, 787–793. [Europe PMC free article] [Abstract] [Google Scholar]
- Georgieff MK, Wobken JK, Welle J, Burdo JR, & Connor JR (2000). Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta, 21, 799–804. [Abstract] [Google Scholar]
- Ghio AJ, Piantadosi CA, Wang X, Dailey LA, Stonehuerner JD, Madden MC et al. (2005). Divalent metal transporter-1 decreases metal-related injury in the lung. American Journal of Physiology Lung Cellular and Molecular Physiology, 289, L460–L467. [Abstract] [Google Scholar]
- Goswami T, Bhattacharjee A, Babal P, Searle S, Moore E, Li M et al. (2001). Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochemical Journal, 354, 511–519. [Europe PMC free article] [Abstract] [Google Scholar]
- Govoni G, Gauthier S, Billia F, Iscove NN, & Gros P (1997). Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. Journal of Leukocyte Biology, 62, 277–286. [Abstract] [Google Scholar]
- Graham RM, Chua AC, Herbison CE, Olynyk JK, & Trinder D (2007). Liver iron transport. World Journal of Gastroenterology, 13, 4725–4736. [Europe PMC free article] [Abstract] [Google Scholar]
- Griffiths WJH, Kelly AL, Smith SJ, & Cox TM (2000). Localization of iron transport and regulatory proteins in human cells. Quarterly Journal of Medicine, 93, 575–587. [Abstract] [Google Scholar]
- Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, & Gros P (1999). The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. Journal of Experimental Medicine, 189, 831–841. [Europe PMC free article] [Abstract] [Google Scholar]
- Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F et al. (2001). Iron-dependent regulation of the divalent metal ion transporter. FEBS Letters, 509, 309–316. [Abstract] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio ÁO, Direnzo C, Robine S, & Andrews NC (2005a). Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. Journal of Clinical Investigation, 115, 1258–1266. [Europe PMC free article] [Abstract] [Google Scholar]
- Gunshin H, Jin J, Fujiwara Y, Andrews NC, Mims M, & Prchal J (2005b). Analysis of the E399D mutation in SLC11A2. Blood, 106, 2221–2222. [Abstract] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF et al. (1997). Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature, 388, 482–488. [Abstract] [Google Scholar]
- Harrison SA, & Bacon BR (2003). Hereditary hemochromatosis: Update for 2003. Journal of Hepatology, 38, S14–S23. [Abstract] [Google Scholar]
- Hartman KR, & Barker JA (1996). Microcytic anemia with iron malabsorption: An inherited disorder of iron metabolism. American Journal of Hematology, 51, 269–275. [Abstract] [Google Scholar]
- Heilig E, Molina R, Donaghey T, Brain JD, & Wessling-Resnick M (2005). Pharmacokinetics of pulmonary manganese absorption: Evidence for increased susceptibility to manganese loading in iron deficient rats. American Journal of Physiology Lung Cellular and Molecular Physiology, 288, L887–L893. [Abstract] [Google Scholar]
- Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, & Wessling-Resnick M (2006). Manganese and iron transport across pulmonary epithelium. American Journal of Physiology Lung Cellular and Molecular Physiology, 290, L1247–L1259. [Abstract] [Google Scholar]
- Hershko C (2010). Pathogenesis and management of iron toxicity in thalassemia. Annals of the New York Academy of Science, 1202, 1–9. [Abstract] [Google Scholar]
- Hirayama BA, Loo DDF, & Wright EM (1994). Protons drive sugar transport through the Na+/glucose cotransporter (SGLT1). Journal of Biological Chemistry, 269, 21407–21410. [Abstract] [Google Scholar]
- Horonchik L, & Wessling-Resnick M (2008). The small-molecule iron transport inhibitor ferristatin/NSC306711 promotes degradation of the transferrin receptor. Chemistry and Biology, 15, 647–653. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang E, Ong W-Y, Go M-L, & Connor JR (2006). Upregulation of iron regulatory proteins and divalent metal transporter-1 isoforms in the rat hippocampus after kainate induced neuronal injury. Exp. Brain Res, 170, 376–386. [Abstract] [Google Scholar]
- Hubert N, & Hentze MW (2002). Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proceedings of the National Academy of Sciences of the United States of America, 99, 12345–12350. [Europe PMC free article] [Abstract] [Google Scholar]
- Illing AC, Shawki A, Cunningham CL, & Mackenzie B (2012). Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. Journal of Biological Chemistry, 287, 30485–30496. [Europe PMC free article] [Abstract] [Google Scholar]
- Iolascon A, d’Apolito M, Servedio V, Cimmino F, Piga A, & Camaschella C (2006). Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1. Blood, 107, 349–354. [Abstract] [Google Scholar]
- Jabado N, Canonne-Hergaux F, Gruenheid S, Picard V, & Gros P (2002). Iron transporter Nramp2/DMT-1 is associated with the membrane of phagosomes in macrophages and Sertoli cells. Blood, 100, 2617–2622. [Abstract] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, & Gros P (2000). Natural resistance to intracellular infections: Natural resistance-associated macrophage protein 1 (NRAMP1) functions as a pH-dependent manganese transporter at the phagosomal membrane. Journal of Experimental Medicine, 192, 1237–1248. [Europe PMC free article] [Abstract] [Google Scholar]
- Jamieson SE, White JK, Howson JM, Pask R, Smith AN, Brayne C et al. (2005). Candidate gene association study of solute carrier family 11a members 1 (SLC11A1) and 2 (SLC11A2) genes in Alzheimer’s disease. Neuroscience Letters, 374, 124–128. [Abstract] [Google Scholar]
- Jenkitkasemwong S, Wang C-Y, Mackenzie B, & Knutson MD (2012). Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals, 25, 643–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Kelleher T, Ryan E, Barrett S, Sweeney M, Byrnes V, O’Keane C et al. (2004). Increased DMT1 but not IREG1 or HFE mRNA following iron depletion therapy in hereditary haemochromatosis. Gut, 53, 1174–1179. [Europe PMC free article] [Abstract] [Google Scholar]
- Knöpfel M, Smith C, & Solioz M (2005a). ATP-driven copper transport across the intestinal brush border membrane. Biochemical and Biophysical Research Communications, 330, 645–652. [Abstract] [Google Scholar]
- Knöpfel M, Zhao L, & Garrick MD (2005b). Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry, 44, 3454–3465. [Abstract] [Google Scholar]
- Knutson M, Menzies S, Connor J, & Wessling-Resnick M (2004). Developmental, regional, and cellular expression of SFT/UbcH5A and DMT1 mRNA in brain. Journal of Neuroscience Research, 76, 633–641. [Abstract] [Google Scholar]
- Knutson MD (2007). Steap proteins: Implications for iron and copper metabolism. Nutrition Reviews, 65, 335–340. [Abstract] [Google Scholar]
- Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J et al. (2001). Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proceedings of the National Academy of Sciences of the United States of America, 98, 12491–12496. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuhn DE, Lafuse WP, & Zwilling BS (2001). Iron transport into Mycobacterium avium-containing phagosomes from an Nramp1Gly169-transfected RAW264.7 macrophage cell line. Journal of Leukocyte Biology, 69, 43–49. [Abstract] [Google Scholar]
- Kumfu S, Chattipakorn S, Srichairatanakool S, Settakorn J, Fucharoen S, & Chattipakorn N (2011). T-type calcium channel as a portal of iron uptake into cardiomyocytes of β-thalassemic mice. European Journal of Haematology, 10–0609. [Abstract] [Google Scholar]
- Kwik-Uribe CL, Reaney S, Zhu Z, & Smith D (2003). Alterations in cellular IRP-dependent iron regulation by in vitro manganese exposure in undifferentiated PC12 cells. Brain Research, 973, 1–15. [Abstract] [Google Scholar]
- Lam-Yuk-Tseung S, Camaschella C, Iolascon A, & Gros P (2006a). A novel R416C mutation in human DMT1 (SLC11A2) displays pleiotropic effects on function and causes microcytic anemia and hepatic iron overload. Blood Cells Molecules and Disease, 36, 347–354. [Abstract] [Google Scholar]
- Lam-Yuk-Tseung S, Govoni G, & Gros P (2003). Iron transport by NRAMP2/DMT1: pH regulation of transport by two histidines in transmembrane domain 6. Blood, 101, 3699–3707. [Abstract] [Google Scholar]
- Lam-Yuk-Tseung S, Mathieu M, & Gros P (2005). Functional characterization of the E399D DMT1/NRAMP2/SLC11A2 protein produced by an exon 12 mutation in a patient with microcytic anemia and iron overload. Blood Cells Molecules and Disease, 35, 212–216. [Abstract] [Google Scholar]
- Lam-Yuk-Tseung S, Picard V, & Gros P (2006b). Identification of a tyrosine-based motif (YGSI) in the amino terminus of Nramp1 (Slc11A1) that is important for lysosomal targeting. Journal of Biological Chemistry, 281, 31677–31688. [Abstract] [Google Scholar]
- Lee PL, Gelbart T, West C, Halloran C, & Beutler E (1998). The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Molecules and Disease, 24, 199–215. [Abstract] [Google Scholar]
- Li F, Li H, Hu L, Kwan M, Chen G, He QY et al. (2005). Structure, assembly, and topology of the G185R mutant of the fourth transmembrane domain of divalent metal transporter. Journal of the Amercan Chemical Society, 127, 1414–1423. [Abstract] [Google Scholar]
- Li H, Gu JD, & Sun H (2008). Structure, topology and assembly of a 32-mer peptide corresponding to the loop 3 and transmembrane domain 4 of divalent metal transporter (DMT1) in membrane-mimetic environments. Journal of Inorganic Chemistry. [Abstract] [Google Scholar]
- Li H, Li F, Qian ZM, & Sun H (2004). Structure and topology of the transmembrane domain 4 of the divalent metal transporter in membrane-mimetic environments. European Journal of Biochemistry, 271, 1938–1951. [Abstract] [Google Scholar]
- Li H, Li F, Sun H, & Qian ZM (2003). Membrane-inserted conformation of transmembrane domain 4 of divalent-metal transporter. Biochemical Journal, 372, 757–766. [Europe PMC free article] [Abstract] [Google Scholar]
- Li YQ, Bai B, Cao XX, Zhang YH, Yan H, Zheng QQ et al. (2012). Divalent metal transporter 1 expression and regulation in human placenta. Biological Trace Element Research, 146, 6–12. [Abstract] [Google Scholar]
- Linder MC, Moriya M, Whon A, Kassa A, & Gilley C (2006). Vesicular transport of Fe and interaction with other metal ions in polarized Caco2 cell monolayers. Biological Research, 39, 143–156. [Abstract] [Google Scholar]
- Lis A, Barone TA, Paradkar PN, Plunkett RJ, & Roth JA (2004). Expression and localization of different forms of DMT1 in normal and tumor astroglial cells. Brain Research Molecular Brain Research, 122, 62–70. [Abstract] [Google Scholar]
- Lis A, Paradkar PN, Singleton S, Kuo HC, Garrick MD, & Roth JA (2005). Hypoxia induces changes in expression of isoforms of the divalent metal transporter (DMT1) in rat pheochromocytoma (PC12) cells. Biochemical Pharmacology, 69, 1647–1655. [Abstract] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, & Cousins RJ (2006). Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proceedings of the National Academy of Sciences of the United States of America, 103, 13612–13617. [Europe PMC free article] [Abstract] [Google Scholar]
- Loo DDF, Hazama A, Supplisson S, Turk E, & Wright EM (1993). Relaxation kinetics of the Na+/glucose cotransporter. Proceedings of the National Academy of Sciences of the United States of America, 90, 5767–5771. [Europe PMC free article] [Abstract] [Google Scholar]
- Mackenzie B, & Garrick MD (2005). Iron Imports. II. Iron uptake at the apical membrane in the intestine. American Journal of Physiology Gastrointestinal and Liver Physiology, 289, G981–G986. [Abstract] [Google Scholar]
- Mackenzie B, & Hediger MA (2004). SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflügers Archiv European Journal of Physiology, 447, 571–579. [Abstract] [Google Scholar]
- Mackenzie B, Illing AC, & Hediger MA (2008). Transport model of the human Na+-coupled L-ascorbic acid (vitamin C) transporter SVCT1. American Journal of Physiology Cell Physiology, 294, C451–C459. [Abstract] [Google Scholar]
- Mackenzie B, Loo DDF, Fei YJ, Liu W, Ganapathy V, Leibach FH et al. (1996a). Mechanisms of the human intestinal H+-coupled oligopeptide transporter hPEPT1. Journal of Biological Chemistry, 271, 5430–5437. [Abstract] [Google Scholar]
- Mackenzie B, Loo DDF, Panayotova-Heiermann M, & Wright EM (1996b). Biophysical characteristics of the pig kidney Na+/glucose cotransporter SGLT2 reveal a common mechanism for SGLT1 and SGLT2. Journal of Biological Chemistry, 271, 32678–32683. [Abstract] [Google Scholar]
- Mackenzie B, Shawki A, Ghio AJ, Stonehuerner JD, Zhao L, Ghadersohi S et al. (2010). Calcium-channel blockers do not affect iron transport mediated by divalent metal-ion transporter-1. Blood, 115, 4148–4149. [Europe PMC free article] [Abstract] [Google Scholar]
- Mackenzie B, Shawki A, Kim R, Anthony SR, Knight PB, Bradford EM et al. (2011). Intestinal brush-border Na+/H+ exchangers are required for iron homeostasis in the mouse. FASEB Journal, 25, 238.1–238.1 (abstract). [Google Scholar]
- Mackenzie B, Takanaga H, Hubert N, Rolfs A, & Hediger MA (2007). Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochemical Journal, 403, 59–69. [Europe PMC free article] [Abstract] [Google Scholar]
- Mackenzie B, Ujwal ML, Chang M-H, Romero MF, & Hediger MA (2006). Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflügers Archiv European Journal of Physiology, 451, 544–558. [Abstract] [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, & Peyssonnaux C (2009). HIF-2α, but not HIF-1α, promotes iron absorption in mice. Journal of Clinical Investigation, 119, 1159–1166. [Europe PMC free article] [Abstract] [Google Scholar]
- McKie AT (2008). The role of Dcytb in iron metabolism: an update. Biochemical Society Transactions, 36, 1239–1241. [Abstract] [Google Scholar]
- Mena NP, Esparza A, Tapia V, Valdes P, & Núñez MT (2008). Hepcidin inhibits apical iron uptake in intestinal cells. American Journal of Physiology Gastrointestinal and Liver Physiology, 294, G192–G198. [Abstract] [Google Scholar]
- Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P et al. (2005). Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood, 105, 1337–1342. [Abstract] [Google Scholar]
- Moos T, & Morgan EH (2004a). The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Annals of the New York Academy of Science, 1012:14–26., 14–26. [Abstract] [Google Scholar]
- Moos T, & Morgan EH (2004b). The significance of the mutated divalent metal transporter (DMT1) on iron transport into the Belgrade rat brain. Journal of Neurochemistry, 88, 233–245. [Abstract] [Google Scholar]
- Moos T, Trinder D, & Morgan EH (2000). Cellular distribution of ferric iron, ferritin, transferrin and divalent metal transporter 1 (DMT1) in substantia nigra and basal ganglia of normal and beta2-microglobulin deficient mouse brain. Cellular and Molecular Biology, 46, 549–561. [Abstract] [Google Scholar]
- Moos T, & Morgan EH (1998). Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. Journal of Neuroscience Research, 54, 486–494. [Abstract] [Google Scholar]
- Nevo Y, & Nelson N (2004). The mutation F227I increases the coupling of metal-ion transport in DCT1. Journal of Biological Chemistry, 279, 53056–53061. [Abstract] [Google Scholar]
- Núñez MT, Tapia V, Rojas A, Aguirre P, Gomez F, & Nualart F (2010). Iron supply determines apical/basolateral membrane distribution of intestinal iron transporters DMT1 and ferroportin 1. American Journal of Physiology Cell Physiology, 298, C477–C485. [Abstract] [Google Scholar]
- Núñez MT, Urrutia P, Mena N, Aguirre P, Tapia V, & Salazar J (2012). Iron toxicity in neurodegeneration. Biometals, 25, 761–776. [Abstract] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J et al. (2005). Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nature Genetics, 37, 1264–1269. [Europe PMC free article] [Abstract] [Google Scholar]
- Okazaki Y, Ma Y, Yeh M, Yin H, Li Z, Yeh KY et al. (2012). DMT1 (IRE) expression in intestinal and erythroid cells is regulated by peripheral benzodiazepine receptor-associated protein 7. American Journal of Physiology Gastrointestinal and Liver Physiology, 302, G1180–G1190. [Europe PMC free article] [Abstract] [Google Scholar]
- Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C et al. (2003). L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nature Medicine, 9, 1187–1194. [Abstract] [Google Scholar]
- Pantopoulos K (2004). Iron metabolism and the IRE/IRP regulatory system: an update. Annals of the New York Academy of Science, 1012, 1–13. [Abstract] [Google Scholar]
- Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, Macfarlane J et al. (2005). Hepcidin in iron overload disorders. Blood, 105, 4103–4105. [Europe PMC free article] [Abstract] [Google Scholar]
- Paradkar PN, & Roth JA (2006). Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-kappaB. Journal of Neurochemistry, 96, 1768–1777. [Abstract] [Google Scholar]
- Park JD, Cherrington NJ, & Klaassen CD (2002). Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicological Sciences, 68, 288–294. [Abstract] [Google Scholar]
- Pelizzoni I, Macco R, Morini MF, Zacchetti D, Grohovaz F, & Codazzi F (2011). Iron handling in hippocampal neurons: Activity-dependent iron entry and mitochondria-mediated neurotoxicity. Aging Cell, 10, 172–183. [Abstract] [Google Scholar]
- Pelizzoni I, Zacchetti D, Smith CP, Grohovaz F, & Codazzi F (2012). Expression of divalent metal transporter 1 in primary hippocampal neurons: Reconsidering its role in non-transferrin-bound iron influx. Journal of Neurochemistry, 120, 269–278. [Abstract] [Google Scholar]
- Picard V, Govoni G, Jabado N, & Gros P (2000). Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. Journal of Biological Chemistry, 275, 35738–35745. [Abstract] [Google Scholar]
- Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N et al. (2011). Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. American Journal of Physiology Cell Physiology, 301, C862–C871. [Europe PMC free article] [Abstract] [Google Scholar]
- Priwitzerova M, Pospisilova D, Prchal JT, Indrak K, Hlobilkova A, Mihal V et al. (2004). Severe hypochromic microcytic anemia caused by a congenital defect of the iron transport pathway in erythroid cells. Blood, 103, 3991–3992. [Abstract] [Google Scholar]
- Priwitzerova M, Pospisilova D, Sheftel AD, Divoky V, & Ponka P (2005). Functional consequences of the human DMT1 mutation on protein expression and iron uptake. Blood, 106, 3985–3987. [Abstract] [Google Scholar]
- Rivera-Mancía S, Ríos C, & Montes S (2011). Manganese accumulation in the CNS and associated pathologies. Biometals, 24, 811–825. [Abstract] [Google Scholar]
- Rolfs A, Bonkovsky HL, Kohlroser JG, McNeal K, Sharma A, Berger UV et al. (2002). Intestinal expression of genes involved in iron absorption in humans. American Journal of Physiology Gastrointestinal and Liver Physiology, 282, G598–G607. [Abstract] [Google Scholar]
- Roth JA (2006). Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biological Research, 39, 45–57. [Abstract] [Google Scholar]
- Roth JA, & Garrick MD (2003). Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochemical Pharmacology, 66, 1–13. [Abstract] [Google Scholar]
- Roth JA, Horbinski C, Feng L, Dolan KG, Higgins D, & Garrick MD (2000). Differential localization of divalent metal transporter 1 with and without iron response element in rat PC12 and sympathetic neuronal cells. Journal of Neuroscience, 20, 7595–7601. [Europe PMC free article] [Abstract] [Google Scholar]
- Rouault TA, Zhang DL, & Jeong SY (2009). Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metabolic Brain Disease, 24, 673–684. [Europe PMC free article] [Abstract] [Google Scholar]
- Rouault TA, & Cooperman S (2006). Brain iron metabolism. Semin. Pediatr. Neurol, 13, 142–148. [Abstract] [Google Scholar]
- Ruvin Kumara VM, & Wessling-Resnick M (2012). Olfactory ferric and ferrous iron absorption in iron-deficient rats. American Journal of Physiology Lung Cellular and Molecular Physiology, 302, L1280–L1286. [Europe PMC free article] [Abstract] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M et al. (2008). Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America, 105, 18578–18583. [Europe PMC free article] [Abstract] [Google Scholar]
- Saroussi S, & Nelson N (2009). Vacuolar H+-ATPase—an enzyme for all seasons. Pflügers Archiv European Journal of Physiology. [Abstract] [Google Scholar]
- Shah YM, Matsubara T, Ito S, Yim SH, & Gonzalez FJ (2009). Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metabolism. [Europe PMC free article] [Abstract] [Google Scholar]
- Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE et al. (2006). Mitoferrin is essential for erythroid iron assimilation. Nature, 440, 96–100. [Abstract] [Google Scholar]
- Shawki A, Anthony SR, Nose Y, Barrientos De Renshaw T, Thiele DJ, & Mackenzie B (2012). Intestinal divalent metal-ion transporter-1 is critical for iron homeostasis but is not required for maintenance of Cu or Zn. FASEB Journal, 26, 1112.2 (abstract). [Google Scholar]
- Shawki A, Illing AC, & Mackenzie B (2006). Molecular impact of human divalent metal-ion transporter DMT1 mutations associated with disease phenotypes. FASEB Journal, 20, A1278–A1278 (abstract). [Google Scholar]
- Shawki A, Illing AC, & Mackenzie B (2008). Molecular impact of divalent metal-ion transporter (DMT1) mutations (V114del and G212V) found in a compound heterozygote with microcytic anemia and hepatic iron overload. FASEB Journal, 22, 1192.3–1192.3 (abstract). [Google Scholar]
- Shawki A, & Mackenzie B (2010). Interaction of calcium with the human divalent metal-ion transporter-1. Biochemical and Biophysical Research Communications, 393, 471–475. [Europe PMC free article] [Abstract] [Google Scholar]
- Shindo M, Torimoto Y, Saito H, Motomura W, Ikuta K, Sato K et al. (2006). Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatology Research, 35, 152–162. [Abstract] [Google Scholar]
- Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, Connor JR et al. (2003). Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatric Research, 53, 800–807. [Abstract] [Google Scholar]
- Smith CP, & Thévenod F (2009). Iron transport and the kidney. Biochimica et Biophysica Acta, 1790, 724–730. [Abstract] [Google Scholar]
- Snyder AM, & Connor JR (2009). Iron, the substantia nigra and related neurological disorders. Biochimica et Biophysica Acta, 1790, 606–614. [Abstract] [Google Scholar]
- Soe-Lin S, Apte SS, Andriopoulos B, Andrews MC, Schranzhofer M, Kahawita T et al. (2009). Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proceedings of the National Academy of Sciences of the United States of America, 106, 5960–5965. [Europe PMC free article] [Abstract] [Google Scholar]
- Soe-Lin S, Apte SS, Mikhael MR, Kayembe LK, Nie G, & Ponka P (2010). Both Nramp1 and DMT1 are necessary for efficient macrophage iron recycling. Experimental Hematology, 38, 609–617. [Abstract] [Google Scholar]
- Soe-Lin S, Sheftel AD, Wasyluk B, & Ponka P (2008). Nramp1 equips macrophages for efficient iron recycling. Experimental Hematology, 36, 929–937. [Abstract] [Google Scholar]
- Song N, Jiang H, Wang J, & Xie JX (2007). Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. Journal of Neuroscience Research, 35, 3118–3126. [Abstract] [Google Scholar]
- Su MA, Trenor CC, Fleming JC, Fleming MD, & Andrews NC (1998). The G185R mutation disrupts function of the iron transporter Nramp2. Blood, 92, 2157–2163. [Abstract] [Google Scholar]
- Suzuki T, Momoi K, Hosoyamada M, Kimura M, & Shibasaki T (2008). Normal cadmium uptake in microcytic anemia mk/mk mice suggests that DMT1 is not the only cadmium transporter in vivo. Toxicology and Applied Pharmacology, 227, 462–467. [Abstract] [Google Scholar]
- Tabuchi M, Yanatori I, Kawai Y, & Kishi F (2010). Retromer-mediated direct sorting is required for proper endosomal recycling of the mammalian iron transporter DMT1. Journal of Cell Science, 123, 756–766. [Abstract] [Google Scholar]
- Tabuchi M, Tanaka N, Nishida-Kitayama J, Ohno H, & Kishi F (2002). Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Molecular Biology of the Cell, 13, 4371–4387. [Europe PMC free article] [Abstract] [Google Scholar]
- Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B et al. (2000). Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. Journal of Biological Chemistry, 275, 1023–1029. [Abstract] [Google Scholar]
- Techau ME, Valdez-Taubas J, Popoff JF, Francis R, Seaman M, & Blackwell JM (2007). Evolution of differences in transport function in Slc11a family members. Journal of Biological Chemistry, 282, 35646–35656. [Abstract] [Google Scholar]
- Thompson BAV, Sharp PA, Elliott R, & Fairweather-Tait SJ (2010). Inhibitory effect of calcium on non-heme iron absorption may be related to translocation of DMT-1 at the apical membrane of enterocytes. Journal of Agricultural and Food Chemistry, 58, 8414–8417. [Abstract] [Google Scholar]
- Thompson K, Molina RM, Brain JD, & Wessling-Resnick M (2006). Belgrade rats display liver iron loading. Journal of Nutrition, 136, 3010–3014. [Europe PMC free article] [Abstract] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Brain JD, & Wessling-Resnick M (2007a). Iron absorption by Belgrade rat pups during lactation. American Journal of Physiology Gastrointestinal and Liver Physiology, 293, G640–G644. [Abstract] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, & Wessling-Resnick M (2007b). Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB Journal, 21, 223–230. [Europe PMC free article] [Abstract] [Google Scholar]
- Tjälve H, & Henriksson J (1999). Uptake of metals in the brain via olfactory pathways. Neurotoxicology, 20, 181–195. [Abstract] [Google Scholar]
- Touret N, Furuya W, Forbes J, Gros P, & Grinstein S (2003). Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrin receptor. Journal of Biological Chemistry, 278, 25548–25557. [Abstract] [Google Scholar]
- Touret N, Martin-Orozco N, Paroutis P, Furuya W, Lam-Yuk-Tseung S, Forbes J et al. (2004). Molecular and cellular mechanisms underlying iron transport deficiency in microcytic anemia. Blood, 104, 1526–1533. [Abstract] [Google Scholar]
- Trinder D, Batey RG, Morgan EH, & Baker E (1990). Effect of cellular iron concentration on iron uptake by hepatocytes. American Journal of Physiology, 259, G611–G617. [Abstract] [Google Scholar]
- Trinder D, Oates PS, Thomas C, Sadleir J, & Morgan EH (2000). Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut, 46, 270–276. [Europe PMC free article] [Abstract] [Google Scholar]
- Vesey DA (2010). Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicology Letters, 198, 13–19. [Abstract] [Google Scholar]
- Vidal SM, Pinner E, Lepage P, Gauthier S, & Gros P (1996). Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. Journal of Immunology, 157, 3559–3568. [Abstract] [Google Scholar]
- Vidal S, Malo D, Vogan K, Skamene E, & Gros P (1993). Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell, 73, 469–485. [Abstract] [Google Scholar]
- Wallander ML, Leibold EA, & Eisenstein RS (2006). Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochimica et Biophysica Acta, 1763, 668–689. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang B, He L, Dong H, Dalton TP, & Nebert DW (2011a). Generation of a Slc39a8 hypomorph mouse: Markedly decreased ZIP8 Zn2+/(HCO−3)2 transporter expression. Biochemical and Biophysical Research Communications, 410, 289–294. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B et al. (2012a). ZIP8 is an iron and zinc transporter whose cell-surface expression is upregulated by cellular iron loading. Journal of Biological Chemistry, 287, 34032–34043. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B et al. (2012b). ZIP8 is an iron and zinc transporter whose cell-surface expression is upregulated by cellular iron loading. Journal of Biological Chemistry (in press). [Europe PMC free article] [Abstract] [Google Scholar]
- Wang D, Song Y, Li J, Wang C, & Li F (2011b). Structure and metal ion binding of the first transmembrane domain of DMT1. Biochimica et Biophysica Acta, 1808, 1639–1644. [Abstract] [Google Scholar]
- Wang K, Zhou B, Kuo YM, Zemansky J, & Gitschier J (2002a). A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. American Journal of Human Genetics, 71, 66–73. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang X, Ghio AJ, Yang F, Dolan KG, Garrick MD, & Piantadosi CA (2002b). Iron uptake and Nramp2/DMT1/DCT1 in human bronchial epithelial cells. American Journal of Physiology Lung Cellular and Molecular Physiology, 282, L987–L995. [Abstract] [Google Scholar]
- Wang XS, Ong WY, & Connor JR (2002c). A light and electron microscopic study of divalent metal transporter-1 distribution in the rat hippocampus, after kainate-induced neuronal injury. Exp. Neurol, 177, 193–201. [Abstract] [Google Scholar]
- Wareing M, Ferguson CJ, Green R, Riccardi D, & Smith CP (2000). In vivo characterization of renal iron transport in the anaesthetized rat. Journal of Physiology, 524, 581–586. [Abstract] [Google Scholar]
- West AR, Thomas C, Sadlier J, & Oates PS (2006). Haemochromatosis protein is expressed on the terminal web of enterocytes in proximal small intestine of the rat. Histochemistry and Cell Biology, 125, 283–292. [Abstract] [Google Scholar]
- Wetli H, Buckett PD, & Wessling-Resnick M (2006). Chemical genetic screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chemistry and Biology, 13, 965–972. [Europe PMC free article] [Abstract] [Google Scholar]
- Williams K, Wilson MA, & Bressler J (2000). Regulation and developmental expression of the divalent metal-ion transporter in the rat brain. Cellular and Molecular Biology, 46, 563–571. [Abstract] [Google Scholar]
- Wolff NA, Abouhamed M, Verroust PJ, & Thévenod F (2006). Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured renal proximal tubule cells. Journal of Pharmacology and Experimental Therapeutics, 318, 782–791. [Abstract] [Google Scholar]
- Worthington MT, Browne L, Battle EH, & Luo RQ (2000). Functional properties of transfected human DMT1 iron transporter. American Journal of Physiology Gastrointestinal and Liver Physiology, 279, G1265–G1273. [Abstract] [Google Scholar]
- Xiao S, Li J, Wang Y, Wang C, Xue R, Wang S et al. (2010). Identification of an “α-helix-extended segment-α-helix” conformation of the sixth transmembrane domain in DMT1. Biochimica et Biophysica Acta, 1758, 1556–1564. [Abstract] [Google Scholar]
- Xiao S, Wang C, Li J, & Li F (2011). Folding and assembly of TMD 6-related segments of DMT 1 in trifluoroethanol aqueous solution. J. Pept. Sci, 17, 505–511. [Abstract] [Google Scholar]
- Xu H, Jin J, DeFelice LJ, Andrews NC, & Clapham DE (2004). A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway. PLoS Biology, 2, E50. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, & Gouaux E (2005). Crystal structure of a bacterial homologue of Na+/Cl–-dependent neurotransmitter transporters. Nature, 437, 215–223. [Abstract] [Google Scholar]
- Yanatori I, Tabuchi M, Kawai Y, Yasui Y, Akagi R, & Kishi F (2010). Heme and non-heme iron transporters in non-polarized and polarized cells. BMC Cell Biology, 11, 39. [Europe PMC free article] [Abstract] [Google Scholar]
- Zampighi GA, Kreman M, Boorer KJ, Loo DDF, Bezanilla F, Chandy G et al. (1995). A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. Journal of Membrane Biology, 148, 65–78. [Abstract] [Google Scholar]
- Zhang A-S, Canonne-Hergaux F, Gruenheid S, Gros P, & Ponka P (2008). Use of Nramp2-transfected Chinese hamster ovary cells and reticulocytes from mk/mk mice to study iron transport mechanisms. Experimental Hematology, 36, 1227–1235. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang Z, Kodumuru V, Sviridov S, Liu S, Chafeev M, Chowdhury S et al. (2012). Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorganic & Medicinal Chemistry Letters, 22, 5108–5113. [Abstract] [Google Scholar]
- Zhao N, Gao J, Enns CA, & Knutson MD (2010). ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. Journal of Biological Chemistry, 285, 32141–32150. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng W, Xin N, Chi ZH, Zhao BL, Zhang J, Li JY et al. (2009). Divalent metal transporter 1 is involved in amyloid precursor protein processing and Aβ generation. FASEB Journal, 23, 4207–4217. [Abstract] [Google Scholar]
- Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G et al. (2001). Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology, 120, 1412–1419. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/b978-0-12-394316-3.00005-3
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7027397?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/b978-0-12-394316-3.00005-3
Article citations
Knockdown of microglial iron import gene, Slc11a2, worsens cognitive function and alters microglial transcriptional landscape in a sex-specific manner in the APP/PS1 model of Alzheimer's disease.
J Neuroinflammation, 21(1):238, 27 Sep 2024
Cited by: 0 articles | PMID: 39334471 | PMCID: PMC11438269
Divalent metal content in diet affects severity of manganese toxicity in Drosophila.
Biol Open, 13(1):bio060204, 05 Jan 2024
Cited by: 0 articles | PMID: 38117005 | PMCID: PMC10810561
Microglial-specific knockdown of iron import gene, Slc11a2, blunts LPS-induced neuroinflammatory responses in a sex-specific manner.
Brain Behav Immun, 116:370-384, 22 Dec 2023
Cited by: 3 articles | PMID: 38141840
Slc11 Synapomorphy: A Conserved 3D Framework Articulating Carrier Conformation Switch.
Int J Mol Sci, 24(20):15076, 11 Oct 2023
Cited by: 0 articles | PMID: 37894758 | PMCID: PMC10606218
The Endolysosomal Transporter DMT1 is Required for Morphine Regulation of Neuronal Ferritin Heavy Chain.
J Neuroimmune Pharmacol, 18(3):495-508, 04 Sep 2023
Cited by: 0 articles | PMID: 37661197 | PMCID: PMC10577102
Go to all (53) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
RefSeq - NCBI Reference Sequence Database (Showing 8 of 8)
- (1 citation) RefSeq - NM_001174128.1
- (1 citation) RefSeq - NM_001174127.1
- (1 citation) RefSeq - NM_001174126.1
- (1 citation) RefSeq - NM_001174125.1
- (1 citation) RefSeq - NM_000617.2
- (1 citation) RefSeq - NR_033422.1
- (1 citation) RefSeq - NR_033421.1
- (1 citation) RefSeq - NG_021139.1
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1).
Biochem J, 403(1):59-69, 01 Apr 2007
Cited by: 94 articles | PMID: 17109629 | PMCID: PMC1828886
Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese.
Am J Physiol Gastrointest Liver Physiol, 309(8):G635-47, 20 Aug 2015
Cited by: 71 articles | PMID: 26294671 | PMCID: PMC4609933
Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes.
Pflugers Arch, 451(4):544-558, 10 Aug 2005
Cited by: 79 articles | PMID: 16091957
Divalent metal transporter 1.
Hematology, 10(4):339-345, 01 Aug 2005
Cited by: 62 articles | PMID: 16085548
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (3)
Grant ID: R01 DK080047
Grant ID: DK060444
Grant ID: T35 DK060444




