Abstract
Purpose
Chemokines are involved in cancer-related inflammation and malignant progression. In this study, we evaluated expression of CCR8 and its natural cognate ligand CCL1 in patients with urothelial carcinomas of bladder and renal cell carcinomas.Experimental design
We examined CCR8 expression in peripheral blood and tumor tissues from patients with bladder and renal carcinomas. CCR8-positive myeloid cells were isolated from cancer tissues with magnetic beads and tested in vitro for cytokine production and ability to modulate T-cell function.Results
We show that monocytic and granulocytic myeloid cell subsets in peripheral blood of patients with cancer with urothelial and renal carcinomas display increased expression of chemokine receptor CCR8. Upregulated expression of CCR8 is also detected within human cancer tissues and primarily limited to tumor-associated macrophages. When isolated, CD11b(+)CCR8(+) cell subset produces the highest levels of proinflammatory and proangiogenic factors among intratumoral CD11b myeloid cells. Tumor-infiltrating CD11b(+)CCR8(+) cells selectively display activated Stat3 and are capable of inducing FoxP3 expression in autologous T lymphocytes. Primary human tumors produce substantial amounts of the natural CCR8 ligand CCL1.Conclusions
This study provides the first evidence that CCR8(+) myeloid cell subset is expanded in patients with cancer. Elevated secretion of CCL1 by tumors and increased presence of CCR8(+) myeloid cells in peripheral blood and cancer tissues indicate that CCL1/CCR8 axis is a component of cancer-related inflammation and may contribute to immune evasion. Obtained results also implicate that blockade of CCR8 signals may provide an attractive strategy for therapeutic intervention in human urothelial and renal cancers.Free full text

Expansion of CCR8+ inflammatory myeloid cells in cancer patients with urothelial and renal carcinomas
Abstract
Purpose
Chemokines are involved in cancer-related inflammation and malignant progression. In this study we evaluated expression of CCR8 and its natural cognate ligand CCL1 in patients with urothelial carcinomas of bladder and renal cell carcinomas.
Experimental Design
We examined CCR8 expression in peripheral blood and tumor tissues from patients with bladder and renal carcinomas. CCR8-positive myeloid cells were isolated from cancer tissues with magnetic beads and tested in vitro for cytokine production and ability to modulate T cell function.
Results
We demonstrate that monocytic and granulocytic myeloid cell subsets in peripheral blood of cancer patients with urothelial and renal carcinomas display increased expression of chemokine receptor CCR8. Up-regulated expression of CCR8 is also detected within human cancer tissues and primarily limited to tumor-associated macrophages (TAMs). When isolated, CD11b+CCR8+ cell subset produces the highest levels of pro-inflammatory and pro-angiogenic factors among intratumoral CD11b myeloid cells. Tumor-infiltrating CD11b+CCR8+ cells selectively display activated Stat3 and are capable of inducing FoxP3 expression in autologous T lymphocytes. Primary human tumors produce substantial amounts of the natural CCR8 ligand CCL1.
Conclusions
This study provides the first evidence that CCR8+ myeloid cell subset is expanded in cancer patients. Elevated secretion of CCL1 by tumors, increased presence of CCR8+ myeloid cells in peripheral blood and cancer tissues indicate that CCL1/CCR8 axis is a component of cancer-related inflammation and may contribute to immune evasion. Obtained results also implicate that blockade of CCR8 signals may provide an attractive strategy for therapeutic intervention in human urothelial and renal cancers.
Introduction
Emerging evidence indicates importance of inflammation in tumor initiation and progression. However, information on specific mechanisms or mediators of cancer-related inflammation in human cancers is still limited (1, 2). Recent studies demonstrate that a substantial portion of inflammatory cells in human tumor tissues is represented by CD11b+ myeloid cells that include large populations of tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) (3). TAMs represent an abundant and heterogeneous cell population in the tumor microenvironment and they play a key role in tumor development (4, 5). For example, although M1-oriented TAMs constitute a critical component of the anti-tumor immune response, they are frequently subverted in the tumor microenvironment into alternatively activated M2 type that promotes tumor progression.
Chemokines and their receptors are involved in malignant progression (2, 6). Some chemokines, like CCL1, CCL2, CCL17 and CCL22, have been shown to promote M2 and Th2 polarization in tumors that subvert the immune system by establishing a microenvironment of immune cells and cytokines that suppress specific anti-tumor responses. Hence, it is critical to study the mechanisms by which specific chemokines and their receptors mediate inflammatory cells traffic into tumor tissue and their functions. Despite the fact that chemokines are abundantly expressed in tumors, there is little information concerning chemokine-receptor expression in circulating or tumor-infiltrating leukocytes in human cancer patients.
CCR8 is a chemokine receptor that was initially described as a Th2 cell-restricted receptor (7, 8). CCR8 is believed to mediate a broad range of cellular activities including Th2 and T regulatory cell recruitment in allergic inflammation (9, 10), recruitment of inflammatory macrophages in mice with experimental hepatitis (11), and chemotaxis of endothelial as well as vascular smooth muscle cells (12, 13). These data suggest involvement of CCR8-expressing cells in inflammatory responses. However, whether CCR8+ cells contribute to cancer-related inflammation associated with progression of human cancers remains unknown.
In the current study we demonstrate that monocytic and granulocytic myeloid cells obtained from peripheral blood of patients with urothelial and renal carcinomas display increased expression of CCR8. Up-regulated expression of CCR8 was also detected in tumor-infiltrating leukocytes. Remarkably, CCR8 expression in cancer tissues was enriched in tumor-infiltrating CD11b myeloid cells and primarily to TAMs. We also found that the tumor-infiltrating CD11b+CCR8+ cell subset is responsible for production of majority pro-inflammatory (e.g. IL-6, CCL3, CCL4) and pro-angiogenic (e.g. VEGF) factors among intratumoral CD11b+ myeloid cells. CD11b+CCR8+ cells are capable of inducing FoxP3 expression in T lymphocytes. In addition, we show that primary human tumors secrete substantial amounts of the natural CCR8 ligand CCL1. Taken together, these results demonstrate a dramatic increase of CCR8+CD11b+ myeloid regulatory cells in peripheral blood and tumor tissue. Obtained data suggest that CCL1/CCR8 axis exhibits critical signals of immune evasion in cancer and identifies CCR8 and CCL1 as factors that associate with cancer-related inflammation in human cancer.
Materials and Methods
Human subjects
Fifty two cancer patients (thirty patients with urothelial carcinoma of bladder and twenty two patients with renal cell carcinoma) were enrolled in this study. Detailed information on these cancer patients is shown in Supplementary Table S1. Peripheral blood and tumor tissue were collected following cystectomy or nephrectomy procedures performed at the Department of Urology, University of Florida, Gainesville, FL. Control blood was collected from healthy donors. Clinical specimens were obtained following informed consent, as approved by the Institutional Review Board. All patients selected for the study were not previously treated with chemo- or adjuvant-therapy.
Assays
Reagents, cell isolation from peripheral blood and tumor tissues, preparation of tumor-conditioned medium, Western blot analysis, Ca2+ influx assay, RT-PCR analysis, flow cytometry, Multiplex cytokine assay and ELISA were performed as described in the section Supplemental Methods.
Statistical analysis
Values are expressed as mean ± SD. Unpaired Student t test was used to determine statistical significance between groups (GraphPad Prism; GraphPad Software, Inc). The criterion for significance was set at P < 0.05. The flow cytometry data shown are representative of at least three separate determinations.
Results
CCR8-expressing myeloid cell subset in peripheral blood of cancer patients
Tumors are known to secrete large amounts and a wide range of chemokines that affect function of host’s immune and inflammatory cell subsets, including myeloid cells of bone marrow origin (14). Given enhanced production of chemokines by tumors we hypothesized that in order to respond to those tumor-derived chemokines, some myeloid cell subsets in cancer patients might display up-regulated expression of certain chemokine receptor(s). To examine this possibility, we measured expression of several chemokine receptors including CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CXCR2 and CXCR4 in myeloid cells obtained from peripheral blood of patients that have been diagnosed with bladder cancer or renal cell carcinoma (Supplementary Table S2, A). As exemplified by paired healthy donor and cancer patients in Fig. 1A and Supplementary Fig. S1, among screened receptors CCR8 expression was markedly up-regulated in patients with bladder and kidney cancers. As can be seen, CD33+ myeloid cells in cancer patients constitute of two cell subpopulations (CD33high and CD33low). This reflects the presence of monocytic and granulocytic myeloid-derived suppressor cell (MDSC) populations in peripheral blood of cancer patients (15). Significantly, both monocytic CD33highCD11b+ and granulocytic CD33lowCD11b+ MDSCs in peripheral blood of cancer patients displayed increased expression of CCR8. Together, in twelve cancer patients we consistently observed elevated expression of CCR8 in CD11b+ myeloid cells in comparison to healthy donors (Supplementary Table S2, B).
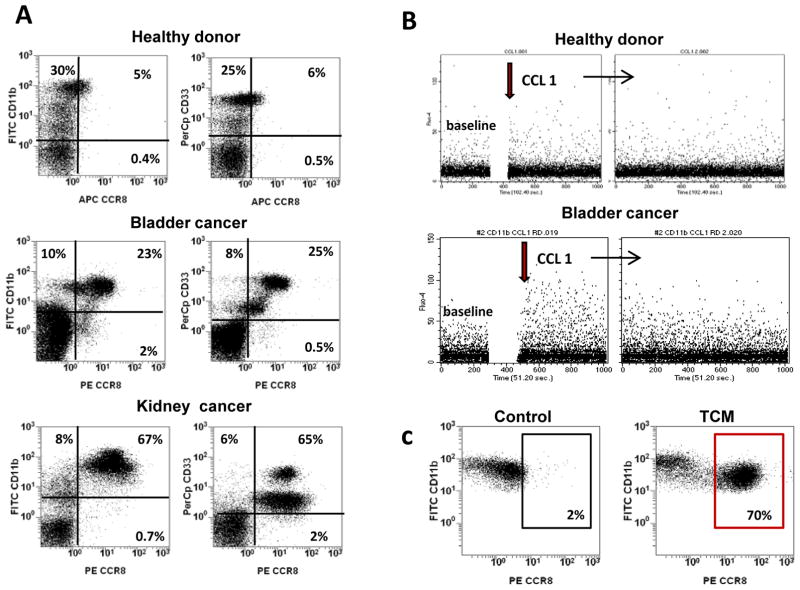
A: Peripheral blood mononuclear cells (PBMC) from cancer patients and healthy donors were separated using Lymphoprep density gradient centrifugation. PBMC were triple stained with anti-CD11b-FITC, CD33-PerCp and CCR8-PE antibodies. Percentage of CCR8 cells was estimated by flow cytometry. B: Calcium mobilization experiments were performed in myeloid cells isolated from PBMC of cancer patients or healthy donors. Freshly isolated CD11b cells were loaded with the fluorescent dye Fluo-4. Calcium mobilization was measured after stimulation with recombinant human CCL1 using flow cytometry as described in Methods. C: CD11b cells were isolated from peripheral blood of healthy donors using magnetic beads and cultured in the presence or absence of bladder tumor-conditioned medium (TCM). After 24 hours cells were collected and expression of CCR8 was assessed using flow cytometry. Results of one representative experiment out of three are shown.
Characterization of CCR8-expressing myeloid cells obtained from peripheral blood
We examined whether CCR8 is functional by treating peripheral blood mononuclear cells (PBMCs) obtained from bladder cancer patients with CCR8 ligand CCL1 and measuring CCL1-elicited intracellular Ca+2 influx using Fluo-4 based assay. Results presented in Fig. 1B demonstrate that in vitro stimulation of PBMCs from a bladder cancer patient (lower panel) but not from a healthy donor (upper panel) with recombinant human CCL1 induced Ca+2 influx in gated CD11b cells. Since CD11b cells also express variable levels of another chemokine receptor CXCR4, we compared the Ca+2 influx induced by CCL1 and the CXCR4 ligand SDF-1. As shown in Fig. S2 both chemokines stimulated the Ca+2 mobilizations, albeit SDF1 elicited a stronger response. Collectively, these results demonstrated that CCR8-expressing cells readily respond to the stimulation with CCL1 to induce Ca+2 influx suggesting that myeloid cells in cancer patients express functional CCR8. In addition to Ca+2 mobilization, we also examined whether CCL1-mediated signaling could stimulate production of reactive oxygen species (ROS) in myeloid cells. Obtained results indicate that treatment of CD11b cells isolated from peripheral blood of cancer patients with CCL1 had no effect on ROS production (data not shown).
In separate experiments, we found that expression of CCR8 also can be induced in myeloid cells by culturing them in the presence of primary bladder tumor-conditioned medium (TCM) (Fig. 1C and Supplementary Fig. Table S3, A) and the addition of CCL1 to cells pre-treated with TCM induced Ca+2 influx (Supplementary Fig. S2, C). To test whether TCM-mediated induction of CCR8 expression in myeloid cells associates with activation of specific transcription factors, we compared phosphorylation status of ERK, Stat1 and Stat3 in TCM-treated CD11b cells that were isolated from peripheral blood of healthy donors. Results presented in Fig. 2A and Supplementary Table S3, B indicate that exposure of normal myeloid cells to the TCM promoted a strong increase in Stat3 phosphorylation, a relatively weak ERK phosphorylation, and no effect on Stat1 phosphorylation. Addition of Stat3 inhibitor S3I-201 completely prevented the TCM-mediated up-regulation of CCR8 in the myeloid cells (Fig. 2B). Distinctly, the inhibition of active ERK with U0126 or PD098059 showed no impact on the TCM-regulated expression levels of CCR8. These data indicate that TCM-induced CCR8 expression in myeloid cells depends on Stat3 activity.
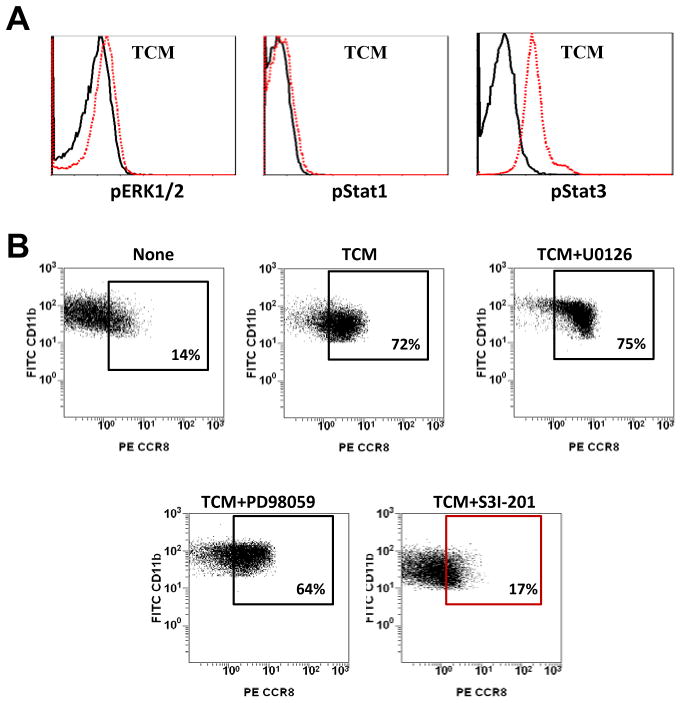
A: PBMC from healthy donors were incubated in the presence of TCM or culture medium alone in ultra-low attachment plate for 24 hours. Cells were collected, stained for CD11b and/or CCR8 and fixed. The CD11b+cells were gated and further analyzed. The intracellular flow cytometric analysis of pStat1, pStat3 and pERK1/2 in CD11b cells was performed using BD Phosflow technology. B: Stat3 inhibitor S3I-201 or ERK inhibitors U0126 and PD98059 were added into cell cultures and expression of CCR8 on CD11b cells was estimated by flow cytometry. Results of one representative experiment out of three are shown.
To evaluate the immune function of CCR8-expressing cells, we examined effects of CCL1 on cytokine production by myeloid cells obtained from peripheral blood of cancer patients. To this end, CD11b+ cells were isolated from PBMC of healthy donors and cancer patients and were cultured in the presence or absence of CCL1 for 24 hours. Cell supernatants were collected and analyzed for the presence of ten cytokines and chemokines, including IL-1β, TNF-α, IL-6, IL-8, VEGF, basic FGF, PDGF-BB, CCL2, CCL3 and CCL4 using Multiplex cytokine assay. Obtained results indicate that treatment of peripheral myeloid cells from both bladder (Fig. 3A) and kidney (Fig. 3B) cancer patients exhibited enhanced IL-6 production. Furthermore, we found that CCL1 stimulates IL-6 production by peripheral CD11b+ cells from cancer patients in a dose-dependent manner (Fig. 3C and Supplementary Fig. S3). Remarkably, CCL1-induced IL-6 production appears to be chemokine-specific and it was not observed following in vitro treatment with, for example, SDF-1 (Fig. 3D).

A, B: CD11b cells were isolated from peripheral blood of patients with bladder cancer (n=4) or RCC (n=3) using magnetic beads. Purified cells were cultured in the absence or presence of CCL1 (100 ng/ml) for 24 hours. Concentration of IL-6 in cell-free culture supernatants was measured using Multiplex cytokine assay. Results from individual patients are shown. C: CD11b myeloid cells were isolated from cancer patients and cultured in the presence of various amounts of CCL1 (0–200 ng/ml) for 24 hours. Concentration of IL-6 was determined in cell-free supernatants using commercial ELISA kit. Results are shown for one patient. D: CD11b cells were enriched from PBMC of a bladder cancer patient using magnetic beads. Isolated cells were cultured in the presence of CCL1 or SDF-1 for 24 hours. Concentration of IL-6 in cell culture supernatants was measured using IL-6 ELISA kit in triplicates. Results of one representative experiment out of three are shown. For all panels, each point represents the mean ± SD; *, p < 0.05.
CCR8+ cell subset can be found among CD11b myeloid cells infiltrating human cancer tissues
In addition to the peripheral blood, we also measured the levels of CCR8 expression in human tumor infiltrates (Fig. 4 and Supplementary Table S4). Analysis of freshly obtained, surgically removed high-grade invasive urothelial carcinoma of bladder revealed that a large portion of tumor-infiltrating CD11b myeloid cells co-expressed CCR8 (Fig. 4A, left panel). Specifically, in bladder cancer tissues expression of CCR8 was associated with CD11b+CD206+ tumor-associated macrophages (TAMs) but not with tumor-infiltrating CD3+ T lymphocytes (TILs) (Fig 4B, left panel). Similarly, expression of CCR8 in human renal cell carcinoma tissue was predominantly associated with CD11b+HLA-DR+CD68+ TAMs (Fig. 4A, right panel) and, again, not with CD3+ TILs (Fig. 4B, right panel). These results indicate that in human cancer tissues CCR8 expression is limited to tumor-infiltrating CD11b myeloid cell subsets including TAMs. It has to be noted that TAMs arise from recruited blood monocytes or myeloid-derived suppressor cells (16), suggesting that circulating CCR8+ myelomonocytic cells in blood (Fig. 1A) might be precursors of CCR8+ TAMs.

A: Tumor tissues were obtained from cancer patients with invasive urothelial carcinoma of bladder or RCC. Single cell suspensions were prepared from tumor tissues as specified in Methods and stained with fluorochome-labeled antibodies against surface markers CD11b, CCR8, CD206, HLA-DR, as well as intracellular CD68. 7-AAD positive cells in tumor cell suspension were excluded from analysis. Expression of indicated markers was assessed using flow cytometry. B: Tumor single cell suspensions were labeled with fluorochome-conjugated antibodies against surface markers CD3 and CCR8. Expression of indicated markers was assessed using flow cytometry. C: Cytomorphologic characterization of tumor-infiltrating CCR8+ myeloid cell subset. CCR8+ myeloid cells were enriched from human RCC tumor tissues. Purity of isolated cell population exceeded 85%. Upper left panel: Expression of CCR8 in CCR8-enriched cell fraction. Upper right panel: Isolated CCR8+CD11b+ cells were cultured on chamber vessel tissue culture glass slide (BD Falcon) and then stained with the Hema3 Stat Pack Kit (Fisher Scientific). Low panels: microphotograps of freshly isolated and H&E-stained tumor-infiltrating CCR8+ (left) and CCR8− (right) myeloid cell populations. Results are shown for one patient. Similar results were obtained from three patients.
Characterization of tumor-infiltrating CD11b+CCR8+ myeloid subset
We next investigated the functional characteristics of CCR8-expressing myeloid cells infiltrating human cancer tissue. CD11b+CCR8+ and CD11b+CCR8− myeloid cell subsets were isolated from RCC tissues using magnetic beads. Freshly isolated and Hematoxylin/Eosin stained CCR8+ myeloid cells strongly resembled tissue macrophages, whereas similarly treated CCR8− fraction of tumor-infiltrating myeloid cells exhibited heterogeneous composition that was comprised of various types of myeloid cells, including polymorphonuclear neutrophils (Fig. 4C). Admittedly, we could not isolate the tumor-infiltrating CCR8+ cells from human bladder cancer due to the small size of available tumor tissues. CCR8+ and CCR8− cell subsets isolated from RCC tissues were cultured for 24 hours and collected cell supernatants were assayed for presence of cytokines and chemokines using 10-plex cytokine assay. Analysis of cytokine production by CCR8+ and CCR8− myeloid cell subsets is shown in Fig. 5A. It appears that tumor-infiltrating CCR8+ cells secreted significantly more IL-6, VEGF, CCL3 and CCL4, but twice less PDGF-BB compared to their CCR8− counterparts. No significant differences were observed between those cell subsets in the production of TNF-α, IL-1β, IL-8, CCL2 and basic FGF (data not shown).

A: Cytokine/chemokine production. Tumor-infiltrating CCR8+ and CCR8− CD11b myeloid cells were isolated from human RCC tumor tissues (n=4) using magnetic beads. Purity of isolated cell populations exceeded 85%. Cells were cultured for 24 hours and collected cell-free supernatants were assayed for presence IL-6, VEGF, CCL3, CCL4 and PDGF-BB using Multiplex cytokine assay. Results from four cancer patients are shown. Average mean ± SD are shown; *, p<0.05. B: CCR8+ myeloid cells induce FoxP3 expression in T lymphocytes. CCR8+ and CCR8− myeloidsubsets were purified from RCC tissues using magnetic beads. Autologous T lymphocytes were obtained using T cell-enrichment columns. 1 × 106 purified T cells (control) or 5 × 105 of each myeloid subset and 1 × 106 of autologous T cells were cultured together. T cells were stimulated with CD3/CD28 antibodies. Forty eight hours later, cells were collected. Expression of FoxP3 in CD4 cells was evaluated using flow cytometry. Results are shown for one patient. Similar results were obtained from three RCC patients.
We recently demonstrated that macrophage infiltrating human RCC exhibit potent regulatory activity (17). Specifically, isolated TAMs were able to induce tolerogenic transcription factor FoxP3+ in T lymphocytes as well as to enhance IL-10 production. In order to evaluate the effects of CCR8+ myeloid cells on T lymphocytes, we isolated CD11b+CCR8+, CD11b+CCR8− myeloid subsets as well as total CD11b cells from RCC tissue and co-cultured them with autologous T cells for 48 hours. Intracellular expression of FoxP3 in CD4 cells was measured using flow cytometry. In addition, we collected cell supernatants and measured the concentration of IL-10 using ELISA. Data presented in Fig. 5B and Supplementary Fig. S4 clearly indicate that enriched CCR8+ myeloid cell subset exhibits superior ability to induce FoxP3 in comparison to CCR8− myeloid cells or total tumor-infiltrating CD11b cells. However, we were not able to obtain consistent data to demonstrate that CCR8+ myeloid cells have a superior ability to induce IL-10 (data not shown). Overall, these data indicate that tumor-infiltrating CD11b+CCR8+ cell subset may contribute to immune evasion and progression of human cancers through enhanced secretion of pro-inflammatory (IL-6, CCL3, CCL4) and pro-angiogenic (VEGF) factors as well as through induction of FoxP3 expression in autologous T lymphocytes.
Primary human cancers secrete large amounts of CCL1
Having demonstrated that expression of CCR8 in myeloid cells during tumor progression is increased, we next evaluated the levels of natural CCR8 ligand CCL1 in cancer patients. Established human bladder and RCC cancer cell lines, freshly obtained primary bladder and kidney cancer tissues and CD11b cells isolated from RCC tissues were cultured for 24 hours. Cell-free supernatants were collected and analyzed for CCL1 presence using commercial ELISA. As shown in Fig. 6A, both bladder and RCC human cancer cell lines (SW780 and A498, respectively) did not secrete detectable levels of CCL1, while primary human tumors secreted substantial amounts of the chemokine (bladder cancer: 118 ± 44 pg/ml and renal carcinoma: 30.5 ± 7 pg/ml). Moreover, tumor-infiltrating myeloid CD11b+ cells were the primary source of CCL1 among the tumor cell suspension as CD11b+ myeloid cells isolated from renal carcinoma tissues produced 98 ± 36 pg/ml of CCL1. In addition, we measured levels of CCL1 in peripheral blood (plasma) obtained from cancer patients. Results indicate that levels of CCL1 in plasma of cancer patients with urothelial or renal carcinomas are very low (< 5 pg/ml) and in most cases undetectable (data not shown). These results indicate that primary human cancers secrete substantial amounts of CCL1 and tumor-infiltrating myeloid cells represent a major source of the chemokine.
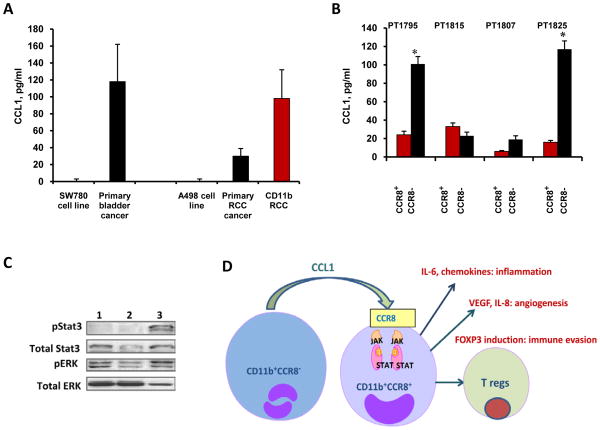
A: Single cell suspensions of RCC and bladder tumor tissue, bladder tumor cell line SW780 or RCC tumor cell line A480 (1×106 cells/ml) were cultured for 24 hours. Collected cell-free supernatants were assayed for CCL1 using ELISA. Average mean ± SD from five patients are shown. B: CCR8−CD11b+ cells are the primary source of CCL1 in tumor tissue. CCR8+ and CCR8− myeloid cells were isolated from human RCC tissues and cultured for 24 hours. Purity of isolated cell population exceeded 85%. Concentration of CCL1 in cell supernatants was measured using ELISA. Individual data from four RCC patients are shown. Average mean ± SD are shown, *, P < 0.05. C: Total tumor-infiltrating CD11b cells (line 1), as well as CCR8− (line 2) and CCR8+ (line 3) cell subsets were purified from RCC tissues. Whole cell lysates were analyzed for expression of phospho-Stat3 and phospho-ERK using Western Blotting. Results are shown for one patient. Similar results were obtained from two other RCC patients. D: Proposed model of immune evasion mediated by CCR8+ myeloid cells. This schematic depicts contribution of CCR8-expressing myeloid cells to the cancer-promoting inflammation, angiogenesis and immune evasion.
To elucidate what cell subset among tumor-infiltrating CD11b cells produce CCL1, we measured CCL1 levels in CCR8+ and CCR8- myeloid cells isolated from human RCC tissues. As can be seen in Fig. 6B, CCR8− myeloid cells secreted significantly more CCL1 than CCR8+ subsets in three out four examined cancer patients. Therefore, it is likely that certain myeloid cell type among intra-tumoral CCR8− CD11b cells (Fig. 4C) represents a major source of CCL1 in RCC tissue, suggesting existence of paracrine mechanisms involved in the regulation of CCL1-induced IL-6 production and CCR8 expression in tumor-infiltrating myeloid cells. A recent study demonstrated that CCL1 expression can be stimulated in human monocytes/macrophages by treatment with another chemokine (CXCL12) implying that CXCL12 could potentially promote elevated levels of CCL1 in tumor tissues (18). Our data, however, indicate that primary RCC tumors secrete negligible levels of CXCL12 (data not shown).
IL-6 is known to induce Stat3 activation (19). In previous experiments (Fig. 2) we demonstrated that CCR8 expression in myeloid cells is dependent upon Stat3 activation. Therefore, we next evaluated Stat3 phosphorylation status in tumor-infiltrating CD11b+CCR8+ cell subset isolated from human RCC. Data presented in Fig. 6C convincingly demonstrate that phosphorylated Stat3 is predominantly and selectively present in tumor-infiltrating CCR8+CD11b+ cell subset. In addition, we found that levels of ERK phosphorylation are higher in the CCR8+ than in their CCR8− counterparts (Fig. 6C). Together, these data demonstrate the CCR8-expressing myeloid cells have activated Stat3 and ERK pathways.
Discussion
Tumor growth is associated with abnormal myelopoiesis and enhanced cancer inflammation as a result of secretion by tumor cells of various bioactive substances including growth factors, cytokines, chemokines and lipids. These factors stimulate mobilization of heterogeneous myeloid cells into blood circulation, promote the accumulation of myeloid-derived suppressor cells (MDSCs) in tumor hosts, and provide constant recruitment of myeloid cells to the tumor site. Increased presence of myeloid cells within tumor tissues associates with poor prognosis through increased tumor angiogenesis, tissue remodeling and suppression of antitumor immune responses (20–25). We and others have demonstrated earlier that MDSCs mediate immune evasion in tumor host by inducing antigen-specific CD8 T-cell tolerance (26–28). Mechanistically, these cells are capable of suppressing T-cell responses through peroxynitrite production in an arginase and/or iNOS-dependent manner (27, 29–31). Furthermore, MDSCs directly promote angiogenesis and tissue remodeling via enhanced production of pro-angiogenic mediators such as VEGF and MMP9 (21, 32). Upon recruitment to tumor tissue, MDSCs frequently differentiate into more mature myeloid cell subsets such as TAMs. The TAMs represent an abundant and heterogeneous cell population in the tumor microenvironment and they play a key role in tumor development (4, 33). Several lines of evidence suggest that TAMs promote tumor invasion, angiogenesis, tissue remodeling and metastasis, and associate with poor prognosis. TAMs also can have an inhibitory role in the development of anti-tumor immunity through the production of immune suppressive cytokines. Despite recent advances in our understanding of the multiple roles of bone marrow-derived myeloid cells in tumor progression, heterogeneity of myeloid cells in tumor tissues as well as lack of specific markers for discrete cell subsets still represent significant obstacles for further progress. Establishing such biomarkers has the potential to enhance clinical prognosis and provide insights into relevant pathways of immune suppression utilized by the specific cancer.
In this study we measured expression of multiple chemokine receptors (CCR1, CCR2, CCR3 CCR4, CCR5, CCR6, CCR7, CCR8, CXCR2 and CXCR4) in myeloid cells obtained from peripheral blood of patients with bladder cancer and renal cell carcinoma. We found that among screened chemokine receptors only CCR8 expression was consistently and strongly up-regulated in CD11b myeloid cells from cancer patients as compared to healthy donors. Importantly, both monocytic CD33highCD11b and granulocytic CD33lowCD11b MDSCs in blood obtained from the cancer patients showed increased expression of CCR8. Up-regulated expression of CCR8 was also detected in tumor tissues obtained from patients with bladder cancer and renal cell carcinoma. Furthermore, CCR8 expression in cancer tissues appears to be limited to the tumor-infiltrating CD11b myeloid cells and primarily to TAMs. In addition, it should be noted that CD3 T lymphocytes infiltrating human renal and bladder tumors did not express CCR8.
At the functional level, we showed that CD11b+CCR8+ cell subset may contribute to immune evasion and progression of human cancers through elevated secretion of pro-inflammatory (IL-6, CCL3, CCL4) and pro-angiogenic (VEGF, IL-8) factors as well as through induction of FoxP3 expression in autologous T lymphocytes. Mechanistically, we found that tumor-derived factors promote CCR8 expression in myeloid cells in a Stat3 activation-dependent manner, and CCR8+ myeloid cells isolated from human cancer tissue display phosphorylated Stat3. Signaling by Stat3 is a major intrinsic pathway for cancer inflammation because it is frequently activated in malignant cells and is capable of regulating the expression of a large number of genes that are crucial for inflammation (1). Activation of Stat3, which is a leading pathway in regulation of both cancer-related inflammation and tumor immunity (1, 34), can be achieved with several pro-inflammatory agents including IL-6, which is produced primarily by bone marrow-derived myeloid cells (1). Here we demonstrate that human renal carcinoma tumor-infiltrating CCR8+ myeloid cells represent a major source of IL-6 and, moreover, IL-6 production by CCR8+ cells can be stimulated by treatment with CCL1. Importantly, elevated levels of these cytokines are frequently observed in cancer and are associated with cancer related inflammation (35). Taken together, our observations provide evidence for enhanced CCL1/CCR8 signal pathway in cancer and suggest that CCL1/CCR8 axis represents an important regulatory component in inflammation that associates with human cancer progression.
In addition to the infiltration with CCR8-expressing myeloid cells, we also observed that primary human tumors secreted substantial amounts of CCL1. We found that RCC-infiltrating myeloid cells produced significantly higher amounts of CCL1 than whole RCC tissue or epithelial cancer cell lines. Previous studies demonstrated that CCL1 exhibits a unique pattern of regulation associated with a distinct form of M2 type monocyte activation that participates in macrophage-dependent regulatory circuits of innate and adaptive immunity (36). CCL1 also induces regulatory T cell recruitment (37) and promotes Th2 immune response (38, 39). Interestingly, in mice subjected to chronic hepatic injury, CCR8-expressing liver macrophages were necessary for CCL1-directed migration of inflammatory, but not classically activated monocytes (11). CCR8 deficiency also protected the liver against injury, ameliorating inflammatory responses and hepatic fibrogenesis. Furthermore, absence of CCR8 enhances innate immunity during septic peritonitis (40) and a recent report indicated that mouse macrophages could up-regulate CCR8 in an autocrine manner upon CCL1 binding (41).
However, information available on the role of CCL1 and CCR8 in the progression of tumors particularly of human cancers is very limited. A recent study, based on measurement of gene expression and single nucleotide polymorphism analyses, evidenced a link between CCL1 expression and development of breast cancer in women (42). Antibody-mediated neutralization of CCL1 in mice harboring mammary carcinomas significantly reduced the rate of CD4 T cell conversion into regulatory T cells (43), suggesting a role for CCL1 (presumably through cognate receptor CCR8) in tumor-associated immune suppression. Other studies showed that CCL1 exhibits pro-angiogenic activity through stimulation of chemotaxis of endothelial and vascular smooth muscle cells (12, 13, 44), reinforcing the possible involvement in cancer progression.
Based on our results, we propose a working model for the contribution of CCR8-expressing myeloid cells to immune evasion in cancer (Fig. 6D). CCL1 is produced primarily by CCR8− tumor-infiltrating myeloid cells. Upon binding to the CCR8 on CCR8+ myeloid cells, CCL1 promotes production of IL-6 that, in turn, activates Stat3 in target cells. Activation of Stat3 promotes expression of a whole range of pro-inflammatory, pro-angiogenic and immunosuppressive genes. In addition, CCR8+ myeloid cells are highly effective in induction of FoxP3 expression in T lymphocytes. Overall, the emerging picture suggests that CD11b+CCR8+ myeloid cell subset could exert a marked tumor-promoting effect.
Overall, we conclude that enhanced production of CCL1 by primary human cancers and increased numbers of CCR8+ myeloid cells represent a common feature of human bladder and renal carcinomas thereby suggesting the contribution of CCR8/CCL1 axis to cancer inflammation, immune evasion and tumor angiogenesis via stimulation of pro-inflammatory and pro-angiogenic cytokines/chemokines and induction of tolerogenic transcription factor Foxp3 in CD4 T lymphocytes. Further studies are needed to evaluate possible correlation of CCR8 expression with clinical parameters and overall contribution of CCR8-expressing cells and its ligand CCL1 to the development and progression of cancers. Lastly, our data also suggest that blockade of CCR8 signaling may provide a novel strategy for therapeutic intervention in human cancers.
Supplementary Material
1
10
2
3
4
5
6
7
8
9
Acknowledgments
Grant Support
This work was supported, in part, by grants 10KN-10 from the James and Esther Biomedical Research Program (to S.K.) and RO1 CA129155 from the US National Institutes of Health (to Y.D).
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/1078-0432.ccr-12-2091
Read article for free, from open access legal sources, via Unpaywall:
https://clincancerres.aacrjournals.org/content/clincanres/19/7/1670.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Targeting the glucocorticoid receptor-CCR8 axis mediated bone marrow T cell sequestration enhances infiltration of anti-tumor T cells in intracranial cancers.
Cell Mol Immunol, 21(10):1145-1157, 23 Jul 2024
Cited by: 0 articles | PMID: 39044027
Molecular understanding and clinical aspects of tumor-associated macrophages in the immunotherapy of renal cell carcinoma.
J Exp Clin Cancer Res, 43(1):242, 22 Aug 2024
Cited by: 0 articles | PMID: 39169402 | PMCID: PMC11340075
Review Free full text in Europe PMC
Reduced chemokine C-C motif ligand 1 expression may negatively regulate colorectal cancer progression at liver metastatic sites.
J Cell Mol Med, 28(7):e18193, 01 Apr 2024
Cited by: 0 articles | PMID: 38506205 | PMCID: PMC10952021
Identification of a non-canonical chemokine-receptor pathway suppressing regulatory T cells to drive atherosclerosis.
Nat Cardiovasc Res, 3:221-242, 22 Jan 2024
Cited by: 2 articles | PMID: 39044999 | PMCID: PMC7616283
Identification of AKI signatures and classification patterns in ccRCC based on machine learning.
Front Med (Lausanne), 10:1195678, 24 May 2023
Cited by: 0 articles | PMID: 37293297 | PMCID: PMC10244623
Go to all (38) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1.
J Immunol, 179(9):5760-5767, 01 Nov 2007
Cited by: 54 articles | PMID: 17947648
CCR8+FOXp3+ Treg cells as master drivers of immune regulation.
Proc Natl Acad Sci U S A, 114(23):6086-6091, 22 May 2017
Cited by: 117 articles | PMID: 28533380 | PMCID: PMC5468670
Chemokine (C-C Motif) Ligand 1 Derived from Tumor-Associated Macrophages Contributes to Esophageal Squamous Cell Carcinoma Progression via CCR8-Mediated Akt/Proline-Rich Akt Substrate of 40 kDa/Mammalian Target of Rapamycin Pathway.
Am J Pathol, 191(4):686-703, 16 Jan 2021
Cited by: 14 articles | PMID: 33460563
Chemokines and cancer: new immune checkpoints for cancer therapy.
Curr Opin Immunol, 51:140-145, 24 Mar 2018
Cited by: 81 articles | PMID: 29579623
Review





