Abstract
Background
Numerous meta-analyses have investigated the effect of exercise in different populations and for single cardiovascular risk factors, but none have specifically focused on the metabolic syndrome (MetS) patients and the concomitant effect of exercise on all associated cardiovascular risk factors.Objective
The aim of this article was to perform a systematic review with a meta-analysis of randomized and clinical controlled trials (RCTs, CTs) investigating the effect of exercise on cardiovascular risk factors in patients with the MetS.Methods
RCTs and CTs ≥4 weeks investigating the effect of exercise in healthy adults with the MetS and published in a peer-reviewed journal up to November 2011 were included. Primary outcome measures were changes in waist circumference (WC), systolic and diastolic blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides and fasting plasma glucose. Peak oxygen uptake ([Formula: see text]) was a secondary outcome. Random and fixed-effect models were used for analyses and data are reported as means and 95% confidence intervals (CIs).Results
Seven trials were included, involving nine study groups and 206 participants (128 in exercise group and 78 in control group). Significant mean reductions in WC -3.4 (95% CI -4.9, -1.8) cm, blood pressure -7.1 (95% CI -9.03, -5.2)/-5.2 (95% CI -6.2, -4.1) mmHg and a significant mean increase in HDL-C +0.06 (95% CI +0.03, +0.09) mmol/L were observed after dynamic endurance training. Mean plasma glucose levels -0.31 (95% CI -0.64, 0.01; p = 0.06) mmol/L and triglycerides -0.05 (95% CI -0.20, 0.09; p = 0.47) mmol/L remained statistically unaltered. In addition, a significant mean improvement in [Formula: see text] of +5.9 (95% CI +3.03, +8.7) mL/kg/min or 19.3% was found.Conclusions
Our results suggest that dynamic endurance training has a favourable effect on most of the cardiovascular risk factors associated with the MetS. However, in the search for training programmes that optimally improve total cardiovascular risk, further research is warranted, including studies on the effects of resistance training and combined resistance and endurance training.Free full text
The Effect of Exercise on the Cardiovascular Risk Factors Constituting the Metabolic Syndrome
Abstract
Background
Numerous meta-analyses have investigated the effect of exercise in different populations and for single cardiovascular risk factors, but none have specifically focused on the metabolic syndrome (MetS) patients and the concomitant effect of exercise on all associated cardiovascular risk factors.
Objective
The aim of this article was to perform a systematic review with a meta-analysis of randomized and clinical controlled trials (RCTs, CTs) investigating the effect of exercise on cardiovascular risk factors in patients with the MetS.
Methods
RCTs and CTs ≥4 weeks investigating the effect of exercise in healthy adults with the MetS and published in a peer-reviewed journal up to November 2011 were included. Primary outcome measures were changes in waist circumference (WC), systolic and diastolic blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides and fasting plasma glucose. Peak oxygen uptake (
Results
Seven trials were included, involving nine study groups and 206 participants (128 in exercise group and 78 in control group). Significant mean reductions in WC −3.4 (95% CI −4.9, −1.8) cm, blood pressure −7.1 (95% CI −9.03, −5.2)/−5.2 (95% CI −6.2, −4.1) mmHg and a significant mean increase in HDL-C +0.06 (95% CI +0.03, +0.09) mmol/L were observed after dynamic endurance training. Mean plasma glucose levels −0.31 (95% CI −0.64, 0.01; p = 0.06) mmol/L and triglycerides −0.05 (95% CI −0.20, 0.09; p = 0.47) mmol/L remained statistically unaltered. In addition, a significant mean improvement in 
Conclusions
Our results suggest that dynamic endurance training has a favourable effect on most of the cardiovascular risk factors associated with the MetS. However, in the search for training programmes that optimally improve total cardiovascular risk, further research is warranted, including studies on the effects of resistance training and combined resistance and endurance training.
Introduction
The ‘metabolic syndrome’ (MetS) or ‘syndrome X’ is a constellation of cardiovascular risk factors including abdominal obesity, impaired glucose control, hypertension and dyslipidaemia [1]. The MetS components separately increase the risk for cardiovascular disease (CVD) and all-cause mortality, but the full syndrome is associated with an increase in risk that is greater than the risk of each separate risk factor [2]. Therapeutic lifestyle changes, including exercise, are recommended as firstline strategy in the treatment of cardiovascular risk factors [3]. Previous meta-analyses demonstrated that dynamic endurance exercise decreases blood pressure (BP) in hypertensive individuals [4, 5], causes a significant reduction in blood lipids and lipoproteins in patients with hyperlipidaemia [6, 7], reduces glycated haemoglobin (HbA1c; percentage) in patients with type 2 diabetes mellitus [7] and is associated with improved body composition in obese individuals [7]. However, to the best of the authors’ knowledge, meta-analyses have not been performed to determine the concomitant effect of exercise on all risk factors of the MetS in patients with the MetS.
Therefore, the aim of this study was to perform a systematic review with a meta-analysis of RCTs or CTs investigating the effect of exercise on cardiovascular risk factors in patients with the MetS.
Research Design and Methods
Literature Search
We conducted a systematic literature search with the MEDLINE computerized database from its inception to November 2011 using the following medical subject headings (MeSH terms): ‘Metabolic Syndrome X’ AND (‘Exercise’ OR ‘Motor Activities’). A second literature search was done in MEDLINE using the following title terms: ‘Metabolic Syndrome’ OR ‘Syndrome X’ AND ‘Exercise’. In addition, the reference lists from published original and review articles were searched manually to identify other possible eligible studies.
Inclusion Criteria
We included studies in the meta-analysis if the following requirements were met: (i) (RCTs) or CTs investigating the effect of exercise (dynamic endurance exercise—dynamic resistance exercise—or a combination); (ii) the duration of intervention was at least 4 weeks; (iii) intervention in adults (≥18 years) with the MetS (WHO 1998 [8], National Cholesterol Education Program Adult Treatment Panel III [NCEP/ATP III] 2001 [9] or International Diabetes Federation [IDF] 2005 [10]) but with no other CVD; (iv) full publication in a peer-reviewed journal up to November 2011.
Measured Outcomes
The primary outcomes were changes in cardiovascular risk factors associated with the MetS: i.e. waist circumference (WC), fasting plasma glucose, systolic and diastolic blood pressure (SBP, DBP), high-density lipoprotein cholesterol (HDL-C) and triglycerides. Secondary outcomes included peak oxygen uptake (
Data Extraction
Two unblinded reviewers (N.P. and S.R.T.E.) independently conducted data extraction. A specifically developed data extraction sheet was used to extract data on study source, study design, characteristics of the participants, the exercise characteristics and the different outcomes in each study. Disagreements were solved by consensus or by a third reviewer (V.C.).
Study Quality
Study quality was assessed using an adapted Physiotherapy Evidence Database (PEDro) scale [11], which is an 11-item questionnaire designed to collect data on eligibility criteria, random allocation, concealed allocation, similarity of baseline values, blinding of therapists and/or assessors, key outcomes, intent-to-treat analysis, between group differences, and point and variability measures. We regarded the quality criteria ‘blinding of participants’ and ‘blinding of therapists’ as not applicable in exercise intervention studies, and omitted both criteria. All questions were binary (yes [1] or no [0]). The minimum score was 0 and the maximum was 9, with a higher number reflecting a better study quality. The PEDro-scale has been reported to be valid and reliable [12, 13].
Statistical Analysis
Statistical analyses were performed using SAS® version 9.3 (SAS Institute, Cary, NC, USA) and Review Manager Software (RevMan 5.1; Cochrane Collaboration, Oxford, UK). Descriptive data are reported as mean ± standard deviation (SD) or median and range. The mean baseline values were calculated by combining mean values from the intervention and control groups, weighted by the total number of participants in each study group. Since we worked with continuous data, we took the approach of assuming that randomization would adjust for baseline differences and used endpoint data only in the meta-analytical statistics. Each effect was weighted by the inverse of its variance. The results were combined using fixed and random effect models and presented with 95% confidence intervals (CI). Heterogeneity between trial results was tested with a standard Chi-squared (χ²) test. The I² parameter was used to quantify any inconsistency (I² = [(Q − df)] × 100%, where Q is the χ² statistic and df are the degrees of freedom). A value for I² > 50% has been considered to be substantial heterogeneity. To examine the influence of each study on the overall results, analyses were also performed with each study deleted from the model. In addition, we performed a simple meta-regression analysis to investigate whether changes in the primary outcomes were related to changes in WC. Two-sided tests for overall effects were considered significant at p ≤ 0.05. Finally, funnel plots were used to assess the potential of small publication bias.
Results
Literature Search
From 822 potentially relevant studies retrieved from our search, 9 trials met our inclusion criteria [14–22]. A flow diagram of literature search and selection is presented in Fig. 1. Two studies were a duplicate of another study, i.e. using the same population and intervention, and so we included the most complete publication [17–19, 22]. Hence, seven trials were included in the final analysis. Some of the trials applied different training regimens [17, 18] so that a total of nine dynamic endurance training interventions, one dynamic resistance training group and two combined dynamic endurance/resistance training groups were available for qualitative analysis. We used the meta-analytic techniques only on the nine dynamic endurance training groups.
Characteristics of the Participants and Study Designs
Characteristics of the 12 included intervention groups are summarized in Table 1. All studies were published between 2003 and 2010. Five of them were RCTs [15, 17, 18, 20, 21] and two of them were CTs [14, 16]. Diagnosis of the MetS was based on WHO [8] criteria [14, 18], ATP III [9] criteria [16] or IDF [10] criteria [17, 20, 21]. One trial [15] used a mixture of the three definitions i.e. stage 1 or 2 hypertension (SBP 140–159 or ≥160 mmHg; DBP 90–99 or ≥100 mmHg); plasma insulin concentration greater than 80 μU/mL (>556 pmol/L) 2 h after the ingestion of 75 g of glucose; dyslipidaemia, defined as high triglyceride (≥150 mg/dL; ≥1.7 mmol/L) and/or low HDL-C levels (men ≤40 mg/dL; ≤1.04 mmol/L, women ≤50 mg/dL; ≤1.3 mmol/L).
Table 1
Descriptive characteristics of the study groups at baseline and characteristics of the intervention
| Study (year) | Study design | MetS criteria | Medication | Group | Pre/post (n) | Sex (M/F) | Mean age (years) | Duration (weeks) | Intervention | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Dynamic endurance training | ||||||||||
| Dumortier et al. [14] (2003) | CT | WHO 1998 | No | Ex | 28/28 | 7/21 | 52 | 8 | F: 3×/week | MetS: WC, plasma glucose, HDL-C, TG |
| Con | 11/11 | 4/7 | 53 | I: maximal lipid oxidation (defined by exercise calorimetry) | Other:  , WHR, BW, BMI, FM, FFM (PBF), plasma insulin, TC, LDL-C , WHR, BW, BMI, FM, FFM (PBF), plasma insulin, TC, LDL-C | |||||
| T: 40 min | ||||||||||
| T: aerobic continuous training (cycling) | ||||||||||
| Watkins et al. [15] (2003) | RCT | Othera | No | Ex | 21/14 | Both | NA | 26 | F: 3–4×/week | MetS: fasting glucose, SBP, DBP, HDL-C, TG |
| Con | 11/11 | Both | NA | I: 70–85% HRres | Other:  , BW, BMI, PBF, fasting insulin, TC, LDL-C , BW, BMI, PBF, fasting insulin, TC, LDL-C | |||||
| T: 55 min (10 min warm-up, 35 min at training intensity, 10 min cool-down) | ||||||||||
| T: aerobic continuous training (cycling, walking, jogging) | ||||||||||
| Gomes et al. [16] (2008) | CT | ATP III 2001 | Yes, except lipid medication | Ex | 18/18 | NA | 46 | 12 | F: 3×/week | MetS: WC, glucose, SBP, DBP, HDL-C, TG |
| Con | 10/10 | NA | 46 | I: HR on anaerobic threshold, 10% below RCP | Other: BMI, TC, LDL-C, HR | |||||
| T: 45 min | ||||||||||
| T: aerobic continuous training (cycling) | ||||||||||
| Irving et al. [17] (2008) | RCT | IDF 2005 | No | Ex 1 | 12/9 | 0/9 | 51 | 16 | F: 5×/week | MetS: WC, fasting glucose, SBP, DBP, HDL-C, TG |
| Ex 2 | 15/11 | 0/11 | 51 | 16 | I: 2 sessions below lactate threshold (RPE 10–12) and 3 sessions between lactate threshold and  (RPE 15–17) (RPE 15–17) | Other:  , BW, BMI, FM, FFM (PBF) , BW, BMI, FM, FFM (PBF) | ||||
| Con | 9/9 | 0/9 | 51 | T: time depending on energy expenditure | ||||||
| T: high-intensity aerobic continuous training (walking, running) | ||||||||||
| F: 5×/week | MetS: WC, fasting glucose, SBP, DBP, HDL-C, TG | |||||||||
| I: below lactate threshold (RPE 10–12) | Other:  , BW, BMI, FM, FFM (PBF) , BW, BMI, FM, FFM (PBF) | |||||||||
| T: time depending on energy expenditure | ||||||||||
| T: low-intensity aerobic continuous training (walking, running) | ||||||||||
| Tjønna et al. [18] (2008) | RCT | WHO 1998 | Yes | Ex 1 | 12/11 | 4/7 | 55 | 16 | F: 3×/week | MetS: WC, WHR, fasting glucose, insulin, SBP, DBP, HDL-C, TG |
| Ex 2 | 10/8 | 4/4 | 52 | 16 | I: 10 min warm-up 70% HRpeak, 4 × 4 min 90% HRpeak and 3 × 3 min 70% HRpeak, 5 min min cool-down | Other  , BW, BMI , BW, BMI | ||||
| Con | 10/9 | 5/4 | 50 | T: 40 min | ||||||
| T: aerobic interval training (walking, running uphill) | ||||||||||
| F: 3×/week | MetS: WC, fasting glucose, SBP, DBP, HDL-C, TG | |||||||||
| I: 70% HRpeak | Other:  , WHR, BW, BMI, insulin , WHR, BW, BMI, insulin | |||||||||
| T: 47 min | ||||||||||
| T: aerobic continuous training (walking, running uphill) | ||||||||||
| Balducci et al. [20] (2010) | RCT | IDF 2005 | Yes | Ex 1 | 20/18 | 12/8 | 64 | 52 | F: 2×/week | MetS: WC, SBP, DBP, HDL-C, TG |
| Con | 20/20 | 11/9 | 61 | I: 70-80% 
| ||||||
| T: 60 min | Other:  , BW, BMI, PBF, TC, LDL-C, HbA1c, HOMA-index , BW, BMI, PBF, TC, LDL-C, HbA1c, HOMA-index | |||||||||
| T: aerobic continuous training (walking, jogging, cycling) | ||||||||||
| Stensvold et al. [21] (2010) | RCT | IDF 2005 | Yes | Ex 1 | 11/11 | Both | 50 | 12 | F: 3×/week | MetS: WC, glucose, SBP, DBP, HDL-C, TG |
| Con | 11/11 | Both | 47 | I: 10 min warm-up 70% HRpeak, 4 × 4 min 90–95% HRpeak and 4 × 3 min 70% HRpeak, 5 min cool-down | Other:  , BW, BMI, FM, FFM (PBF), TC, HbA1c , BW, BMI, FM, FFM (PBF), TC, HbA1c
| |||||
| T: 43 min | ||||||||||
| T: aerobic interval training (walking, running) | ||||||||||
| Dynamic resistance training | ||||||||||
| Stensvold et al. [21] (2010) | RCT | IDF 2005 | Yes | Ex 2 | 11/10 | Both | 51 | 12 | F: 3×/week (2 × programme 1, 1 × programme 2) | MetS: WC, glucose, SBP, DBP, HDL-C, TG |
| Con | 11/11 | Both | 47 | I: warm-up 2 sets, 15–20 repetitions, 40–50% 1RM, week 1: 60% 1RM; from week 2 on: 3 sets, 8–12 repetitions, 80% 1RM | Other:  , BW, BMI, FM, FFM (PBF), TC, HbA1c , BW, BMI, FM, FFM (PBF), TC, HbA1c
| |||||
| T: programme 1: 40 min; programme 2: 50 min | ||||||||||
| T: dynamic resistance training, programme 1: 3 exercises; programme 2: 5 exercises | ||||||||||
| Combined intervention: dynamic endurance + dynamic resistance training | ||||||||||
| Balducci et al. [20] (2010) | RCT | IDF 2005 | Yes | Ex 2 | 22/20 | 14/8 | 61 | 52 | F: 2×/week | MetS: WC, SBP, DBP, HDL-C, TG |
| Con | 20/20 | 11/9 | 61 | I: aerobic endurance training 70–80%  dynamic resistance training 80% 1RM dynamic resistance training 80% 1RM | Other:  , BW, BMI, PBF, TC, LDL-C, HbA1C, HOMA-index , BW, BMI, PBF, TC, LDL-C, HbA1C, HOMA-index | |||||
| T: aerobic endurance training 40 min dynamic resistance training 20 min | ||||||||||
| T: aerobic continuous training (walking, jogging, cycling) dynamic resistance training (4 exercises) | ||||||||||
| Stensvold et al. [21] (2010) | RCT | IDF 2005 | Yes | Ex 3 | 10/9 | Both | 53 | 12 | F: 3×/week (2 × aerobic endurance training, 1 × dynamic resistance training) | MetS: WC, glucose, SBP, DBP, HDL-C, TG |
| Con | 11/11 | Both | 47 | I: aerobic endurance training 10 min warm-up 70% HRpeak, 4 × 4 min 90–95% HRpeak and 4 × 3 min 70% HRpeak, 5 min cool-down dynamic resistance training warm-up 2 sets, 15–20 repetitions, 40–50% 1RM, week 1: 60% 1RM; from week 2 on: 3 sets, 8–12 repetitions, 80% 1RM | Other:  , BW, BMI, FM, FFM (PBF), TC, HbA1c , BW, BMI, FM, FFM (PBF), TC, HbA1c
| |||||
| T: aerobic endurance training 43 min dynamic resistance training 40 min | ||||||||||
| T: aerobic interval training (walking, running) dynamic resistance training (3 exercises) | ||||||||||
1RM one repetition maximum, ATP III Adult Treatment Panel III, BMI body mass index, BW body weight, Con control group, CT controlled trial, DBP diastolic blood pressure, Ex exercise group (numbered as 1, 2 and 3 when more intervention groups within one study), F female, F: I: T: T: frequency: intensity: time: type:, FFM fat free mass, FM fat mass, HbA
1c glycated haemoglobin, HDL-C high-density lipoprotein cholesterol; HR heart rate, HR
peak peak HR; HR
res HR reserve; IDF International Diabetes Federation, LDL-C low-density lipoprotein cholesterol, M males, MetS metabolic syndrome (cardiovascular outcomes constituting the MetS), n number of participants; NA not available, Other additional outcomes of the included trials, PBF percent body fat, Post after the intervention, Pre before the intervention, RCP respiratory compensation point, RCT randomized controlled trial, RPE rate of perceived exertion, SBP systolic blood pressure, TC total cholesterol, TG triglycerides, 
aInclusion criteria of Watkins et al.: stage 1 or 2 hypertension (SBP 140–159 or ≥160 mmHg; DBP 90–99 or ≥100 mmHg); plasma insulin concentration greater than 80 μU/mL (>556 pmol/L) 2 h after the ingestion of 75 g of glucose; dyslipidaemia, defined as high TG (≥150 mg/dL; ≥1.7 mmol/L) and/or low HDL-C levels (men ≤ 40 mg/dL; ≤1.04 mmol/L, women ≤ 50 mg/dL; ≤1.3 mmol/L)
272 participants were randomized or allocated to an exercise group (dynamic endurance training, n = 147; dynamic resistance training, n = 11; combination, n = 32) or a control group (n = 82). Average drop out was 10% (range 0–33%), resulting in a total of 245 participants that could be included in the final analysis: 128 of them completed a dynamic endurance training intervention, 10 completed a dynamic resistance training intervention, 30 completed a combined intervention and 78 were control subjects.
Median age was 52 years (range 46–64). Six study trials included both men and women, and one trial included only women [17] (±38% and ±41% males in the exercise and control group, respectively) [15]. Four trials defined ‘not being sedentary’ as an exclusion criterion [14–17], while this was not specifically reported in the other three trials [18, 20, 21]. Two trials did not report on diabetes status, four study trials excluded individuals with diabetes [14–17] whereas Balducci et al. [20] included only MetS patients with diabetes. The use of medication was reported in three trials [18, 20, 21]; antihypertensive agents, lipid-lowering agents and oral hypoglycaemic agents or insulin use were common pharmacotherapy treatments. Three other trials defined the use of medications as an exclusion criterion [14, 15, 17]. Gomes et al. [16] defined drugs known to affect lipoprotein metabolism as an exclusion criterion.
The 12 interventions lasted between 8 and 52 weeks (median 16). The frequency of training varied between two and five training sessions weekly (median 3), with an average of 47.5 min per session (range 40–60). All trials used walking, jogging or cycling as the modes of exercise. Two dynamic endurance training groups performed interval training [18, 21], while the other seven endurance training groups reported using a continuous training programme. Intensities ranged from moderate (e.g. ‘maximal lipid oxidation’) to high (e.g. intervals at 90–95% of heart rate peak [HRpeak]). The exercise programmes were supervised in all studies except for Dumortier et al. [14] where only the first training sessions were supervised and the patients continued the training at home afterwards.
In Table 2, the results of the adapted PEDro-scale are represented. The median PEDro score was 6, with a range from 5 [15, 17] to 8 [20].
Table 2
PEDro scores for the included intervention trials
| Study (year) | Eligibility criteria | Randomly allocated | Allocated concealed | Similar baseline values | Blinding of assessors | Key outcomes in 85% of included subjects | Intent to treat | Between-group statistical comparison | Point + variability measure | Total PEDro score |
|---|---|---|---|---|---|---|---|---|---|---|
| Dumortier et al. [14] (2003) | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Watkins et al. [15] (2003) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Gomes et al. [16] (2008) | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Irving et al. [17] (2008) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Tjønna et al. [18] (2008) | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Balducci et al. [20] (2010) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Stensvold et al. [21] (2010) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
PEDro Physiotherapy Evidence Database
Primary Outcomes: Effect of Exercise on Cardiovascular Risk Factors Associated with the Metabolic Syndrome
Dynamic endurance training resulted in a mean reduction in WC of 3.36 cm (Fig. 2; baseline 104.62; 95% CI −4.87, −1.84; p < 0.0001; I² = 35%), a mean BP decrease of 7.11 (Fig. 3; baseline 137.97; 95% CI −9.03, −5.18; p < 0.0001; I² = 0%) mmHg/5.15 mmHg (Fig. 4; baseline 87.60; 95% CI −6.16, −4.13; p < 0.0001; I² = 28%) and a mean increase in HDL-C of 0.06 (Fig. 5; baseline 1.04; 95% CI +0.03, +0.09; p < 0.0001; I² = 38%) mmol/L. By contrast, levels of triglycerides (Fig. 6; −0.05; baseline 2.05; 95% CI −0.20, +0.09; p = 0.47; I² = 0%) mmol/L and plasma glucose (Fig. 7; −0.31; baseline 5.98; 95% CI −0.64, +0.01; p = 0.06; I² = 0%) mmol/L remained statistically unaltered.
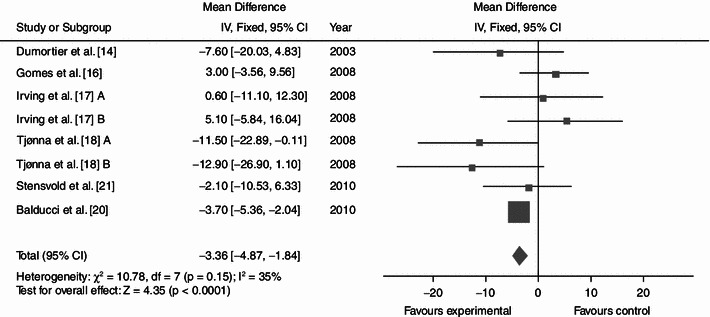
Average net changes and corresponding 95% confidence intervals for waist circumference. Tjønna et al. [18] A and B represents moderate continuous training programme and aerobic interval training programme, respectively; and Irving et al. [17] A and B represents high-intensity training and low-intensity training, respectively. CI confidence intervals, df degrees of freedom, IV intervention, χ 2 Chi-squared
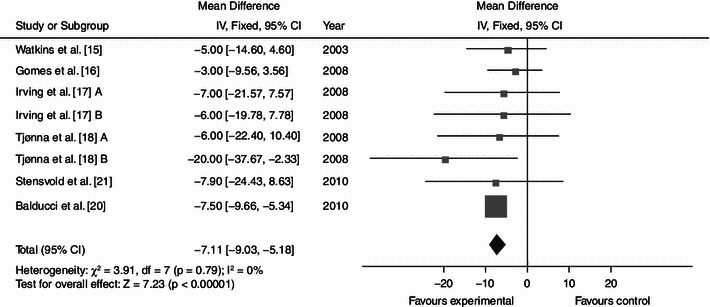
Average net changes and corresponding 95% confidence intervals for systolic blood pressure. Tjønna et al. [18] A and B represents moderate continuous training programme and aerobic interval training programme, respectively; and Irving et al. [17] A and B represents high-intensity training and low-intensity training, respectively. CI confidence intervals, df degrees of freedom, IV intervention, χ 2 Chi-squared
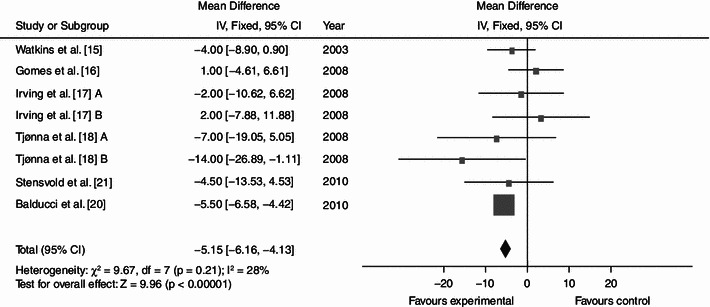
Average net changes and corresponding 95% confidence intervals for diastolic blood pressure. Tjønna et al. [18] A and B represents moderate continuous training programme and aerobic interval training programme, respectively; and Irving et al. [17] A and B represents high-intensity training and low-intensity training, respectively. CI confidence intervals, df degrees of freedom, IV intervention, χ 2 Chi-squared
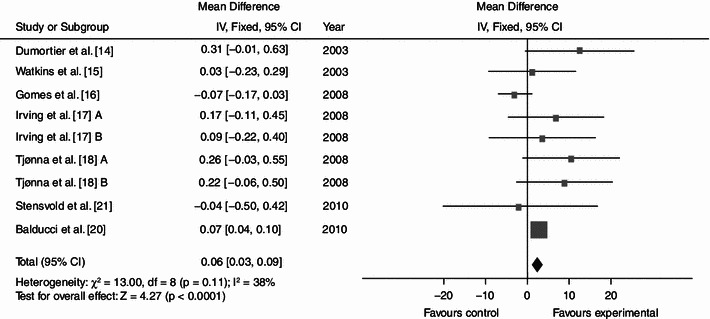
Average net changes and corresponding 95% confidence intervals for HDL-C. Tjønna et al. [18] A and B represents moderate continuous training programme and aerobic interval training programme, respectively; and Irving et al. [17] A and B represents high-intensity training and low-intensity training, respectively. CI confidence intervals, df degrees of freedom, IV intervention, χ 2 Chi-squared
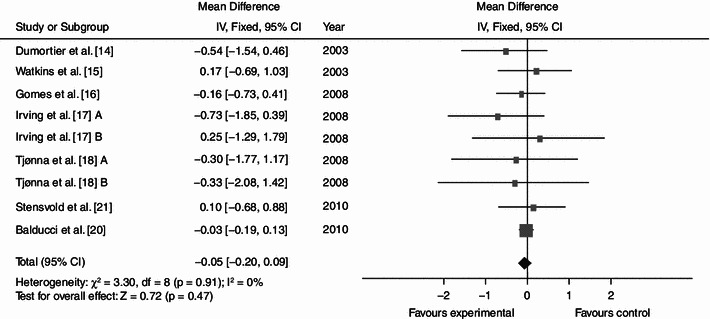
Average net changes and corresponding 95% confidence intervals for triglycerides. Tjønna et al. [18] A and B represents moderate continuous training programme and aerobic interval training programme, respectively; and Irving et al. [17] A and B represents high-intensity training and low-intensity training, respectively. CI confidence intervals, df degrees of freedom, IV intervention, χ 2 Chi-squared
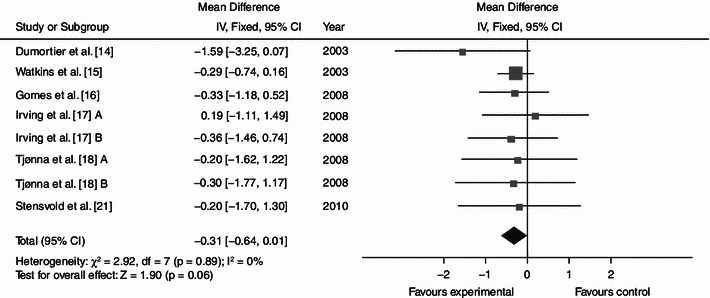
Average net changes and corresponding 95% confidence intervals for plasma glucose. Tjønna et al. [18] A and B represents moderate continuous training programme and aerobic interval training programme, respectively; and Irving et al. [17] A and B represents high-intensity training and low-intensity training, respectively. CI confidence intervals, df degrees of freedom, IV intervention, χ 2 Chi-squared
To assess the effect of individual dynamic endurance studies on the summary estimate we performed a sensitivity analysis, in which the pooled estimates for the different outcomes were recalculated omitting one or two studies at a time. When we excluded the two aerobic interval training (AIT) studies, results were similar. However, when we omitted the only trial in which all patients had type 2 diabetes [17], overall changes in DBP, HDL-C and WC were no longer statistically significant (p = 0.07; p = 0.44; p = 0.40, respectively).
The results of the meta-regression showed that decreases in DBP were significantly associated with reductions in WC (r = 0.91; p = 0.001). A non-significant trend was found for the association of WC with SBP (r = 0.67; p = 0.10) and for WC with HDL-C (r = −0.68; p = 0.06). triglyceride and plasma glucose were not related to changes in WC (p > 0.20 for all).
Only one study investigated the effect of dynamic resistance training on components of the MetS [21]. They reported a significant reduction of WC (from mean ± SD 111.5 ± 10.8 to 110.1 ± 11.0 cm; p < 0.05) whereas HDL-C, triglycerides, plasma glucose, SBP and DBP remained statistically unaltered (p > 0.05 for all).
Two trials, involving 33 participants investigated the effect of combined dynamic endurance and dynamic resistance training in patients with the MetS [20, 21]. Both trials observed a significant reduction of WC. In addition, Balducci et al. [20] reported positive findings for HbA1c (from mean ± SD 7.29 ± 1.35 to 6.34 ± 0.96%; p < 0.0001) and HDL-C (from mean ± SD 44.1 ± 2.0 to 47.6 ± 1.85 mg/dL; p < 0.001) whereas this remained statistically unaltered in the study of Stensvold et al. [21]. Finally, none of the combined exercise studies found a statistically significant effect on triglycerides, SBP or DBP.
Secondary Outcomes: Effect of Dynamic Endurance Exercise on Other Cardiovascular Risk Factors
As shown in Table 3, dynamic endurance training also positively affected other blood lipids (LDL-C, total cholesterol), some anthropometric characteristics (body weight, BMI) and resulted in a significant increase of 
Table 3
Effect of dynamic endurance training on secondary outcomes
| Outcome | Mean baseline value | Mean effect | 95% CI | Significance | Interventions (n) | Heterogeneity I² (%) | Model used |
|---|---|---|---|---|---|---|---|
| Blood lipids | |||||||
| LDL-C (mmol/L) |  3.34 3.34 | −0.38 | −0.75, −0.01 | p = 0.05 | 4 | 60 | RE |
| TC (mmol/L) |  5.55 5.55 | −0.53 | −0.64, −0.42 | p < 0.001 | 5 | 23 | FE |
| Anthropometric characteristics | |||||||
| Weight (kg) | 92.06 | −3.51 | −5.40, −1.61 | p < 0.001 | 8 |  0 0 | FE |
| BMI (kg/m²) | 32.18 | −1.65 | −2.81, −0.48 | p < 0.01 | 9 |  0 0 | FE |
| FM (kg) | 38.73 | −1.92 | −6.41, +2.57 | NS | 4 |  0 0 | FE |
| Glucose/insulin parameters | |||||||
| Plasma insulin (mmol/L) | 18.68 | −0.98 | −4.51 to +2.56 | NS | 4 |  0 0 | FE |
| Other secondary outcomes | |||||||
 (mL/kg/min) (mL/kg/min) | 26.61 | +5.86 | +3.03, +8.69 | p < 0.001 | 8 | 58 | RE |
BMI body mass index, CI confidence interval, FE fixed effect model, FM fat mass, LDL-C low-density lipoprotein cholesterol, NS non-significant, RE random effect model, TC total cholesterol, 
Publication Bias
Funnel plots did not show any significant publication bias for all of the primary and secondary outcomes, meaning that there was no asymmetric relationship between treatment effects and study size (data not shown).
Discussion
The main findings of this meta-analysis suggest that in patients with the MetS, dynamic endurance training is associated with favourable effects on most cardiovascular risk factors related to the MetS, that is WC, HDL-C, SBP and DBP. Our results further demonstrate that in these patients dynamic endurance training also favourably affects other important cardiovascular risk factors including LDL-C, total cholesterol, BMI and 
It is generally accepted that we need better and more affordable prevention and treatment strategies to improve wide-scale cardiovascular health outcome and to prevent the epidemic of MetS from reaching global proportions and straining public health and the economy [18]. Exercise is a key component in the treatment of patients with the MetS and in the prevention of CVD morbidity and mortality. The results of this study are in line with previous meta-analyses and extensive reviews that focused on the effect of exercise on single risk factors in populations with different cardiovascular risk factors [4–7].
First of all, we observed a significant improvement in measures of body composition including reduction in abdominal obesity, assessed by means of WC, body weight and BMI and a non-significant reduction in fat mass. Improvements in body composition characteristics are suggested to be associated with beneficial changes in lipids and lipoproteins through mechanisms related to insulin resistance [23, 24]. Indeed, the results of our meta-regression show a trend (p = 0.06) between changes in WC and HDL-C. In line with the meta-analysis of Kelley et al. [25], we observed significant increases in HDL-C and decreases in LDL-C and total cholesterol, without an effect on triglycerides. Wilson et al. [26] found that an HDL-C level between 41 and 46 mg/dL (1.06–1.19 mmol/L) for men resulted in a relative risk (RR) of all-cause death of 1.27 and HDL-C levels between 23 and 44 mg/dL (0.59–1.14 mmol/L) in an RR of 1.47 for women, compared with groups with values of more than 55 and 70 mg/dL, respectively. In our meta-analysis, HDL-C levels rose from 1.05 mmol/L to 1.13 mmol/L in a sex-mixed population, still showing non-optimal HDL-C plasma levels. However, it is stated in a meta-analysis of Kelley et al. [25] that a decrease of 1% in HDL-C would be associated with an increase in the risk for coronary heart disease of 2–3%. Assuming that the reverse is true, the approximate increase of 8% observed in our meta-analysis should decrease the coronary heart disease risk by 16–24%.
In addition, patients with the MetS do have type 2 diabetes or at least have an increased risk for developing it. Whereas plasma insulin levels remained statistically unaltered, we observed a trend towards lower values of mean plasma glucose following dynamic endurance exercise (p = 0.06). Earlier, Snowling and Hopkins [27] reported in patients with type 2 diabetes significant reductions in fasting glucose, HbA1c and insulin sensitivity after dynamic endurance training, with some evidence of small additional benefits resulting from combining endurance and resistance training. Moreover, it was suggested that the effects were somewhat larger for those with a more severe disease status, in particular for HbA1c. The lack of a significant effect on glucose-insulin dynamics in the current meta-analysis might be partly explained by the fact that the majority of participants were pre-diabetic but did not have diabetes. Indeed, the study of Balducci et al. [20] in patients with type 2 diabetes and the MetS did result in significant decreases in HbA1c after 12 months of exercise training, which confirms our suggestion. In addition, measuring plasma glucose and plasma insulin after an oral glucose tolerance test is often a better marker of changes in glucose tolerance after an exercise or dietary intervention. In the study of Watkins et al. [15], significant decreases in 2 h of plasma insulin were found in the dynamic endurance exercise group but not in the control group after 26 weeks, while there were no changes in fasting glucose and fasting insulin.
Following dynamic endurance training, a mean reduction in BP of 7/5 mmHg was achieved. These mean differences are statistically significant but more importantly, the magnitude of these differences is likely to be clinically relevant. Results from large, prospective intervention studies suggested that small reductions in resting SBP and DBP of 3 mmHg can reduce coronary heart disease risk by 5%, stroke by 8% and all-cause mortality by 4% [28].
Although the observed effect on each of the individual risk factors might seem small, when we combine all the effect sizes, that is a BP reduction to a mean of 131/82 mmHg, a decrease in WC to an average of 101 cm and for plasma glucose and triglycerides to a mean of 5.67 and 2.0 mmol/L, respectively and an increase of HDL-C to 1.1 mmol/L, a large number of patients would probably no longer be classified as having the MetS. Earlier, Katzmarzyk et al. [29] showed that 30.5% of patients (mean age 44.7 years) with the MetS at baseline, were no longer classified as having the MetS after a 20-week supervised dynamic endurance training programme. This reduction was mostly due to an improvement in triglycerides (43%), BP (38%) and WC (28%) whereas beneficial effects on HDL-C and plasma glucose were seen in fewer patients (16 and 9%, respectively). Similarly, Anderssen et al. [30] reported that 23.5% of 34 patients no longer had the MetS after an exercise intervention of 1 year. The only study included in this meta-analysis reporting on the number of patients with the MetS showed similar decreases with a 37.5% (from 8/8 to 5/8) and a 45% decrease (from 11/11 to 6/11) following 16 weeks of continuous and interval training, respectively [18]. Although it is difficult to quantify exactly the overall risk reduction associated with all observed changes, these results are compatible with an overall improvement of cardiovascular risk. Moreover, the results of this meta-analysis study are supported by studies that have examined the cross-sectional relationship between physical activity and the MetS. For example, in 1298 healthy police employees of the Utrecht Police Lifestyle Intervention Fitness and Training (UPLIFT) study, average physical activity intensity, average time spent at physical activity, physical activity volume and physical fitness, were each associated with reduced odds of the MetS [31]. Taken together, the results of these studies, and those from the present meta-analysis, reinforce the notion that physical activity is an important treatment option for the MetS, as stated in Katzmarzyk et al. [29].
Finally, low aerobic capacity has been shown to be a stronger predictor of CVD and mortality compared with other established risk factors [32]. The five studies that reported on the effect of dynamic endurance training on 
With regard to the effect of other training characteristics on cardiovascular risk factors in patients with the MetS, more research is warranted to allow for appropriate exercise prescriptions. That is, only one intervention group investigated the effect of dynamic resistance training on the clustering of cardiovascular risk factors in individuals with the MetS and found that only WC was decreased significantly [21]. In a study of Banz et al. [34], with obese subjects, the effect of dynamic resistance training also resulted in significant changes in WC, but in no other significant beneficial cardiovascular effects. Smutok et al. [35] found significant changes in glucose and insulin responses after an oral glucose tolerance test was administered after a dynamic resistance training programme in individuals with two cardiovascular risk factors, but blood lipids and lipoproteins or BP outcomes didn’t improve significantly. It seems that dynamic resistance training has potential beneficial effects on the cardiovascular risk profile, but that dynamic endurance training is more beneficial [34, 35]. In a large meta-analysis of Cornelissen et al. [36], the effect of resistance training on BP and other cardiovascular risk factors was reviewed. They found positive effects on SBP, DBP and 
The effect of combined exercise on the clustering of cardiovascular risk factors in patients with MetS was discussed in two studies [20, 21]. Balducci et al. [20] concluded that combined endurance and resistance training had larger beneficial effects on upper body strength and body weight than dynamic endurance training only. No significant differences were found for the other outcome measures [20]. Stensvold et al. [21] found slightly larger benefits for the dynamic endurance training on SBP and DBP than that of combined exercise. The training responses were similar for WC, HDL-C, triglycerides and plasma [21]. However, more research is needed to investigate the potential benefits of combined training programmes.
The results of this meta-analysis should be interpreted within the context of its limitations: First, only one study fulfilling the inclusion criteria included a dynamic resistance intervention [21], and only two trials used a combined intervention of dynamic resistance training and endurance training [20, 21]. Further research about the effect of dynamic resistance training only or in combination with dynamic endurance exercise training is warranted in patients with the MetS. Second, a sensitivity analysis demonstrated that omitting the only trial in which all patients had type 2 diabetes [20], the effect of exercise on DBP, HDL-C and WC were no longer significant (p = 0.07; p = 0.44; p = 0.40, respectively). The severity of the MetS risk factors or a pathological state (type 2 diabetes) can probably influence the effects of exercise training, with more severe CV risk factors resulting in potentially larger improvements. Third, due to the small number of study groups, a subgroup analysis for sex or medication intake was not possible. More research is needed on the effect of variables that could potentially affect the outcomes of an exercise programme, especially the effect of sex and medication. Further, the mean age of participants was 52 years with a range between 46 and 64 years; therefore it may not be possible to generalize these results for younger or older individuals with the MetS. Finally, it has been suggested that one exercise dose does not fit for all components of the MetS and that recommendations should vary regarding the different risk factors [37]. Therefore, it is necessary to strike a balance for the most optimal exercise programme to improve the cluster of cardiovascular risk factors in patients with the MetS.
Conclusion
It can be concluded that exercise interventions have beneficial effects on most of the cardiovascular risk factors in patients with the MetS, but further research is needed to expand the knowledge and to strengthen the results found in this meta-analysis.
Acknowledgements
No potential conflicts of interest directly relevant to this content of this article were reported. Véronique A. Cornelissen is supported as a Postdoctoral Fellow by Research Foundation Flanders (F.W.O.). Prof. Luc Vanhees is the holder of the faculty chair ‘Lifestyle and Health’ at the University of Applied Sciences, Utrecht, the Netherlands; Nele Pattyn is a doctoral researcher at the KU Leuven, Department of Rehabilitation Sciences; Saeed R. Toghi Eshghi was a Master’s student at KU Leuven, at the time of the preparation of this article.
Footnotes
N. Pattyn and V. A. Cornelissen are joint first authors.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s40279-012-0003-z
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s40279-012-0003-z.pdf
Citations & impact
Impact metrics
Article citations
The effects of high-intensity interval training versus moderate-intensity continuous training on athletes' aerobic endurance performance parameters.
Eur J Appl Physiol, 124(8):2235-2249, 21 Jun 2024
Cited by: 0 articles | PMID: 38904772
Review
Effects of a co-designed exercise and sport intervention on cardiorespiratory fitness and metabolic syndrome components among individuals living in a refugee camp in Greece: A randomized controlled trial.
J Migr Health, 9:100227, 27 Mar 2024
Cited by: 0 articles | PMID: 38577627 | PMCID: PMC10992696
Development and effectiveness of a mobile-based autonomy support program for the prevention of metabolic syndrome in middle-aged women.
Front Public Health, 12:1334988, 07 Feb 2024
Cited by: 0 articles | PMID: 38384883 | PMCID: PMC10879428
Effect of resistance training volume on body adiposity, metabolic risk, and inflammation in postmenopausal and older females: Systematic review and meta-analysis of randomized controlled trials.
J Sport Health Sci, 13(2):145-159, 01 Oct 2023
Cited by: 2 articles | PMID: 37788790 | PMCID: PMC10980902
Review Free full text in Europe PMC
Approach to the Patient: The Evaluation and Management of Men ≥50 Years With Low Serum Testosterone Concentration.
J Clin Endocrinol Metab, 108(9):e871-e884, 01 Aug 2023
Cited by: 1 article | PMID: 36995891 | PMCID: PMC10438885
Go to all (138) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of exercise training on metabolic syndrome risk factors in post-menopausal women - A systematic review and meta-analysis of randomised controlled trials.
Clin Nutr, 42(3):337-351, 10 Jan 2023
Cited by: 7 articles | PMID: 36736057
Review
Exercise is good for your blood pressure: effects of endurance training and resistance training.
Clin Exp Pharmacol Physiol, 33(9):853-856, 01 Sep 2006
Cited by: 98 articles | PMID: 16922820
The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis.
Cardiovasc Diabetol, 16(1):110, 30 Aug 2017
Cited by: 105 articles | PMID: 28854979 | PMCID: PMC5577843
Review Free full text in Europe PMC
Effect of vegetarian diets on the presentation of metabolic syndrome or its components: A systematic review and meta-analysis.
Clin Nutr, 38(3):1117-1132, 06 Jun 2018
Cited by: 26 articles | PMID: 29907356
Review







