Abstract
Free full text

Norovirus Gastroenteritis in Immunocompromised Patients
Infectious gastroenteritis is a common, acute illness that is characteristically self-limiting, but it can become debilitating and life-threatening in immunocompromised patients.1 Noroviruses are major pathogens among the microbes associated with gastroenteritis in both immunocompetent and immunocompromised hosts1–4 (Table 1). In the United States, noroviruses are the single most common cause of acute gastroenteritis in adults that results in a visit to the hospital emergency department,2 and they are second only to rotaviruses as a major cause of severe diarrhea in infants and young children.5 In developing countries, noroviruses are estimated to cause more than 200,000 deaths annually among children younger than 5 years of age, and it is predicted that these viruses will become the predominant cause of diarrhea in all age groups worldwide once rotavirus infection is controlled through vaccination.6
Table 1
Infectious Causes of Gastroenteritis.
| Type of Pathogen | Immunocompetent Hosts | Immunocompromised Hosts |
|---|---|---|
| Viruses | Norovirus | Norovirus |
| Rotavirus | Rotavirus | |
| Astrovirus | Astrovirus | |
| Adenovirus | Adenovirus | |
| Herpesvirus | ||
| Human immunodeficiency virus (AIDS enteropathy) | ||
| Cytomegalovirus | ||
| Bacteria | Escherichia coli (pathogenic) | E. coli (pathogenic) |
| Salmonella spp. | Salmonella spp. | |
| Campylobacter jejuni | C. jejuni | |
| Clostridium difficile | C. difficile | |
| Shigella spp. | Shigella spp. | |
| Chlamydia trachomatis | ||
| Mycobacterium tuberculosis, M. avium complex | ||
| Parasites | Cryptosporidium spp. | Cryptosporidium spp. |
| Entamoeba histolytica | E. histolytica | |
| Giardia lamblia | G. lamblia | |
| Cystoisospora belli | ||
| Blastocystis hominis | ||
| Cyclospora spp. | ||
| Strongyloides stercoralis | ||
| Fungi | Microsporidium spp. | |
| Histoplasma spp. | ||
| Candida spp. |
Noroviruses are increasingly recognized as an important cause of chronic gastroenteritis in immunocompromised patients, as reflected by the growing number of clinical case reports.7–9 A comparison of the known features of norovirus gastroenteritis in immunocompetent versus immunocompromised hosts highlights the potentially serious outcome of this illness in persons who cannot adequately clear the virus (Table 2). The purpose of this review is to summarize recent developments in norovirus research that are relevant to the prevention and management of norovirus gastroenteritis in immunocompromised patients.
Table 2
Characteristics of Norovirus Gastroenteritis in Immunocompetent versus Immunocompromised Hosts.
| Characteristic | Immunocompetent Hosts | Immunocompromised Hosts |
|---|---|---|
| Prevalence | Leading cause of gastroenteritis worldwide | Not established; estimated at about 17 to 18% |
| Seasonality | Peak in winter months | Year-round |
| Clinical features | Acute onset, duration of 24 to 48 hr | Acute onset, indefinite duration |
| Viral shedding | 20 to 40 days | Weeks to years |
| Level of virus | 108 to 109 genome copies per gram of stool | 105 to 108 genome copies per gram of stool, depending on level of immunosuppressive therapy |
| Evolution of virus in host | Small number of stable variants | Markedly diverse variants |
| Tissue tropism | Small intestine | Small intestine |
| Complications | Dehydration | Dehydration, malnutrition, dysfunction of intestinal barrier |
| Treatment | Infection is usually self-limiting; rehydration, if needed | No virus-specific treatment is available; supportive care, adjustment of immunosuppressive therapy |
| Prognosis | Usually excellent, but the infection can be life-threatening | Poor to excellent; chronic infection is common |
NOROVIRUS CLASSIFICATION AND STRUCTURE
Noroviruses are small, nonenveloped viruses with a single-stranded RNA genome that make up the genus norovirus of the family Caliciviridae.4 They are divided into six major genogroups designated GI through GVI. GI and GII contain the majority of norovirus strains associated with human disease and are further divided into about 30 genotypes.10 A single genotype, GII.4, has been associated with the majority of global outbreaks since the mid-1990s, when active surveillance with molecular diagnostic techniques was initiated.11,12
The norovirus genome encodes seven nonstructural and two structural proteins (Fig. 1).5,13 The majority of reverse-transcriptase–polymerase-chain-reaction (RT-PCR) diagnostic assays target the RNA polymerase region of the genome because of its higher sequence conservation among strains. VP1 is the major structural protein of the virus that self-assembles into viruslike particles (VLPs), which are being evaluated as possible vaccines; VP2 is a minor structural protein.5,14,15 Noroviruses bind saccharides of the human histo–blood group antigens (HBGAs) within their VP1 protruding 2 (P2) domain,13 a proposed mechanism for facilitating viral entry into the epithelial cells of the gastrointestinal tract (Fig. 1). It is thought that susceptibility to norovirus in humans is determined by allelic variation of HBGAs,13 with each norovirus strain presenting a characteristic HBGA-binding profile. Thus, a certain genetic background might confer resistance to the infection, as in the case of persons classified as nonsecretors (i.e., persons in whom these carbohydrates are not expressed on the surface of intestinal epithelial cells), who are resistant to infection with Norwalk virus, a GI.1 strain.13

The RNA genome of the prototype norovirus strain, Norwalk virus (shown at the top), is organized into three open reading frames (ORF1, ORF2, and ORF3) that encode the designated nonstructural and structural proteins. Most diagnostic primers used in reverse-transcriptase–polymerase-chain-reaction assay target conserved areas in the RNA-dependent RNA polymerase region (NS7POL). VP1, the major capsid protein (shown below), is further organized into the N-terminal (N), shell (S), and protruding (P) domains defined by the indicated VP1 amino acid residues. The P2 region of the P domain (blue) is exposed on the surface of the capsid protein and is the site where histo–blood group antigens (HBGAs) (magenta) interact with the virion (dashed box).5,13
NOROVIRUSES IN IMMUNOCOMPROMISED PATIENTS
Prolonged norovirus and illness have been reported in persons who are immunosuppressed as a result of congenital immunodeficiency, immunosuppressive therapy for the purpose of maintaining an organ allograft, cancer chemotherapy, and infection with the human immunodeficiency virus (HIV).16,17 Immunocompromised patients can be exposed to noroviruses from many sources, including family members, health care workers, contaminated food or water, and the environment (including nosocomial sources).5 The overall incidence of norovirus gastroenteritis in hospital and community settings has not yet been determined. An increasing number of studies show that immunosuppressive therapy is a risk factor for norovirus infection. According to one report, 18% of patients who underwent allogeneic hematopoietic stem-cell transplantation (HSCT) contracted norovirus over a 1-year period, many after they had received intensified immunosuppressive regimens for suspected graft-versus-host disease (GVHD).18 A 2-year survey of renal-transplant recipients showed that 17% of the patients were chronically infected with norovirus and had intermittent diarrhea.19
Noroviruses are highly resistant to harsh environmental conditions, and the infectious oral dose is estimated to be less than 20 viral particles.4 In immunocompetent adults, norovirus gastroenteritis is characteristically acute (24 to 48 hours in duration) and self-limiting, but in immunocompromised adults, the disease can become chronic and can persist for weeks to years5,9 (Table 2). A marked predominance of wintertime norovirus infections has been widely described in the general population,5 and common names are winter-vomiting disease and stomach flu. In contrast, in a case–control study of children with cancer9 and in a case series of patients who had undergone HSCT,18 the rate of illness was reported to be unchanged throughout the year.
It is not yet clear whether noroviruses are transmissible to immunocompetent adults from patients with chronic viral shedding, the latter having been proposed as a possible reservoir of novel genetic variants.20 Surveillance studies suggest that most nosocomial norovirus infections are acquired in the community; nosocomial outbreaks in which persons with immunodeficiency disorders are the source are rare.21,22 Vomiting and diarrhea have been linked to high viral loads in patients undergoing immunosuppressive therapy, whereas asymptomatic shedding was associated with lower viral loads.9 These findings, as well as those involving immunocompetent hosts, suggest that in most cases the virus is transmitted from symptomatic persons, even when high levels of the virus are shed in the stool for prolonged periods after symptoms have resolved.23
Chronic norovirus gastroenteritis can present specific clinical challenges in patients with an impaired immune response, as compared with immunocompetent hosts. For example, norovirus-induced diarrhea in immunosuppressed renal-transplant recipients is characterized by dramatic weight loss and lasts considerably longer than treatable bacteria- or parasite-induced diarrhea (i.e., an average of 9 months vs. 1 month).24 The malnutrition, dehydration, and altered intestinal mucosal barrier associated with prolonged norovirus-related diarrhea can increase morbidity and worsen the outcome of the underlying disease.21 Noroviruses were reported to be the cause of death in one patient 49 days after the onset of symptoms21 and in another patient after 1 year of unresolved gastroenteritis.18
DIAGNOSIS OF NOROVIRUS GASTROENTERITIS
It is difficult to diagnose norovirus gastroenteritis on the basis of clinical features alone. Diarrhea is a common complication in transplant recipients17: gastroenteritis develops in 80% of patients who have undergone allogeneic HSCT, as a result of conditioning therapy, GVHD, drugs, or infectious agents.25 Symptoms of acute norovirus disease can include diarrhea, fever, and projectile vomiting, in contrast to the characteristic combination of diarrhea and nausea (without vomiting) observed in GVHD.5,18 Although a provisional diagnosis might be made, use of a reliable diagnostic assay is crucial to distinguish infectious diarrhea from clinical complications such as graft rejection and GVHD, since these conditions require diametrically opposed approaches to management (i.e., decreasing immunosuppression in infectious diarrhea and increasing it in graft rejection and GVHD).26
Noroviruses are shed in stool, and norovirus-specific antigens and RNA can be detected in stool samples. Regular or quantitative real-time RT-PCR assay is the most widely used laboratory method for diagnosing norovirus gastroenteritis, but several other assays are now available.4,27 Computed tomography has been reported to aid in discriminating between norovirus infection and GVHD, since norovirus-infected patients have pronounced bowel-wall edema restricted to the small intestine, which is infrequently seen in patients with intestinal cytomegalovirus infection or GVHD.21,28 Precise and timely diagnosis of norovirus infection by means of laboratory testing is essential in intestinal-transplant recipients, since the pathological characteristics of norovirus infection are similar to those seen in allograft rejection, with chronic inflammatory change, apoptotic cells, and blunted villi.22 The diagnosis of gastrointestinal GVHD, a well-described complication of HSCT, also relies on histopathological findings that could be mistaken for those associated with norovirus infection, in which numerous apoptotic bodies are observed.29
NOROVIRUS DIVERSIT Y AND EVOLUTION IN IMMUNOCOMPROMISED PATIENTS
The diversity of norovirus genotypes circulating in the community, where GII.4 is most prevalent, is reflected in the genotypes detected in persons with an impaired immune system.18,30,31 There have been no reported strain differences among the norovirus genotypes with regard to symptoms, severity, or progression to chronicity in immunocompromised hosts.
Intrahost variation and evolution of the viral genome over extended periods of shedding have been studied in detail in several patients persistently infected with norovirus.19,30–34 An analysis of the viral variants present in the stool of infected persons showed that in immunocompetent persons in whom the infection resolved during the acute phase, a single major variant predominated, whereas immunocompromised patients with chronic norovirus infection had a diverse viral population (Fig. 2).34 These data indicate that a chronic infection without immune pressure allows the generation of a diverse norovirus population in the host; however, at present there is no epidemiologic evidence to suggest that these variants become prevalent as epidemic strains in the community. Despite the increased viral heterogeneity generated during a chronic norovirus infection, the amino acid residues that interact with HBGA ligands remain conserved — a finding that is consistent with the proposed importance of this interaction in the binding of virus to intestinal epithelial cells.35
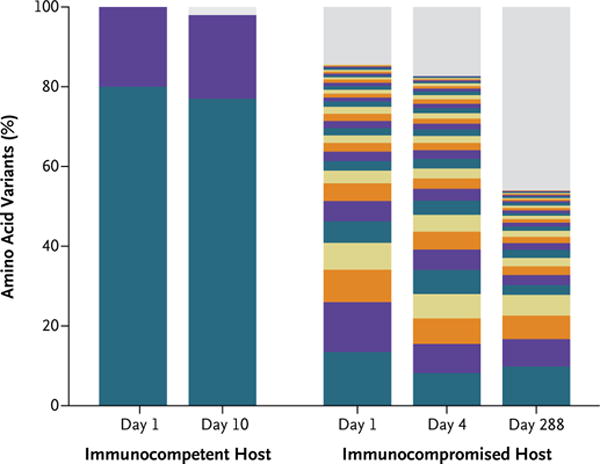
The bar graph shows the frequency distribution of VP1 norovirus variants, identified through next-generation sequencing, in an immunocompetent host and an immunocompromised host. Each unique variant is represented by a different color. Given the limited color palette, repeated colors represent distinct variants. Low-frequency variants, with an estimated frequency of occurrence below the detection threshold (2%), are shown in gray. Only two variants were detected in the immunocompetent host, and they remained stable throughout the 10 days of infection. No predominant variant was observed in the immunocompromised host. Instead, numerous low-frequency variants coexisted, and their prevalences varied over the course of the infection. Adapted from Bull et al.34
The shedding of norovirus by immunocompromised patients for an extended period of time has provided a unique opportunity to monitor the effects of genetic changes that lead to the accumulation of alterations in the amino acid makeup of the virus.19 Evolution of the norovirus genome in patients infected for extended periods is relatively rapid (3.3% amino acid substitutions per year), considering that GII.4 noroviruses have been shown to have accumulated only a 10% amino acid difference in their viral capsid after circulating in the community for 31 years.36 Accurate determination of a substitution rate could be useful in assessing whether a patient with chronic shedding continues to have the same norovirus strain or has been reinfected with a new strain; it may also help track norovirus transmission among immunocompromised patients in a common setting. This “time-clock” approach may prove useful in establishing the role of nosocomial transmission in immunocompromised patients and in evaluating the efficacy of treatment because knowledge of the rate at which genetic differences are generated can be used to determine whether a persistent strain is under investigation or a new strain has been introduced.19
PREVENTION AND TREATMENT OF NOROVIRUS INFECTION IN IMMUNOCOMPROMISED PATIENTS
No vaccines or specific antiviral agents are currently available to prevent or treat norovirus infection, but progress has recently been made in vaccine development.14,15 Norovirus vaccines have been tested in both humans and chimpanzees, and the results of these studies were used to determine correlates of protection and duration of immune response.14,15 It has also been reported that both T-cell and B-cell responses are necessary to clear norovirus. In a mouse model, CD4+ and CD8+ cells were required for clearance of murine norovirus in the intestine.37,38 Norovirus clearance in patients with chronic infection has been shown to be associated with the recovery of T cells25; in one study, symptoms improved in patients with HIV infection who had an increased CD4+ count.39
Currently, the treatment of patients with norovirus gastroenteritis is primarily supportive and focuses on prevention and reversal of dehydration. Chronic norovirus infections in transplant recipients may also require the adjustment of immunosuppressive therapy during prolonged illness.24 Passive antibody therapies have been tested in individual case studies, and evidence of their efficacy in treating patients with norovirus gastroenteritis is mostly anecdotal. Oral administration of breast milk or immune globulin has yielded mixed results, probably reflecting differences in the quality and quantity of norovirus-specific antibodies in the treatment administered.7,32 Both immune globulin and breast milk have been administered successfully through a duodenal tube (in an attempt to bypass the adverse acidic environment of the stomach) to treat prolonged norovirus infection in a heart-transplant recipient, but this approach failed to clear norovirus in a patient with agammaglobulinemia.40,41
Certain commonly used antiviral drugs such as ribavirin have failed to clear norovirus in chronically infected patients.40 Nitazoxanide (an anti-protozoal drug) was reported to significantly reduce the time to resolution of symptoms related to both rotavirus- and norovirus-induced diarrhea in immunocompetent patients,42 and symptoms of severe norovirus gastroenteritis were described as markedly reduced after 1 day of treatment in a patient who underwent HSCT.43 However, quantification of the exact genomic load in this patient was not reported, and viral shedding persisted for a month after treatment. Further testing will be needed to determine the efficacy of this drug in immunocompromised patients.
Finally, it has been suggested that the class of immunosuppressive drugs provided might affect the clearance of norovirus, since certain drugs also have antiviral properties.30,44 A significant increase in the antiviral properties of the immunosuppressive therapy administered (as measured by the incidence of cytomegalovirus infections in immunodeficient patients) was observed only when a switch was made from an antimetabolite (azathioprine or mycophenolate) to a mammalian target of rapamycin (mTOR) inhibitor (sirolimus or everolimus).45 The incidence of norovirus gastroenteritis in patients being treated with different types of immunosuppressive agents will require more study.
CONCLUSIONS
Given the substantial toll that noroviruses can take on the prognosis for and quality of life of patients with a deficient immune response, appropriate measures should be taken to reduce the risk of norovirus infection. First and foremost, rigorous personal hygiene, especially hand-washing, is the single most effective measure to combat norovirus transmission. This measure is crucial, given the fact that 80% of hospital surfaces were found to be contaminated with 21 different noroviruses during environmental surveillance in a unit for children with immunodeficiency disorders.46 Immunocompromised patients should avoid contact with persons who are acutely ill with gastroenteritis and should follow guidelines designed to prevent infections with enteric pathogens.47 Such patients should consume foods considered to be safe according to the principles designed to minimize the risk of foodborne diseases.48,49 Although it is prudent to isolate patients who have chronic norovirus infection, the virulence of noroviruses shed in the stool in such patients has been called into question, given the absence of reported secondary cases. Finally, norovirus testing can now be included in the care of immunocompromised patients with acute or chronic gastroenteritis of unknown cause. Widespread use of diagnostic assays and continued research will help clarify the precise disease burden and epidemiologic features of norovirus infection in this population and will improve the clinical care of those infected.
Acknowledgments
We thank Dr. Albert Z. Kapikian for his critical review and suggestions and Dr. Colleen Hadigan for expert clinical advice.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
No potential conflict of interest relevant to this article was reported.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmra1207742
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4944753?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1056/nejmra1207742
Article citations
Norovirus-mediated translation repression promotes macrophage cell death.
PLoS Pathog, 20(9):e1012480, 03 Sep 2024
Cited by: 0 articles | PMID: 39226332 | PMCID: PMC11398682
Establishment of a Nucleic Acid Detection Method for Norovirus GII.2 Genotype Based on RT-RPA and CRISPR/Cas12a-LFS.
Pol J Microbiol, 73(2):253-262, 20 Jun 2024
Cited by: 0 articles | PMID: 38905280 | PMCID: PMC11192556
A narrative review of norovirus epidemiology, biology, and challenges to vaccine development.
NPJ Vaccines, 9(1):94, 29 May 2024
Cited by: 3 articles | PMID: 38811605 | PMCID: PMC11137017
Review Free full text in Europe PMC
Evaluation of the detection of diarrhoea-associated RNA viruses in immunocompromised children in Iran.
Infect Prev Pract, 6(3):100370, 15 May 2024
Cited by: 0 articles | PMID: 38855735
Infectious Disease Prophylaxis During and After Immunosuppressive Therapy.
Kidney Int Rep, 9(8):2337-2352, 25 Apr 2024
Cited by: 0 articles | PMID: 39156157 | PMCID: PMC11328545
Review Free full text in Europe PMC
Go to all (200) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Norovirus gastroenteritis.
N Engl J Med, 361(18):1776-1785, 01 Oct 2009
Cited by: 614 articles | PMID: 19864676 | PMCID: PMC3880795
Review Free full text in Europe PMC
[Gastroenteritis due to norovirus].
Nihon Naika Gakkai Zasshi, 93(11):2334-2340, 01 Nov 2004
Cited by: 0 articles | PMID: 15624468
Review
Norovirus in immunocompromised patients.
Oncol Nurs Forum, 40(5):434-436, 01 Sep 2013
Cited by: 9 articles | PMID: 23989016
Review
[Noroviruses: most frequent cause of infectious gastroenteritis].
Dtsch Med Wochenschr, 132(43):2261-2266, 01 Oct 2007
Cited by: 0 articles | PMID: 17940932
Review





