Abstract
Free full text

The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy
Abstract
T cell-mediated rejection of tumors requires signals from the T cell receptor and co-stimulatory molecules to license effector functions of tumor-antigen specific T cells. There is also an array of immune suppressive mechanisms within the tumor microenvironment that can suppress anti-tumor immunity. The use of monoclonal antibodies to overcome this suppression and/or enhance tumor-antigen specific T cell responses has shown promise in clinical trials. In particular, targeting co-stimulatory members of the tumor necrosis factor receptor (TNFR) family with agonist Abs enhances T cell function, which has led to encouraging therapeutic results in cancer-bearing hosts. These encouraging data establish TNFRs as important targets for enhancing tumor-specific immune responses in mice and man. This review will focus on agonists that target the TNFRs OX40, 4-1BB, and CD40.
Introduction
Targeting co-stimulatory and co-inhibitory receptors expressed by immune cells is a promising new approach for treating cancer. The recent FDA approval of the monoclonal antibody (mAb) against the inhibitory molecule CTLA-4 (ipilumimab) demonstrates the proof of principle that enhancing T cell function can have therapeutic effects in cancer patients [1]**. This study provides the foundation for many other monoclonal Ab therapies that are currently in clinical development. Some of these mAbs target members of immune cell surface molecules that are within the tumor necrosis factor (TNF) and TNF receptor super family (TNFRSF). There are approximately 50 members of this protein family that are both membrane bound and soluble and are expressed by cells of the immune system. When these proteins are ligated either by their cognate receptor or by agonist antibodies, a wide range of cellular outcomes has been reported ranging from cell differentiation, proliferation, apoptosis and survival to increased production of cytokines and chemokines [2–7]. In addition, the unique expression of some of the TNFRSF members on antigen-specific T cells has made these molecules ideal targets for novel immunotherapies.
Immunotherapy has been proposed as an effective mechanism for controlling tumor growth since the late 1800’s [8]. In the past few decades, the generation of monoclonal antibodies that target either immune-stimulating receptors or inhibitory receptors has shown to increase anti-tumor immunity in cancer-bearing hosts leading to therapeutic responses. The general premise regarding these therapies is that cancer-bearing hosts have T cells that are specific for tumor Ags residing in their body, however their function is suppressed by the tumor microenvironment. Moreover, the frequency of tumor antigen specific cells is also speculated to be limited. Therefore, these immune stimulating Abs help to overcome this immune suppression by increasing the frequency and function of antigen presenting cells and T cells, which ultimately leads to tumor regression [9]. Many of the TNFR family members have been identified as potential immunotherapy targets.
Optimal activation of naïve T cells requires a strong T cell receptor peptide antigen-MHC interaction along with engagement of costimulatory molecules expressed by antigen presenting cells [10]. In the absence of these costimulatory signals, activated naïve T cells die or are rendered anergic [11]; thus costimulation is indispensible for a functional T cell response. Many costimulatory receptors have been described but signals from CD28, a costimulatory molecule constitutively expressed on naïve T cells, is indispensable for T cell effector function and expansion.
In addition to CD28, there are a number of other costimulatory proteins that are required to generate optimal effector and memory T cells following antigen encounter. Several of these costimulatory proteins are members of the TNFR superfamily. Initially described to be expressed on activated T and B lymphocytes and antigen presenting cells (APCs), ligation of some of these receptors is shown to promote cell division and survival, differentiation, maturation, and provide signals directly to T cells (Table 1). Because of the unique T cell activating features of these receptors, many groups have targeted TNFRs with agonist mAbs to enhance lymphocyte function, particularly in the context of tumor immunotherapy.
Table 1
TNF family members targeted for cancer immunotherapy, their expression patterns, and therapeutic targeting molecules
| TNF Receptors | TNF ligands | ||||||
|---|---|---|---|---|---|---|---|
| Molecule | Expressed by | Expression level | Therapeutic targeting molecule | Molecule | Expressed by | Expression level | Therapeutic targeting molecule |
| OX40 (CD134) | CD4+, CD8+ T cells, NK cells, NKT cells, Foxp3+ Treg cells | Inducible on most cells, constitutively expressed on Treg cells | MEDI6469 (mouse-anti human OX40 agonist mAb) | OX40L (CD252) | APCs, endothelial cells, smooth muscle, mast cells | Inducible on all cells described | N/A |
| GITR (CD357) | CD4+, CD8+ T cells, NK cells, NKT cells, B cells, Foxp3+ Treg cells, Macrophages, DCs | Inducible on most cells described, constitutively expressed on Treg cells | TRX518 (antihuman GITR mAb) | GITRL | APCs, endothelial cells | Constitutively expressed | N/A |
| 4-1BB (CD137) | CD4+, CD8+ T cells, NK, NKT, mast cells, neurophils, Foxp3+ Tregs, DCs, enothelial cells, eosinophils, | Inducible on most cells, constitutively expressed on Treg cells | BMS-666513 (humanized anti-human 4-1BB mAb)/ PF-05082566 (humanized anti-4-1BB mAb) | 4-1BBL | APCs, mast cells, NK cells, smooth muscle, hematopoietic progenitors | Primarily inducible, constitutively expressed by hematopoietic progenitors. | N/A |
| CD40 | APCs, epithelial, endothelial, and smooth muscle cells, fibroblasts, basophils | Both constitutive and inducible expression | 4D11 (anti-human CD40 mAb) | CD40L (CD154) | T cells, B cells, Eosinophils, monocytes, macrophages, endothelial, epithelial, and muscle cells | Inducible on all cells described | Recombinant CD40L |
| CD27 | CD4+, CD8+ T cells, some B cells, Foxp3+ Treg cells, NKT cells, hematopoietic precuro | Usually constitutively expressed on T cells, inducible on B cells | CDX1127 (agonist anti-CD27 mAb) | CD70 | T cells, B cells, DCs, APCs | Induced on lymphocytes and constitutively expressed by APCs | SGN-75 (antibody-drug conjugate targeting CD70+ tumor cells) |
Distinct TNFR subtypes have been described, ones that contain a death domain (DD) (i.e. FAS, DR5, TNFRI), decoy receptors that don’t signal, and receptors that need adaptor molecules to signal, the latter will be the focus of this review. The TNF receptors that need adaptor molecules to signal use intracellular TNF receptor-associated factor (TRAF) proteins that interact with the cytoplasmic tail of these TNFRs. This in turn activates downstream signaling of NK-κB, activation of mitogen-activated protein 3 kinase, and PI3-k signaling to promote effector T cell recruitment and function such as cell survival, proliferation, and activation [12,13].
In this review, we focus on the role of the immune activating TNFRSF members expressed predominantly by T cells as immunotherapeutic targets for an array of cancer malignancies. We will briefly discuss the biology of these receptors and their ability to activate the immune system and then present data on the pre-clinical and clinical findings of targeting TNFRSF members for immunotherapy of cancer. In particular, we will discuss CD40, CD134 (OX40), and CD137 (4-1BB) in detail.
OX40 Background and Tumor Immunotherapy
OX40 was initially described as a T cell activation marker on rat CD4 T cells [14] and shown later to be up-regulated upon TCR engagement [15]. OX40 signaling can promote co-stimulatory signals to T cells leading to enhanced cell proliferation, survival, effector function and migration [3,16]. The ligand for OX40, OX40L, is predominantly expressed on antigen presenting cells (APCs) and its expression can be induced via CD40 and mast cell signaling, toll like receptors (TLRs), as well as inflammatory cytokines. In addition to APCs, non-hematopoietic cells such as smooth muscle and vascular endothelial cells can also express OX40L. In transgenic mice that overexpress the OX40L there is increased T cell activation and when immunized these mice generated an enhanced T cell response [17,18]. This data suggested that OX40L expression was the limiting factor in regards to OX40 signaling in T cells. Therefore our group and others initiated studies to determine whether OX40 agonists (anti-OX40 and OX40L:Ig) could enhance T cell responses in tumor-bearing hosts [19].
Initial experiments showed that injection of OX40 agonists into tumor-bearing mice early after tumor inoculation cured 20–80% of the animals depending on the tumor model [19]. The therapeutic response was dose-dependent and was dependent on both CD4 and CD8 T cells. The mice that were cured following anti-OX40 were resistant to re-challenge with the same tumor. At the same time these experiments were being performed our lab described OX40+ cells within resected tumors from patients with cancer [20]. OX40+ T cells were found in a wide variety of human malignancies [21], which increased the rationale for translating anti-OX40 therapy to the clinic. The phase I trial tested 3 doses of the Ab (0.1, 0.4 and 2.0 mg/kg) and 10 patients were treated per cohort. The results of the study which have been summarized [22] and submitted for publication [23] showed the drug was tolerated, increased T cell activation/proliferation, and caused tumor shrinkage in some patients. Two new OX40-based clinical trials have been initiated at the Providence Cancer Center; one combines chemotherapy (cyclophosphamide) and radiation with the anti-OX40 Ab treatment in prostate cancer patients and the second trial combines high-dose fractionated radiation with anti-OX40 treatment in breast cancer patients.
The mechanism by which anti-OX40 treatment enhances T cell function in animal tumor models has been somewhat controversial. It has been shown that while OX40 is expressed on conventional CD4 (FoxP3neg) and CD8 T cells (Figure 1), it is also on CD4+Foxp3+ (Treg) cells [24]. There is plenty of evidence demonstrating that anti-OX40 has direct effects on CD4 and CD8 T cells to enhance their effector function and memory T cell development and function, but there is also evidence that OX40 agonist stimulation decreases/destabilizes Treg suppressor function [25–27]. However, we and others have also shown that OX40 agonists delivered in vivo can actually stimulate Treg cell proliferation and that Treg cells recovered from OX40 agonist-treated mice are functionally intact [28]. OX40 agonists have stimulatory effects on all T helper cell lineages [29], which includes increasing T cytokine production. Since cytokines are known to have effects on T helper cell plasticity [30]it could be that OX40 agonists shift the balance of cytokines, which in turn have detrimental effects on Treg function and lineage commitment. However, if the cytokine milieu favors Treg cell development, OX40 agonists will expand Tregs and enhance their survival [31,32]. Hence shifting the cytokine balance towards a Th1 milieu in tumor-bearing hosts prior to OX40 agonist administration led to greater therapeutic activity as was demonstrated when OX40 agonists were combined with IL-12 [33] or a TGFβ receptor inhibitor [34]*.
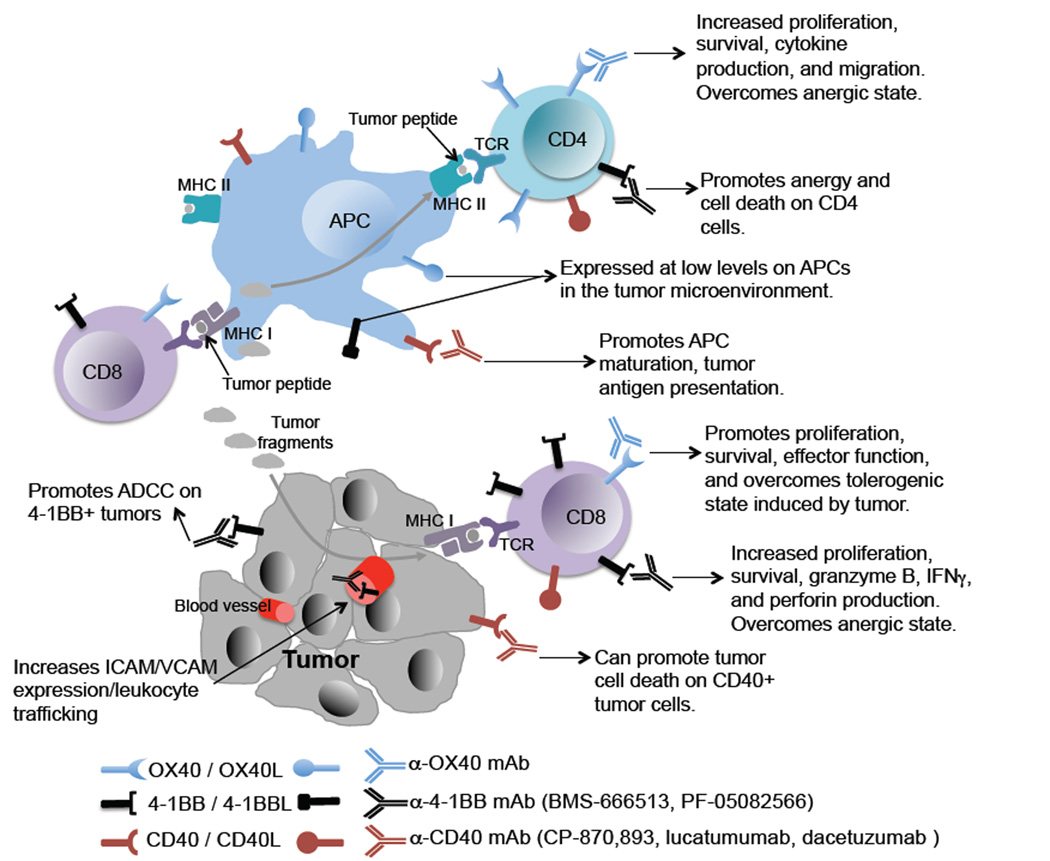
TNFR family members OX40, 4-1BB, and CD40 have unique roles in regulating anti-tumor T cell responses. OX40 is expressed at much higher levels on activated CD4 T cells than CD8 T cells whereas 4-1BB is more highly expressed on CD8 T cells than CD4s. APCs in the tumor microenvironment express low levels of CD40 and activated T cells express both pre-formed CD40L and rapidly increase CD40L on the cell surface after antigen encounter. Tumor cells themselves are also targeted by agonist antibodies via ADCC. Therefore, targeting these TNFR family members with agonist antibodies promotes intra-tumor effector T cell responses and tumor regression.
4-1BB
The first of the TNFR family members to be identified as a possible immunotherapy target was 4-1BB (also known as CD137) [35]. 4-1BB, expressed on activated T cells (Figure 1), NK cells, and a number of other activated cells of hematopoietic and non-hematopoietic origin including endothelial cells of some tumors, binds to its ligand 4-1BBL expressed by activated DCs, B cells, and macrophages. This interaction leads to a co-stimulatory signal that promotes the upregulation of anti-apoptotic molecules such as Bcl2 and Bcl-xl and protects tumor antigen specific cells from activation-induced cell death [36,37]**. In addition, it was shown that ligation of 4-1BB with an agonist antibody can reverse tolerance of CD8+ T cells [38] and promote tumor regression of established tumors [35,39] primarily via CD8 CTL activity and NK cell function. However, the 4-1BB agonist mAb also increases the frequency of anergic CD4 T cells resulting in the loss of humoral immunity in both mouse [40] and non-human primates [41]. Interestingly, a recent study using a chimeric streptavidin-4-1BBL molecule identified that co-stimulation with this molecule rendered effector T cells insensitive to Treg mediated suppression [42] and inhibited the conversion of conventional CD4 T cells to regulatory T cells [43]. This effect promoted tumor eradication and sustained anti-tumor T cell responses in tumor bearing mice. In addition, the anti-tumor immune effects of agonist 4-1BB antibody therapies are CD8 dependent and promote long-term anti-tumor memory T cell survival [44]. Thus, targeting 4-1BB may be a viable approach to promoting tumor antigen specific T cell responses.
In addition to 4-1BB’s role in promoting proliferation and survival of antigen specific T cells, it can be expressed on tumor cells and the vascular endothelium [36]. Interestingly, treatment of tumor-bearing mice with a 4-1BB agonist mAb increased infiltration of activated T cells into the tumor in an intercellular adhesion molecule I (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) dependent fashion [45]. Taken together, this suggests that the mechanism of 4-1BB mediated immunotherapy might involve both immune cell activation as well as enhance accessibility to the tumor by leukocytes. Last, expression of 4-1BB directly by tumor cells themselves also suggests that agonist CD137 antibodies could promote direct tumor cell death via antibody dependent cell mediated cytotoxicity (ADCC) and tumor cell phagocytosis [46,47]*.
Several clinical trials are investigating the role of anti-4-1BB in patients with malignancies. BMS-663513, a fully humanized IgG4 anti-CD137 mAb, was tested in a phase I dose-escalation study in patients with advanced cancer. The study, presented at the 2008 ASCO meeting, showed three partial responses and stable disease in four patients with melanoma. Preliminary biomarker analysis revealed an increased percentage of activated CD8 T cells in the peripheral blood [48]. Based on these results, a randomized, multi-dose, open-label, phase II study of BMS-663513 as a second-line monotherapy in subjects with previously treated unresectable stage III or IV melanoma was designed however, the study was terminated due to a high incidence of grade 4 hepatitis. Another fully humanized anti-CD137, PF-05082566, is currently being tested in clinical trial as either a single agent in patients with solid tumors or in combination with rituximab in patients with CD20 positive B cells Non-Hodgkin’s lymphoma (NCT01307267).
CD40
CD40 is constitutively expressed on APCs and ligation promotes functional maturation leading to an increase in antigen presentation and cytokine production, and a subsequent increase in the activation of antigen specific T cells (Figure 1). Initial studies using an agonist CD40 antibody showed increased cytotoxic lymphocyte (CTL) responses against poorly activating tumor antigens [49,50] and promoted effector function of cytotoxic T cells tolerized by tumor antigens [51]. Thus, targeting CD40 with monoclonal antibodies has potent anti-tumor efficacy [49]. Thus far, T cell co-stimulatory receptors have been discussed as immunotherapy targets. In addition, as was observed with anti-4-1BB antibody therapy, targeting CD40 with an agonist antibody also allowed T cells to overcome tolerance and promote tumor eradication in mouse models of disease [49–51] as CD40 is also expressed at the surface of a wide array of primary tumors. Therefore, targeting CD40 might exert its anti-tumor activity by two distinct mechanisms; directly, anti-CD40 induces antibody-dependent phagocytosis of tumor cells and inhibits CD40-CD40L induced tumor proliferation and indirectly by activating the anti-tumor immune response via APC maturation.
Targeting CD40 with an agonist monoclonal antibody has been tested in patients with both hematological cancers and solid tumors [52–54]*. Several humanized anti-CD40 mAb have completed phase I clinical trial and are currently assessed in phase II trials. The first two trials included patients with B cell malignancies; either chronic lymphocytic leukemia (CLL) or multiple myeloma (MM). Lucatumumab (formerly HCD122), was used in two phase I clinical trials in patients with CLL and with MM [53,54]. In both studies, grade 3 and 4 toxicities were asymptomatic elevated lipase and amylase levels with one case of grade 4 thrombocytopenia in a patient with MM. Immunologically, there was minimal response, prompting the design of combination therapy studies. A phase I clinical trial of patients with CLL and MM [55,56] resulted in minimal clinical activity with dacetuzumab (formerly SGN-40) however, ~30% of patients experienced grade 3–4 toxicities with steroid pre-treatment and anti-CD40 mAb doses of 12mg/kg. Combination trials are currently evaluating bortezomib with dacetuzumab in patients with multiple myeloma (NCT00664898). The results are not yet available.
The only anti-CD40 mAb tested in patients with solid malignancies is CP-870,893. The first single dose trial showed an objective partial response in 14% of patients with melanoma. Subsequently, multiple doses of CP-870,893 was shown to be well tolerated and 26% of the patients had stable disease. The single dose study reported a transient depletion of B cells, with an upregulation of co-stimulatory molecules on the remaining B cells [57]. The weekly dosing of anti-CD40 also induced B cell depletion with increased expression of CD86 and CD54 expression at the surface of B cells. Moreover, a decrease in CD4 and CD8 T cells was observed [58].
In an elegant study in both mice and humans, the agonist anti-CD40 CP-870,893 in combination with Gemcitabine, was shown to be active. The anti-tumor activity was dendritic cell and T cell independent, instead, acting directly on CD40-expressing macrophages [59]. These results demonstrate and highlight the importance of both innate and adaptive immune responses in mediating tumor regression. Moreover, the pleiotropic effects of monoclonal antibody therapies that target this and other TNFR family members, make them ideal targets for single agent and combination immunotherapy. Currently a clinical trial evaluating the role of CP-879,895 in combination with tremelimumab (anti-CTLA-4) in melanoma patients (NCT01103635) is under way.
Conclusions and future perspectives
The promise of immunotherapy for the treatment of cancer has been confirmed by the success reported in recent clinical trials in which co-inhibitory molecules have been targeted [1,60,61]. While these therapies increased the anti-tumor immune responses in patients with a range of malignancies, efficacy often correlated with the immunogenicity of the tumor. Therefore, we propose that successful immunotherapeutic approaches for poorly immunogeneic tumors will require combination with targeted therapy such as chemo or radiation therapy in conjunction with immune cell modulating antibodies or adjuvants as demonstrated with interleukin-2 [62]. A few of these combination therapies have been tested in mouse models or observed in patients and show promise [63–66]. We propose that use of targeted co-stimulatory agonistic antibodies together with inhibitory receptor blocking antibodies are promising approaches to overcoming the suppressive tumor microenvironment and expanding the cohort of patients that benefit from immune mediated cancer therapies.
Acknowledgements
Supported by NIH grants RO1 CA102577 and CA122701 (M.K.B, A.D.W), DOD grant W81XWH-11-1-0345 (A.D.W.), and NIH T32 AI78903 training fellowship (A.E.M.). The authors wish to thank Walter J. Urba for critical reading of this manuscript.
Footnotes
Conflict of Interest
A.D. Weinberg has issued patents regarding the use of OX40 agonists to treat cancer patients. A.E. Moran and M. Kovacsovics-Bankowski declare no conflict of interest related to this work.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.coi.2013.01.004
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3815601?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Emerging druggable targets for immune checkpoint modulation in cancer immunotherapy: the iceberg lies beneath the surface.
Apoptosis, 29(11-12):1879-1913, 01 Oct 2024
Cited by: 0 articles | PMID: 39354213
Review
Preclinical characterization and phase 1 results of ADG106 in patients with advanced solid tumors and non-Hodgkin's lymphoma.
Cell Rep Med, 5(2):101414, 07 Feb 2024
Cited by: 1 article | PMID: 38330942 | PMCID: PMC10897605
Reconciling intrinsic properties of activating TNF receptors by native ligands versus synthetic agonists.
Front Immunol, 14:1236332, 19 Sep 2023
Cited by: 3 articles | PMID: 37795079 | PMCID: PMC10546206
Review Free full text in Europe PMC
Intrinsic RIG-I restrains STAT5 activation to modulate antitumor activity of CD8+ T cells.
J Clin Invest, 133(9):e160790, 01 May 2023
Cited by: 6 articles | PMID: 36927693 | PMCID: PMC10145944
Frequency of Peripheral CD8+ T Cells Expressing Chemo-Attractant Receptors CCR1, 4 and 5 Increases in NPC Patients with EBV Clearance upon Radiotherapy.
Cancers (Basel), 15(6):1887, 21 Mar 2023
Cited by: 0 articles | PMID: 36980772 | PMCID: PMC10047204
Go to all (103) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (3)
- (1 citation) ClinicalTrials.gov - NCT01307267
- (1 citation) ClinicalTrials.gov - NCT00664898
- (1 citation) ClinicalTrials.gov - NCT01103635
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity.
J Autoimmun, 95:77-99, 31 Aug 2018
Cited by: 99 articles | PMID: 30174217 | PMCID: PMC6289740
Review Free full text in Europe PMC
Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function.
J Immunother, 34(3):236-250, 01 Apr 2011
Cited by: 82 articles | PMID: 21389874 | PMCID: PMC3063939
OX40/OX40 ligand and its role in precision immune oncology.
Cancer Metastasis Rev, 43(3):1001-1013, 25 Mar 2024
Cited by: 3 articles | PMID: 38526805 | PMCID: PMC11300540
Review Free full text in Europe PMC
Striving for synergy: how to improve cancer immunotherapy through multiple agonist costimulation.
Immunotherapy, 5(12):1271-1273, 01 Dec 2013
Cited by: 1 article | PMID: 24283834
Funding
Funders who supported this work.
DOD grant (2)
Grant ID: W81XWH-11-1-0345
Grant ID: NIH T32 AI78903
NCI NIH HHS (3)
Grant ID: R01 CA102577
Grant ID: R01 CA122701
Grant ID: CA122701
NIAID NIH HHS (2)
Grant ID: T32 AI078903
Grant ID: T32 AI78903
NIH grants (2)
Grant ID: CA122701
Grant ID: RO1 CA102577





