Abstract
Free full text

Endocannabinoid system: potential novel targets for treatment of schizophrenia
Abstract
Accumulating epidemiological evidences suggest that cannabis use during adolescence is a potential environmental risk for the development of psychosis, including schizophrenia. Consistently, clinical and preclinical studies, using pharmacological approaches and genetically engineered animals to target endocannabinoid signaling, reveal the multiple varieties of endocannabinoid system-mediated human and animal behaviors, including cognition and emotion. Recently, there has been substantial progress in understanding the molecular mechanisms of the endocannabinoid system for synaptic communications in the central nervous system. Furthermore, the impact of endocannabinoid signaling on diverse cellular processes during brain development has emerged. Thus, although schizophrenia has etiological complexities, including genetic heterogeneities and multiple environmental factors, it now becomes crucial to explore molecular pathways of convergence of genetic risk factors and endocannabinoid signaling, which may provide us with clues to find novel targets for therapeutic intervention. In this review, epidemiological, clinical, and pathological evidences on the role of the endocannabinoid system in the pathophysiologies of schizophrenia will be presented. We will also make a brief overview of the recent progress in understanding molecular mechanisms of the endocannabinoid system for brain development and function, with particular focus on cannabinoid receptor type 1 (CB1R)-mediated cascade, the most well-characterized cannabinoid receptor. Lastly, we will discuss the potential of the endocannabinoid system in finding novel therapeutic targets for prevention and treatment of schizophrenia.
Introduction
A recent major progress in schizophrenia research is that psychiatric genetics have finally identified genetic risk factors for schizophrenia via various genetic approaches, such as classical linkage and association studies, cytogenetic approaches, as well as genome wide association and copy number variation studies (Doherty et al., 2012). Accumulating biological information reveals that many risk genes play a role in diverse molecular pathways implicated in fundamental developmental cellular processes ranging from early developmental stages (i.e. pre- and perinatal stages) to late developmental stages (i.e. childhood and adolescence) including adulthood, of which disturbances may result in pathogenic biological events underlying disease etiology (Insel, 2010). Although it is important to examine how genetic risk factors are involved in brain development, of which disturbances lead to aberrant neuronal maturation that may in turn confer vulnerability to schizophrenia, it may be difficult to find effective therapeutic strategies to restore genetic predisposition during early brain developmental processes. In this respect, therapeutic intervention during adolescence, the late stage of postnatal brain development, seems to be more practically feasible. Adolescence is a vulnerable period for environmental stimuli to alter functional and structural organization of the developing brain and likely is a critical time window for the emergence of full-blown onset of schizophrenia in early adulthood (Jaaro-Peled et al., 2009). Thus, despite etiological complexities for schizophrenia encompassing multiple genetic risks and environmental factors, exploring the molecular mechanisms of environmental influences and its convergence with genetic insults during adolescence may provide us with novel therapeutic targets for early intervention and prevention.
In this respect, the clinical effect of the use of cannabis during adolescence, which may disturb proper late brain maturation, is worth investigating as an environmental risk for schizophrenia. Furthermore, recent biological studies have demonstrated that the endocannabinoid system mediates regulation of neurotransmitter systems, including glutamatergic, GABAergic, and dopaminergic synaptic functions (Heifets and Castillo, 2009; Kano et al., 2009; Katona and Freund, 2012). It is also known that the endocannabinoid system plays a key role for multiple cellular processes during brain development, such as neural progenitor proliferation, neuronal migration, and axonal growth (Berghuis et al., 2007; Harkany et al., 2007; Keimpema et al., 2011; Mulder et al., 2008; Williams et al., 2003). In this article, we will summarize epidemiological and clinical evidences regarding the implication of the use of cannabis and the role of endocannabinoid system in the pathophysiologies of schizophrenia. We will also provide a brief overview on the recent progress in biology of endocannabinoid signaling. Finally, we will discuss the potential of the endocannabinoid system as novel therapeutic targets.
Cannabis use and schizophrenia
The pathological link between cannabis use and schizophrenia is generally accepted (Andreasson et al., 1987; van Os et al., 2002). Although there is still a debate on whether cannabis use is an independent risk factor for schizophrenia, or if high prevalence of cannabis use in patients with schizophrenia denotes patient self-medication for its neuroprotective effect (e.g., amelioration of cognitive impairment) (Potvin et al., 2008), substantial epidemiological evidences consistently suggest the use of cannabis during adolescence increases the relative risk for psychotic disorders, including schizophrenia and schizophreniform disorder, compared with non-cannabis users (Andreasson et al., 1987; Arseneault et al., 2002; Henquet et al., 2005; van Os et al., 2002; Zammit et al., 2002). Of interest, several longitudinal prospective studies demonstrated a dose-response relationship between the frequency of cannabis use and the risk for psychotic disorders, including schizophrenia (Arseneault et al., 2002; Henquet et al., 2005). Furthermore, the relative risk for first break psychosis and prodromal symptoms of psychosis is much higher for those who use cannabis during adolescence (Di Forti et al., 2009; Miettunen et al., 2008). Nonetheless, given that the majority of cannabis users do not develop schizophrenia, the use of cannabis is not sufficient to develop full-blown disease onset, but rather cannabis use may contribute as an environmental risk in a specific population vulnerable to schizophrenia with genetic risks and/or other environmental factors. In fact, Caspi et al. have recently reported that a functional polymorphism of catechol-O-methyltransferase (COMT) which encodes a major dopamine degradation enzyme, is associated with the effect of cannabis use on increased risk for psychosis (Caspi et al., 2005; Glatt et al., 2003). A missense mutation of COMT that consists of a valine to methionine substitution at codon 158 (Val158Met) affects its enzymatic activity and is genetically associated with schizophrenia (Tunbridge et al., 2006). The individual homozygous for valine 158 more likely displays psychotic symptoms after use of cannabis during adolescence. Finally, acute administration of delta-9-tetrahydrocannabinol (Δ9-THC), a major psychoactive ingredient of cannabis, into healthy subjects transiently induces psychotic symptoms, such as schizophrenia-like positive and negative symptoms and cognitive impairment, supporting the association of cannabis use and schizophrenia (D’Souza et al., 2004). Collectively, cannabinoid signaling may be implicated in the pathophysiology of schizophrenia.
Clinical evidence for disturbance of endocannabinoid system in schizophrenia
Multiple lines of evidence from clinical research, including genetic, postmortem, neuroimaging studies, as well as studies using cerebrospinal fluid (CSF) demonstrate that the endocannabinoid system is, at least in part, involved in schizophrenia pathology (Table 1). The endocannabinoid signaling is a lipid signaling system, which has multiple regulatory functions in the central nervous system. In addition to Δ9-THC, a major psychoactive component in cannabis, many endogenous ligands, including 2-arachidonoylglycerol (2-AG) and anandamide, have been indentified (Devane et al., 1992; Sugiura et al., 1995). Two G protein-coupled cannabinoid receptors, namely, cannabinoid receptor type 1 (CB1R) and type 2 (CB2R), have major roles in endocannabinoid system, although additional cannabinoid receptors may also be important (Matsuda et al., 1990; Munro et al., 1993).
Table 1
Evidences of potential endocannabinoid pathologies for schizophrenia on postmortem brain studies, neuroimaging studies, and studies using CSF
| Postmortem brain studies | Brain Region | Findings | Reference |
|---|---|---|---|
| [3H]CP-55940 | DLPFC | CB1R in the DLPFC: SZ > Controls | (Dean et al., 2001) |
| [3H]SR141716A | ACC | CB1R in the ACC: SZ > Controls | (Zavitsanou et al., 2004) |
| [3H]CP-55940 | PCC | CB1R in the PCC: SZ > Controls | (Newell et al., 2006) |
| anti CB1R antibody | ACC | CB1R in the ACC: No evidence in differences | (Koethe et al., 2007) |
| riboprobe, anti CB1R antibody | DLPFC | CB1R in the DLPFC: SZ < Controls | (Eggan et al., 2008) |
| anti CB1R antibody | DLPFC | CB1R in the DLPFC: SZ < Controls | (Eggan et al., 2010) |
| Neurological imaging studies | Brain Region | Findings | |
| MRI | Global and regional volume | No significant difference in global and regional volume | (Block et al., 2000) |
| MRI | No significant interaction effect of group with side, except for a difference in left versus right lateral ventricle | (Cahn et al., 2004) | |
| MRI | ACC | ACC volume in patients who used cannabis < both patients who did not use cannabis and healthy volunteers | (Szeszko et al., 2007) |
| MRI | PCC | PCC volume in patients who used cannabis < both patients who did not use cannabis and healthy volunteers | (Bangalore et al., 2008) |
| MRI | Grey matter | Grey matter volume in patients with cannabis use < the other subject groups | (Rais et al., 2008) |
| PET ([11C]MOAR) | PO | a higher VT values in all brain regions of subjects with SZ and significantly increase in the PO | (Wong et al., 2010) |
| CSF studies | endocannabinoid | Findings | |
| GC/MS | AEA, (PEA), 2-AG | AEA, (PEA): SZ > Controls, 2AG: below detection | (Leweke et al., 1999) |
| HPLC/MS | AEA | first-episode paranoid SZ > healthy controls | (Giuffrida et al., 2004) |
| LC/MS | AEA | SZ low-frequency cannabis users > SZ high-frequency users, healthy controls | (Leweke et al., 2007) |
| HPLC/MS | AEA | patient with prodromal state > healthy controls | (Koethe et al., 2009) |
Abbreviations: 2-AG, 2-arachidonoylglycerol; ACC, anterior cingulate cortex; AEA, anandamide; CB1R, cannabinoid receptor type 1; CSF, cerebrospinal fluid; DLPFC, dorsolateral prefrontal cortex; GC, gas chromatography; HPLC, high-performance liquid chromatography; LC, liquid chromatography; MRI, magnetic resonance imaging; MS, mass spectrometry; PCC, posterior cingulate cortex; PEA, palmitoylethanolamide; PO, pons; SZ, schizophrenia; VT, total distribution volume
There are some genetic studies that have reported the association of CB1R gene and schizophrenia, in particular, hebephrenic type (Chavarria-Siles et al., 2008; Ujike et al., 2002). Although further studies are awaited to confirm CB1R as a genetic risk for schizophrenia in a large sample cohort, recent studies using postmortem brains from patients with schizophrenia support the role of CB1R in schizophrenia pathology. Three studies have reported the increase in cannabinoid receptor binding in the prefrontal area of brains from patients with schizophrenia by using in vitro autoradiography. Dean et al. found an increase in cannabinoid receptor binding with [3H]CP-55940 in the dorsolateral prefrontal cortex in patients with schizophrenia (Dean et al., 2001). Another study found the upregulation of CB1R in the anterior cingulate cortex in patients with schizophrenia using a more selective CB1R ligand, [3H]SR141716A (Zavitsanou et al., 2004). Lastly, an increase in CB1R binding density has been found in the posterior cingulate cortex in schizophrenia patients using [3H]CP-55940, another CB1R ligand (Newell et al., 2006). In contrast, expression analysis by immunohistochemistry failed to demonstrate alteration of the expression of CB1R at the protein level in the anterior cingulate cortex from patients with schizophrenia (Koethe et al., 2007). More recently, Lewis’s group reported reduction of CB1R expression, both at the protein and messenger RNA level, in the prefrontal cortex in patients with schizophrenia by using in situ hybridization and immunohistochemical analysis (Eggan et al., 2008; Eggan et al., 2010). The authors discussed that the discrepancy between both lines of data may be explained by the possibility that the data of increase in CB1R binding by autoradiography may result from the conformational change of CB1R which affects binding affinity of radioligands, and may not reflect the amount of CB1R itself (Eggan et al., 2008). Nonetheless, the recent positron emission tomography (PET) study using [11C]OMAR (JHU75528), a novel radioligand tracer for CB1R, demonstrated an increase of [11C]OMAR binding in patients with schizophrenia, at least, in the pons (Horti et al., 2006; Wong et al., 2010). There is significant correlation in the ratio of Brief Psychiatry Rating Score (BPRS) psychosis to withdrawal subscore versus volume of interest measures of [11C]OMAR in the frontal lobe, as well as middle and posterior cingulated cortex (Wong et al., 2010). More recently, Wong and colleagues have reported a voxelwise confirmation of this correlation and most importantly an inverse correlation between [11C]OMAR volume of distribution and BPRS withdrawal subscore, suggesting an important role for negative symptoms with CB1R in vivo imaging (unpublished data; the abstract of Wong et al. in the annual meeting of the Society of Biological Psychiatry at Philadelphia in April 2012).
The data from magnetic resonance imaging (MRI) studies also supports the association of cannabis use and schizophrenia. Although initial studies reported no brain abnormalities in either cannabis users or cannabis-exposed patients with schizophrenia (Block et al., 2000; Cahn et al., 2004), recent evidences demonstrated volume loss of certain brain regions, such as anterior and posterior cingulate cortex in patients with first-episode schizophrenia who use cannabis, and these areas are also known to be rich in CB1R (Bangalore et al., 2008; Szeszko et al., 2007). Also, it has been shown that cannabis use during adolescence causes greater volume loss of whole brain than cannabis use post-adolescence (Wilson et al., 2000).
The effect of the endocannabinoid system on schizophrenia pathology is also supported by studies using cerebrospinal fluid (CSF) from patients with schizophrenia. Leweke and colleagues reported an increase in the concentration of anandamide and palmitoylethanolamide (PEA), endogenous cannabinoids, in CSF from patients with schizophrenia when compared to healthy controls, whereas levels of 2-AG were not observed due to its low concentration under the detectable level (Leweke et al., 1999). The same group further found an increase of anandamide in CSF from patients with drug-naive first episode schizophrenia and even in prodromal stages of psychosis (Giuffrida et al., 2004; Koethe et al., 2009).
Observed data from these clinical studies support the idea that cannabis use during adolescence is an environmental factor which affects subsequent development of schizophrenia. Nonetheless, there are other studies which refute the link between the endocannabinoid system and schizophrenia. Tsai et al. have reported no genetic association between the CB1R gene and schizophrenic disorders (Tsai et al., 2000). Other sources not only state no statistically significant association between mutations in the CB1R gene and a predisposition to develop schizophrenia, but also conclude that even interactions between drug use such as tobacco and marijuana, and the affected genes alpha 7 nicotinic receptor (CHRNA7), CB1R, and COMT, do not play a role in schizophrenia (Seifert et al., 2007; Zammit et al., 2007). However, much like the studies supporting a role of the endocannabinoid system in schizophrenia, these studies admit that there could be much overlooked and unaccounted for including proper sample size, background of subjects, and relevant variations in areas tested. With all studies in mind, the endocannabinoid system is still a relevant an exciting area for the study of novel treatments for schizophrenia. Collectively, in order to explore novel therapeutic targets for schizophrenia in endocannabinoid system, further studies are needed to examine the association of cannabis effect and genetic predisposition for schizophrenia and its underling molecular mechanisms.
Endocannabinoid system in the developmental trajectory
During the past decades, substantial progress has been made towards understanding the molecular basis of the endocannabinoid system for multiple developmental cellular processes and various types of synaptic communication (Di Marzo, 2011; Harkany et al., 2007; Kano et al., 2009; Keimpema et al., 2011). This includes characterization of metabolic pathways for anandamide (AEA) and 2-arachidonoylglycerol (2-AG), two major endocannabinoids which are expressed during and after brain development (Harkany et al., 2007; Kano et al., 2009) (Figure 1). 2-AG is mainly produced via sn-1-diacylglycerol lipase α and β (DAGL α and β)-mediated pathways (Bisogno et al., 2003), whereas the synthetic process of AEA remains controversial. In addition to N-acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), other enzymes, such as protein tyrosine phosphatase, non-receptor type 22 (PTPN22) and α/β hydrolase 4 (ABHD4), are also reportedly involved in AEA production (Harkany et al., 2007). On the other hand, the degrading pathways are well characterized, such that monoglyceride lipase 1/2 (MAGL1/2) and FAAH are major enzymes for catabolizing 2-AG and AEA, respectively, but ABHD6/12 and cyclooxygenase 2 (COX2) also have degrading roles (Harkany et al., 2007). The effect of endocannabinoids and psychoactive compounds of cannabis are mainly mediated by activating CB1R, a major G protein-coupled cannabinoid receptor to control numerous physiological functions, including cognition, emotion, mood, reward-associated behaviors, feeding, and pain (Monory and Lutz, 2009). Recent evidences have further suggested the existence of additional cannabinoid receptors, including the aforementioned CB2R, the transient receptor potential cation channel subfamily V member 1 (TRPV1), and the orphan G-protein-coupled receptor 55 (GPCR55), which have not been fully characterized yet (Harkany et al., 2007; Ohno-Shosaku et al., 2011). Multiple intracellular signaling pathways of CB1R have been identified to regulate developmental processes defining cell morphology and synaptic function in a context dependent manner (Di Marzo, 2011; Harkany et al., 2007; Kano et al., 2009; Keimpema et al., 2011). Collectively, the endocannabinoid system has pleiotropic effects via the complex nature of metabolic pathways and signaling cascades in controlling numerous physiological functions. In this review, we will briefly overview biological bases of the endocannabinoid system at the molecular to behavioral range, with a particular focus on well-studied CB1R-mediated signaling for brain development, synaptic function, and cognition.
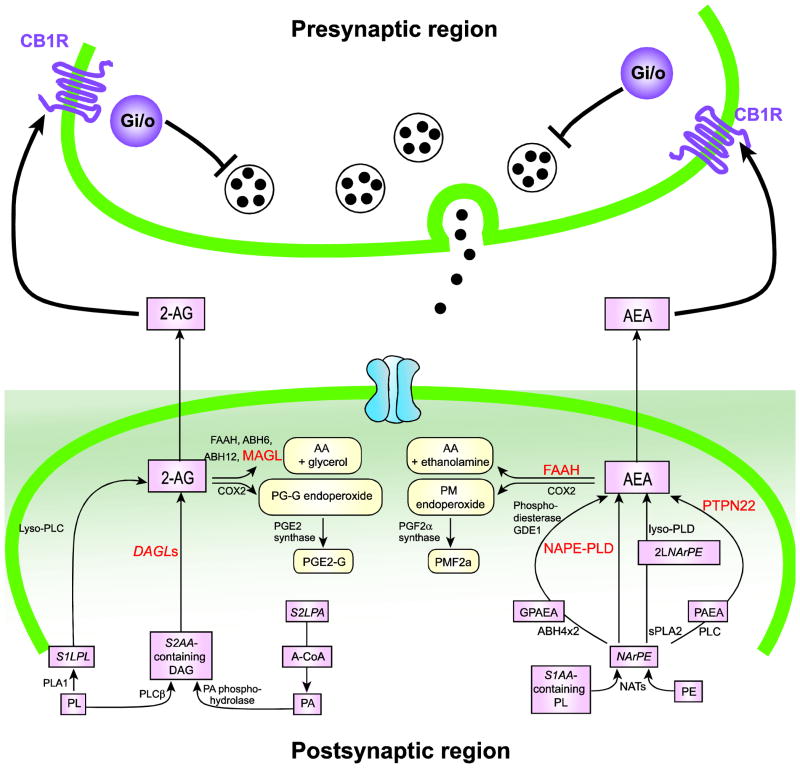
Abbreviations: 2-AG, 2-arachidonoylglycerol; 2LNArPE, 2-l-N-arachidonoyl-phosphatidylethanolamine; AA, arachidonic acid; ABH, A-CoA, arachidonoyl-CoA; αβ-hydrolases; AEA, anandamide; COX2, cyclooxygenase 2; DAGLs, sn-1-selective diacylglycerol lipases; FAAH, fatty acid amide hydrolase; GDE1, glycerophosphodiester phosphodiesterase 1; GPAEA, Glycerophosphoanandamide; MAGL, monoacylglycerol lipase; NAPE-PLD, N-acyl-phosphatidylethanolamine-selective phosphodiesterase; NATs, N-acyltransferases; NArPE, N-arachidonoyl-phosphatidylethanolamine; PA, Phosphatidic acid; PE, Phosphatidylethanolamine; PGE2-G, Prostaglandin E2 glycerol ester; PMF2a, Prostamide F2a; (s)PLA1/2, (soluble) phospholipase A1/2; PLC, phospholipase C; PLCβ, phospholipase Cβ; PLD, phospholipase D;PTPN22, protein tyrosine phosphatase, non-receptor type 22; S1LPL, sn-1-lysophospholipid; S2AA, sn-2-arachidonic acid; S2LPA, sn-2-lysophosphatidic acid.
CB1R-mediated endocannabinoid system during development
The developmental expression of CB1R has been extensively investigated in the rodent and primate brains. In human frontal cortex, hippocampus, and striatum, autoradiographic levels of CB1R increase from prenatal stages to young adulthood (Mato et al., 2003). An increase of CB1R in certain rat brain regions, such as striatum, limbic forebrain, and ventral mesencephalon, with the peak at the postnatal days 30–40 has been reported (Rodriguez de Fonseca et al., 1993). More recently, the developmental increase of CB1R protein expression which achieved at the adult levels by postnatal 1 weeks, has been reported in the dorsolateral prefrontal cortex of macaque monkeys (Eggan et al., 2009). During early brain development, sequential cellular processes, including cell proliferation, migration, and axon/dendrite growth, as well as gliogenesis are required for coordinating proper neuronal architecture (Harkany et al., 2007; Keimpema et al., 2011). The expression of CB1R in certain brain regions, such as cerebral cortex, hippocampus, cerebellum, and basal ganglia from embryonic stages to prenatal period support the importance of endocannabinoid signaling for these developmental cellular events (Berrendero et al., 1999). In fact, disturbance of cell proliferation and neuronal migration in the developing cerebral cortex, as well as postnatal astrogliogenesis was observed in CB1R knockout mice (Aguado et al., 2006; Mulder et al., 2008). Consistently, genetic deletion of FAAH increases neuronal progenitor proliferation with high levels of AEA (Aguado et al., 2006). Convergent action of CB1R-mediated endocannabinoid signaling and other molecular pathways is also suggested to control such developmental cellular processes. For instance, Berghuis and colleagues reported that the treatment of AEA induces the migration of dissociated cultured CB1R-expressing interneuron, which is blocked by AM251, an inverse agonist of CB1R (Berghuis et al., 2005). Interestingly, concomitant treatment of AEA and BDNF accelerate interneuron migration, suggesting the cooperative action of endocannabinoid and BDNF signaling (Berghuis et al., 2005).
The effect of endocannabinoid signaling in axon growth has also been assessed. CB1R is highly expressed in the axonal regions of cortical pyramidal neurons and hippocampal GABAergic interneurons at the early developmental stages, including embryonic stages, when the axonal process is vigorously growing for the establishment of synaptic connections (Berghuis et al., 2007; Mulder et al., 2008). By using conventional and conditional CB1R knockout mice, including pyramidal neuron-specific and GABAergic interneuron-specific ones, as well as pharmacological manipulation of endocannabinoid signaling, Harkany and colleagues have found that endocannabinoids act as axon guidance cues, thereby coordinating proper axon patterning for both local GABAergic interneurons and pyramidal neurons during brain development (Berghuis et al., 2007; Mulder et al., 2008). The proper development of axonal projection regulated by CB1R-mediated endocannabinoid signaling is critical for the long lasting neuronal circuit formation between various brain regions. In fact, despite the lack of CB1R expression in thalamocortical axons, aberrant axon fasciculation and pathfinding is observed in not only corticothalamic projections, but also thalamocortical axons in conditional CB1R knockout mice lacking CB1R in only cortical pyramidal neurons (Wu et al., 2010). These results prove the importance of endocannabinoid signaling in regulation of axon growth, and may hint at further links of the endocannabinoid system and neuronal circuit formation between brain regions.
There are several molecular signaling pathways, such as phosphoinositol 3 kinase (PI3K)/Akt and ERK pathways, which have been reported to have roles in the downstream of CB1R-mediated endocannabinoid signaling (Ozaita et al., 2007; Rueda et al., 2002). Of interest, in silico gene regulatory network approach delineated that the pathway through PI3K to transcription factor paired box 6 (PAX6) acts as the downstream signaling of CB1R-mediated signaling for regulating neurite outgrowth (Bromberg et al., 2008). In spite of such evidences, intracellular mechanisms underpinning their impact on developmental cellular behaviors, such as cell proliferation, neuronal migration, and axon/dendrite growth in in vivo condition, warrants further investigation.
Endocannabinoid system in synapse function
Basic mechanisms of CB1R-mediated endocannabinoid signaling in synaptic function have been extensively investigated. Here we provide a brief overview of the current progress in our understanding of the role of endocannabinoids in synaptic signaling. For detailed physiological mechanisms of the effects of endocannabinoids on synaptic plasticity, including presynaptic regulation of neurotransmitter release via CB1R signaling, please refer to several excellent reviews (Heifets and Castillo, 2009; Kano et al., 2009; Katona and Freund, 2012). CB1R is widely expressed in many brain regions that control cognition and emotion, such as the hippocampus, amygdala, and cerebral cortex, mainly in cholecystokinin (CCK)-containing interneurons and to some extent in pyramidal neurons (Heifets and Castillo, 2009; Ohno-Shosaku et al., 2011). Endocannabinoids regulate synaptic plasticity, namely short-term depression and long-term depression, via CB1R-mediated mechanisms for suppression of neurotransmitter release at both excitatory and inhibitory synapses (Heifets and Castillo, 2009; Ohno-Shosaku et al., 2011). After the initial discovery of the endocannabinoids as a retrograde signaling function to regulate synaptic plasticity in the hippocampus (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001), a similar mechanism for the regulation of depolarization-induced suppression of inhibition and excitation has been reported in many brain regions, including cerebral cortex, cerebellum, striatum, and amygdala ((Heifets and Castillo, 2009; Ohno-Shosaku et al., 2011). Endocannabinoid release from postsynaptic regions in a retrograde fashion is regulated by calcium influx with neuronal depolarization through many effectors, such as the N-methyl-D-aspartate (NMDA) receptor, voltage-dependent calcium channels (VDCC), and calcium release from intracellular stores (Freund et al., 2003). The mechanisms of postsynaptic endocannabinoid release are generally believed to be common in short-term depression and long-term depression (Ohno-Shosaku et al., 2011). Interestingly, a transient suppression of inhibition induced by stimulation of NMDA receptor is not impaired by the treatment of VDCC blockers in cultured hippocampal neurons, although postsynaptic elevation of calcium is required for the suppression of inhibition (Ohno-Shosaku et al., 2007). This suggests that calcium intake via NMDA receptor may regulate the postsynaptic endocannabinoid production/release, independently through VDCC-mediated mechanisms.
In addition, endocannabinoid release is also mediated by the activation of G protein coupled receptors without calcium-driven mechanisms (Heifets and Castillo, 2009; Ohno-Shosaku et al., 2011). For instance, group I metabotropic glutamate receptors (mGluRs), Gq/11 protein-coupled receptors, induces short-term depression via endocannabinoid-mediated regulation (Jung et al., 2005; Maejima et al., 2001; Varma et al., 2001). The activation of mGluRs enhances depolarization-induced suppression of inhibition of which the effect is impaired by both mGluRs and CB1R antagonists in the hippocampal slice cultures (Varma et al., 2001). The role of mGluRs for endocannabinoid-mediated presynaptic inhibition has also been reported in cerebellar Purkinje cells (Maejima et al., 2001). Jung et al. found that activation of mGluR5 receptors induce the production of 2-AG, but not anandamide, in rat corticostriatal and hippocampal slice cultures, indicating that 2-AG may be a major endogenous ligand for mGluR5 receptor-mediated endocannabinoid signaling (Jung et al., 2005). Interestingly, mGluRs-mediated short-term depression was enhanced by the depolarization-induced increase of intracellular calcium concentration (Hashimotodani et al., 2005). These results suggest that these mechanisms also synergistically regulate synaptic transmission in a context dependent manner.
Recent evidences suggest that astrocytes are also involved in the regulation of endocannabinoid-mediated neurotransmission in hippocampal pyramidal neurons (Navarrete and Araque, 2010). Of note, the authors found that the increase of calcium concentration in astrocytes via their CB1R activation induce the release of glutamate from the astrocytes, resulting in the activation of presynaptic mGluRs, thereby potentiating synaptic transmission (Navarrete and Araque, 2010).
Endocannabinoid system and cognition
Consistent with the multiple roles of endocannabinoid signaling for brain developmental processes and neuronal transmission, genetic and pharmacological manipulation of endocannabinoid signaling can elucidate its regulatory effects on various behavioral aspects in rodent models, including cognitive and emotional processes. Here, we provide a brief overview of behavioral characteristics of the endocannabinoid system, with a particular focus on cognitive function (Table 2). For more detailed information on studying the role of endocannabinoid on behaviors, please see the systematic review (Zanettini et al., 2011).
Table 2
The effect of genetic and pharmacological manipulation of endocannabinoid system on cognitive function
| Genetically engineered model | Phenotype | Reference |
|---|---|---|
| CB1R knockout mice | Memory extinction ↓ | (Kamprath et al., 2006; Marsicano et al., 2002; Varvel et al., 2005) |
| CB1R knockout mice | Recognition memory ↑ | (Marsicano et al., 2002) |
| FAAH knockout mice | Memory acquisition ↑ | (Varvel et al., 2007) |
| Astroglial-specific CB1R knockout with Δ9-THC | Deficits in working memory ↓ | (Han et al., 2012) |
| Pharmacological model | ||
| Cannabinoids (Δ9-THC, anandamide) | Working memory ↓ | (Da and Takahashi, 2002; Heyser et al., 1993; Lichtman et al., 1995; Mallet and Beninger, 1998) |
| CB1R agonist (WIN55,212-2) | Working memory ↓, | (Deadwyler et al., 2007) |
| CB1R antagonist (Rimonabant) | Working memory ↑, Recognition memory ↑ | (Deadwyler et al., 2007; Terranova et al., 1996) |
| Cannabinoids (Δ9-THC) | Memory acquisition ↓ | (Da and Takahashi, 2002) |
| CB1R agonist (WIN55,212-2, HU-210) | Memory acquisition ↓ | (Ferrari et al., 1999; Robinson et al., 2010) |
| CB1R agonist (WIN55,212-2, HU-210) | Recognition memory ↓ during adolescence | (Schneider and Koch, 2003) |
| FAAH inhibitor (OL-135) | Memory acquisition ↑, Memory extinction ↓ | (Varvel et al., 2007) |
There are many studies examining the effect of endocannabinoid signaling on working memory using various behavioral paradigms, such as water maze test, radial maze test, and delayed non-matching to place (DNMTP) task, with pharmacological manipulation. Generally, cannabinoid receptor agonists, such as Δ9-THC, anandamide, CP-55,940, and WIN55,212-2 impaired working memory, whereas rimonabant, a CB1R antagonist, enhanced working memory (Da and Takahashi, 2002; Deadwyler et al., 2007; Heyser et al., 1993; Lichtman et al., 1995; Mallet and Beninger, 1998). Multiple studies using cannabinoid receptors agonists and antagonists also reported that endocannabinoid signaling is required for memory acquisition. The administration of Δ9-THC impaired the acquisition of spatial learning and the performance of mice in the working memory task (Da and Takahashi, 2002). Although the aforementioned studies focus mainly on acute drug administration results, other studies focus on chronic drug administration. The chronic treatment of Δ9-THC for 27 days in adolescence (postnatal days 28 to 54) impairs learning and memory in Sprague-Dawley rats when the effects are measured specifically in the “post-acute” period (17 h after drug exposure) (Steel et al., 2011), while Rubino et al. have demonstrated that the chronic Δ9-THC administration in adolescence (postnatal days 35 to 45) can also induce cognitive impairment assessed by radial maze task and altered expression of synaptic proteins in the prefrontal cortex in female adult rats (postnatal days 75) (Rubino et al., 2009). Consistently, other CB1R agonists, such as HU-210 and WIN55,212-2 have commonly impaired memory acquisition (Ferrari et al., 1999; Robinson et al., 2010). Of note, it has also been reported that chronic administration of WIN55,212-2 during adolescence leads to deficits in recognition memory, but not in adulthood (Schneider and Koch, 2003). Although there are some discrepancies in the effect of WIN55,212-2 among previous studies, perhaps due to the differences in experimental procedures, adolescence may nevertheless be considered as a vulnerable period for cognitive changes by pharmacological manipulation of endocannabinoid signaling. Nonetheless, behavioral phenotypes induced by pharmacological manipulation warrant caution in data interpretation. These drugs may affect other molecular processes which regulate high brain function. Furthermore, endocannabinoids may regulate distinct memory processes depending on brain regions and cell types. In fact, chronic hippocampal injection of WIN55,212-2 impaired object recognition memory (Barna et al., 2007), whereas administration of WIN55,212-2 into the basolateral amygdala enhanced memory retention (Campolongo et al., 2009).
Genetically engineered animals of endocannabinoid signaling are available for behavioral assessment. Several groups have reported impairment of memory extinction in CB1R knockout mice (Kamprath et al., 2006; Marsicano et al., 2002; Varvel et al., 2005), providing complementary evidences for the results observed via pharmacological manipulation of endocannabinoid signaling as described above. Furthermore, enhancement of recognition memory was observed in CB1R knockout mice (Maccarrone et al., 2002). Consistently, mice with genetic deletion of FAAH, the hydrolytic enzyme for the endogenous cannabinoid receptor ligand anandamide, displayed enhanced acquisition under certain conditions (Varvel et al., 2007). Aforementioned above, endocannabinoid signaling may mediate multiple cognitive behaviors in a brain region- and cell type- dependent manner. Given that conditional genetically engineered mice of CB1R, such as forebrain GABAergic neurons-specific, cortical glutamatergic neurons-specific, and astrocytes-specific CB1R deletion mice are available, further behavioral characterization is awaited for understanding the complex nature of endocannabinoid signaling for cognitive function. Of note, Zhang and colleagues have recently reported that the impairment of spatial working memory induced by acute Δ9-THC administration is ameliorated in astroglial cell-specific conditional knockout of CB1R, but not in mice lacking CB1R in glutamatergic or GABAergic neurons (Han et al., 2012). The author concluded that the effect of exogenous cannabinoids on working memory is mediated by the activation of CB1R in astroglial cells (Han et al., 2012).
Potential novel therapeutic targets in endocannabinoid system for treatment of schizophrenia
Based on the evidences above, the endocannabinoid system can and has been considered a target of study for novel therapeutic techniques in schizophrenia. In fact, CB1 antagonists have been tested for anti-psychotic properties relevant to treating schizophrenia with varying results in preclinical and clinical studies (Roser et al., 2010; Zanettini et al., 2011). A more recent and exciting development in this avenue is the testing of AVE1625, a CB1 receptor antagonist, in a co-treatment setting with antipsychotics. In this experiment, Black et al. found an improvement in cognitive function and a reduction of typical antipsychotic side effects in rodents (Black et al., 2011). Further research of CB1 antagonists is expected to find novel therapeutic strategies in endocannabinoid system for treatment of schizophrenia.
Since the initial discovery of CB1R and endogenous ligands, such as 2-AG and anandamide in the 1990s, great progress has been made in understanding the roles of endocannabinoid signaling at the molecular to behavioral range, which is extremely valuable when studying pathophysiological mechanisms of the effect of the endocannobinoid system on schizophrenia. As we described above, although endocannabinoid signaling plays critical roles for early brain developmental processes, therapeutic intervention during adolescence, the late stage of postnatal brain maturation, seems to be more practically feasible. Adolescence is the critical period for maturation of many neurotransmitter systems, including dopaminergic, glutametergic, and GABAergic projections along with dynamic changes of receptor expression for these neurotransmitters as well as synaptic density (Kilb, 2011; O’Donnell, 2010). As it has been mentioned above, given that the activation of CB1R suppresses the release of glutamate and GABA in a context dependent manner, aberrant CB1R signaling may hamper full-maturation of the neuronal circuit network via the impairment of proper neuronal communications during adolescence. This is consistent with the notion that cannabis use during adolescence is an environmental risk for disturbance of proper full-maturation of neuronal circuits which might underlie the later development of schizophrenia. Thus, further investigation to elucidate the molecular mechanism of how endocannabinoid signaling contributes to neuronal maturation, including possibly its effect on pruning of synaptic connections during adolescence, is awaited.
Conclusions
Although highly speculative, if cannabis exposure during adolescence may play a role as a “second hit” for the onset of schizophrenia in persons with genetic predisposition in early developmental stages, intervention of endocannabinoid signaling during adolescence might be a useful strategy for prevention of schizophrenia in a certain population. In addition, many genetic risk factors for schizophrenia, including Disrupted-in-schizophrenia-1 (DISC1) and neuregulin-1, are involved in synaptic functions (Jaaro-Peled et al., 2009; Kamiya et al., 2012). Thus, it is important to explore how such genetic risk factors may have a convergent effect with cannabis use on neuronal network maturation during adolescence, of which disturbances may underlie full-blown onset of schizophrenia. In fact, Waddington and colleagues reported that chronic administration of Δ9-THC during adolescence deteriorated the deficits in working memory in mice lacking COMT gene, a risk gene for schizophrenia (O’Tuathaigh et al., 2010). Further investigation of the convergent mechanisms of genetic risk factors and endocannabinoid signaling during the adolescent period may allow us to dissect pathological processes associated with the endocannabinoid system, which may in turn, shed light on the discovery of novel therapeutic intervention and prevention for schizophrenia.
Acknowledgments
We thank Ms. Sandra Zoubovsky for critical reading of the manuscript. We also thank Ms. Yukiko Lema for preparation of the figures. This work was supported by grants from MH091230 (A.K.), MH083728 (M.P.), MH094268 (A.K.; M.P.), and foundation grants from NARSAD (A.K.; M.P.), S-R (A.K.), BSF (A.K.), and SMRI (M.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguado T, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. [Europe PMC free article] [Abstract] [Google Scholar]
- Andreasson S, et al. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. [Abstract] [Google Scholar]
- Arseneault L, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. [Europe PMC free article] [Abstract] [Google Scholar]
- Bangalore SS, et al. Cannabis use and brain structural alterations in first episode schizophrenia--a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. [Abstract] [Google Scholar]
- Barna I, et al. WIN-55,212-2 chronically implanted into the CA3 region of the dorsal hippocampus impairs learning: a novel method for studying chronic, brain-area-specific effects of cannabinoids. Behav Pharmacol. 2007;18:515–520. [Abstract] [Google Scholar]
- Berghuis P, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. [Europe PMC free article] [Abstract] [Google Scholar]
- Berghuis P, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. [Abstract] [Google Scholar]
- Berrendero F, et al. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. [Abstract] [Google Scholar]
- Bisogno T, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. [Europe PMC free article] [Abstract] [Google Scholar]
- Black MD, et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacology (Berl) 2011;215:149–163. [Abstract] [Google Scholar]
- Block RI, et al. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. [Abstract] [Google Scholar]
- Bromberg KD, et al. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. [Europe PMC free article] [Abstract] [Google Scholar]
- Cahn W, et al. Cannabis and brain morphology in recent-onset schizophrenia. Schizophr Res. 2004;67:305–307. [Abstract] [Google Scholar]
- Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. [Europe PMC free article] [Abstract] [Google Scholar]
- Caspi A, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. [Abstract] [Google Scholar]
- Chavarria-Siles I, et al. Cannabinoid receptor 1 gene (CNR1) and susceptibility to a quantitative phenotype for hebephrenic schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147:279–284. [Abstract] [Google Scholar]
- D’Souza DC, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. [Abstract] [Google Scholar]
- Da S, Takahashi RN. SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:321–325. [Abstract] [Google Scholar]
- Deadwyler SA, et al. Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharmacol. 2007;18:571–580. [Abstract] [Google Scholar]
- Dean B, et al. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. [Abstract] [Google Scholar]
- Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. [Abstract] [Google Scholar]
- Di Forti M, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–491. [Europe PMC free article] [Abstract] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9–15. [Abstract] [Google Scholar]
- Doherty JL, et al. Recent genomic advances in schizophrenia. Clin Genet. 2012;81:103–109. [Abstract] [Google Scholar]
- Eggan SM, et al. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. [Europe PMC free article] [Abstract] [Google Scholar]
- Eggan SM, et al. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex. 2009;20:1164–1174. [Europe PMC free article] [Abstract] [Google Scholar]
- Eggan SM, et al. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35:2060–2071. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferrari F, et al. Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacol Biochem Behav. 1999;64:555–561. [Abstract] [Google Scholar]
- Freund TF, et al. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. [Abstract] [Google Scholar]
- Giuffrida A, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. [Abstract] [Google Scholar]
- Glatt SJ, et al. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry. 2003;160:469–476. [Abstract] [Google Scholar]
- Han J, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. [Abstract] [Google Scholar]
- Harkany T, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. [Abstract] [Google Scholar]
- Hashimotodani Y, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. [Abstract] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. [Europe PMC free article] [Abstract] [Google Scholar]
- Henquet C, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330:11. [Europe PMC free article] [Abstract] [Google Scholar]
- Heyser CJ, et al. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [Abstract] [Google Scholar]
- Horti AG, et al. 11C-JHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med. 2006;47:1689–1696. [Abstract] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [Abstract] [Google Scholar]
- Jaaro-Peled H, et al. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. [Europe PMC free article] [Abstract] [Google Scholar]
- Jung KM, et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. [Abstract] [Google Scholar]
- Kamiya A, et al. DISC1 Pathway in Brain Development: Exploring Therapeutic Targets for Major Psychiatric Disorders. Front Psychiatry. 2012;3:25. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamprath K, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. [Europe PMC free article] [Abstract] [Google Scholar]
- Kano M, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. [Abstract] [Google Scholar]
- Katona I, Freund TF. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu Rev Neurosci 2012 [Europe PMC free article] [Abstract] [Google Scholar]
- Keimpema E, et al. Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci. 2011;32:551–561. [Europe PMC free article] [Abstract] [Google Scholar]
- Kilb W. Development of the GABAergic System from Birth to Adolescence. Neuroscientist 2011 [Abstract] [Google Scholar]
- Koethe D, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 2009;194:371–372. [Abstract] [Google Scholar]
- Koethe D, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114:1055–1063. [Abstract] [Google Scholar]
- Leweke FM, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94:29–36. [Abstract] [Google Scholar]
- Leweke FM, et al. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. [Abstract] [Google Scholar]
- Lichtman AH, et al. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. [Abstract] [Google Scholar]
- Maccarrone M, et al. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci. 2002;15:1178–1186. [Abstract] [Google Scholar]
- Maejima T, et al. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. [Abstract] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 1998;140:11–19. [Abstract] [Google Scholar]
- Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. [Abstract] [Google Scholar]
- Mato S, et al. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–1754. [Abstract] [Google Scholar]
- Matsuda LA, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. [Abstract] [Google Scholar]
- Miettunen J, et al. Association of cannabis use with prodromal symptoms of psychosis in adolescence. Br J Psychiatry. 2008;192:470–471. [Abstract] [Google Scholar]
- Monory K, Lutz B. Genetic models of the endocannabinoid system. Curr Top Behav Neurosci. 2009;1:111–139. [Abstract] [Google Scholar]
- Mulder J, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105:8760–8765. [Europe PMC free article] [Abstract] [Google Scholar]
- Munro S, et al. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. [Abstract] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. [Abstract] [Google Scholar]
- Newell KA, et al. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172:556–560. [Abstract] [Google Scholar]
- O’Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. [Abstract] [Google Scholar]
- O’Tuathaigh CM, et al. Chronic adolescent exposure to Delta-9-tetrahydrocannabinol in COMT mutant mice: impact on psychosis-related and other phenotypes. Neuropsychopharmacology. 2010;35:2262–2273. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohno-Shosaku T, et al. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol. 2007;584:407–418. [Abstract] [Google Scholar]
- Ohno-Shosaku T, et al. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. [Abstract] [Google Scholar]
- Ohno-Shosaku T, et al. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2011;18:119–132. [Abstract] [Google Scholar]
- Ozaita A, et al. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. [Abstract] [Google Scholar]
- Potvin S, et al. Contradictory cognitive capacities among substance-abusing patients with schizophrenia: a meta-analysis. Schizophr Res. 2008;100:242–251. [Abstract] [Google Scholar]
- Rais M, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490–496. [Abstract] [Google Scholar]
- Robinson L, et al. WIN55,212-2 induced deficits in spatial learning are mediated by cholinergic hypofunction. Behav Brain Res. 2010;208:584–592. [Europe PMC free article] [Abstract] [Google Scholar]
- Rodriguez de Fonseca F, et al. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. [Abstract] [Google Scholar]
- Roser P, et al. Potential antipsychotic properties of central cannabinoid (CB1) receptor antagonists. World J Biol Psychiatry. 2010;11:208–219. [Abstract] [Google Scholar]
- Rubino T, et al. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res. 2009;15:291–302. [Abstract] [Google Scholar]
- Rueda D, et al. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. [Abstract] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. [Abstract] [Google Scholar]
- Seifert J, et al. No association of CNR1 gene variations with susceptibility to schizophrenia. Neurosci Lett. 2007;426:29–33. [Abstract] [Google Scholar]
- Steel RW, et al. Learning impairment by Delta(9)-tetrahydrocannabinol in adolescence is attributable to deficits in chunking. Behav Pharmacol. 2011;22:837–846. [Abstract] [Google Scholar]
- Sugiura T, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. [Abstract] [Google Scholar]
- Szeszko PR, et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–236. [Abstract] [Google Scholar]
- Terranova JP, et al. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) 1996;126:165–172. [Abstract] [Google Scholar]
- Tsai SJ, et al. Association study of a cannabinoid receptor gene (CNR1) polymorphism and schizophrenia. Psychiatr Genet. 2000;10:149–151. [Abstract] [Google Scholar]
- Tunbridge EM, et al. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. [Abstract] [Google Scholar]
- Ujike H, et al. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry. 2002;7:515–518. [Abstract] [Google Scholar]
- van Os J, et al. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. [Abstract] [Google Scholar]
- Varma N, et al. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. [Europe PMC free article] [Abstract] [Google Scholar]
- Varvel SA, et al. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. [Abstract] [Google Scholar]
- Varvel SA, et al. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. [Abstract] [Google Scholar]
- Williams EJ, et al. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. [Abstract] [Google Scholar]
- Wilson W, et al. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. [Abstract] [Google Scholar]
- Wong DF, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52:1505–1513. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu CS, et al. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci. 2010;32:693–706. [Europe PMC free article] [Abstract] [Google Scholar]
- Zammit S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. [Europe PMC free article] [Abstract] [Google Scholar]
- Zammit S, et al. Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use. Br J Psychiatry. 2007;191:402–407. [Abstract] [Google Scholar]
- Zanettini C, et al. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. [Europe PMC free article] [Abstract] [Google Scholar]
- Zavitsanou K, et al. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:355–360. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.nbd.2012.11.020
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3594631?pdf=render
Citations & impact
Impact metrics
Article citations
Cannabidiol versus placebo as adjunctive treatment in early psychosis: study protocol for randomized controlled trial.
Trials, 24(1):775, 30 Nov 2023
Cited by: 1 article | PMID: 38037108 | PMCID: PMC10691114
Microglial cannabinoid receptor type 1 mediates social memory deficits in mice produced by adolescent THC exposure and 16p11.2 duplication.
Nat Commun, 14(1):6559, 25 Oct 2023
Cited by: 2 articles | PMID: 37880248 | PMCID: PMC10600150
Gene, cell type, and drug prioritization analysis suggest genetic basis for the utility of diuretics in treating Alzheimer disease.
HGG Adv, 4(3):100203, 05 May 2023
Cited by: 2 articles | PMID: 37250495 | PMCID: PMC10209737
The Effects of Peripubertal THC Exposure in Neurodevelopmental Rat Models of Psychopathology.
Int J Mol Sci, 24(4):3907, 15 Feb 2023
Cited by: 4 articles | PMID: 36835313 | PMCID: PMC9962163
Interaction of sex and cannabis in adult in vivo brain imaging studies: A systematic review.
Brain Neurosci Adv, 6:23982128211073431, 19 Jan 2022
Cited by: 3 articles | PMID: 35097219 | PMCID: PMC8793398
Review Free full text in Europe PMC
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Endocannabinoid System: A Multi-Facet Therapeutic Target.
Curr Clin Pharmacol, 11(2):110-117, 01 Jan 2016
Cited by: 56 articles | PMID: 27086601
Review
A systematic review of phytocannabinoid exposure on the endocannabinoid system: Implications for psychosis.
Eur Neuropsychopharmacol, 29(3):330-348, 08 Jan 2019
Cited by: 17 articles | PMID: 30635160
Review
Proteomics and Lipidomics in the Elucidation of Endocannabinoid Signaling in Healthy and Schizophrenia Brains.
Proteomics, 18(18):e1700270, 20 Aug 2018
Cited by: 4 articles | PMID: 30070429
Review
The endocannabinoid system in ageing: a new target for drug development.
Curr Drug Targets, 7(11):1539-1552, 01 Nov 2006
Cited by: 25 articles | PMID: 17100593
Review
Funding
Funders who supported this work.
NIMH NIH HHS (6)
Grant ID: R01 MH091230
Grant ID: MH094268
Grant ID: MH083728
Grant ID: P50 MH094268
Grant ID: MH091230
Grant ID: R01 MH083728





