Abstract
Free full text

The Warburg effect version 2.0
Abstract
When fighting cancer, knowledge on metabolism has always been important. Today, it matters more than ever. The restricted cataloging of cancer genomes is quite unlikely to achieve the task of curing cancer, unless it is integrated into metabolic networks that respond to and influence the constantly evolving cancer stem cell (CSC) cellular states. Once the genomic era of carcinogenesis had pushed the 1920s Otto Warburg’s metabolic cancer hypothesis into obscurity for decades, the most recent studies begin to support a new developing paradigm, in which the molecular logic behind the conversion of non-CSCs into CSCs can be better understood in terms of the “metabolic facilitators” and “metabolic impediments” that operate as proximate openings and roadblocks, respectively, for the transcriptional events and signal transduction programs that ultimately orchestrate the intrinsic and/or microenvironmental paths to CSC cellular states. Here we propose that a profound understanding of how human carcinomas install a proper “Warburg effect version 2.0” allowing them to “run” the CSCs’ “software” programs should guide a new era of metabolo-genomic-personalized cancer medicine. By viewing metabolic reprogramming of CSCs as an essential characteristic that allows dynamic, multidimensional and evolving cancer populations to compete successfully for their expansion on the organism, we now argue that CSCs bioenergetics might be another cancer hallmark. A definitive understanding of metabolic reprogramming in CSCs may complement or to some extent replace, the 30-y-old paradigm of targeting oncogenes to treat human carcinomas, because it can be possible to metabolically create non-permissive or “hostile” metabotypes to prevent the occurrence of CSC cellular states with tumor- and metastasis-initiating capacity.
Over the past 30 y, oncology research has been dominated by a genocentric view of discovery. Information is no longer a bottleneck to understanding the ultimate genetic complexity of cancer diseases, because we have not yet reached a plateau in the advances in genome sequencing and editing. However, the process of discovery and the approval of new oncology drugs have stagnated during the past decade. Today, although a large catalog of human cancer genomes exists, the success rate of new drugs reaching cancer patients is extremely low, with the regulatory agencies approving only a small proportion of the drugs entering phase I clinical trials.1-3 Cancer remains one of the leading causes of death in Europe and the US, and as a consequence of the aging population, the incidence of cancer is rising (as demonstrated in the cancer fact sheet4 published on the World Health Organization website). Although the US Food and Drug Administration (FDA)’s Critical Path Initiative5 was launched in 2004 to streamline the development process of new drugs, the US FDA has not approved a single novel agent in oncology in the last two years, and the success rate of major oncology-pharma and -biotechs is lower than ever. The lack of success in the current cancer drug-discovery progress is further exacerbated by the typical cytostatic, transient response to novel single-pathway-targeting drugs, which has resulted in an unacceptable situation, wherein we are progressing into more personalized approaches for providing effective cancer therapy in a post-genomics world. Rather than recurrently boosting genomics and other human “cancer-omics”-project-like approaches, such as cancer proteomics and cancer epigenomics, “to drive innovation in the scientific processes through which oncology products are developed […],”5 perhaps we should consider that the 30-y-old paradigm of targeting cancer genes to treat human carcinomas is reaching its end.
Because cancer is a very complex, multifactorial disease, it has been assumed that cancer solutions will be generated exclusively by large, expensively managed projects rather than simple approaches.6 Complementary, we hypothesize that it is time to revisit the 1920s and remember Otto Warburg’s original, simple metabolic hypothesis for cancer, which states that
“the prime cause of cancer is the replacement of the respiration of oxygen (oxidation of sugar) in normal body cells by fermentation of sugar.”
This thinking might unexpectedly clarify the modern hypotheses in cancer pathophysiology, one of which states that the molecularly distinct subpopulation of so-called cancer stem cells (hereafter CSCs) are responsible for many, if not all, aspects of tumorigenesis, including the clinical failure of the majority of available oncology therapies and the existence of persistent, deadly metastases. We now propose a new version 2.0 of the Warburg effect that incorporates bioenergetics to the operational properties of CSCs (Fig. 1).
The Warburg effect version 2.0: Coloring an old, black and white picture. Because cancer is a very complex, multifactorial disease, it has been erroneously assumed that cancer solutions will be generated exclusively by large, expensive managed projects rather than simple approaches. Instead, it is time to revisit the 1920s and remember Otto Warburg’s original, simple metabolic hypothesis for cancer, which states “the prime cause of cancer is the replacement of the respiration of oxygen (oxidation of sugar) in normal body cells by fermentation of sugar.” This thinking would now clarify the modern hypotheses in cancer pathophysiology, one of which states that the molecularly distinct subpopulation of so-called CSCs are responsible for many, if not all, aspects of tumorigenesis, including the clinical failure of the majority of available oncology therapies and the existence of persistent, deadly metastases.
The End of a 30-Year-Old Paradigm for Targeting Cancer Genes to Cure Cancer
Current cancer drug discovery: The genome scenario
The fact that regulatory agencies such as the US FDA have not approved a single novel oncology therapy agent in the last two years reflects our inability to discover and develop magic-bullet therapies against the thousands of genes that can be mutated in every cancer type. More than 100,000 point cancer mutations have been identified to date, and more than 350 of these mutations are thought to influence the cancer phenotype.7-12 Targeted therapies, i.e., drugs developed to interfere with specific cancer gene products, have been a dominant research focus in cancer biology over the past 30 y but have translated into few novel oncology treatments. Several explanations may support the current state of cancer research. First, most cancer genes are tumor suppressors, which are either inactivated or completely lost in tumor cells; tumor suppressors are not druggable targets for the easily designed small molecules that are commonly developed to block oncoprotein hyperactivity. Second, many oncogenic gains-of-function, including those for the commonly deregulated non-enzymatic transcription factors, are also difficult to target. Third, most newly identified candidate cancer genes, including genes that represent good drug targets, exhibit very low mutation frequencies, indicating that these genes may represent passenger noise in genetically unstable tumors. Indeed, part of the difficulty in identifying appropriate drug targets is that passenger mutations greatly outnumber the bona fide mutations that are required for cancer development, called driver mutations. For example, recent genome analyses of 500 primary breast cancer samples revealed 27,000 point mutations; however, less than 0.01% of these mutations are thought to represent potential drivers.12 The development of most common cancers requires multiple driver mutations; therefore, these cancers generally develop slowly over many years. Thus, the mutations that facilitate an essential malignant function early in cancer development may no longer be required at the relatively late stage when the tumor is clinically detected.13 These results may compromise the current efforts of the International Cancer Genome Consortium in coordinating a global genotype assessment of 500 tumor samples from 50 cancer types.14 The genotyping for each set of tumor samples is estimated to cost 20 million dollars, which totals over 1 billion dollars for all 50 cancer types, and will facilitate the identification of driver mutations that occur at frequencies as low as 3%.15 With the identification of new genetic cancer subtypes, up to 1,000 tumor samples from each cancer type should be genotyped to identify all of the clinically relevant driver mutations.16 From a cancer drug discovery perspective, it is not surprising that heavy operational bias exists toward targeting signaling pathway kinases; however, because oncogenic networks maintain essential features for robustness, such as modularity and redundancy,16 signaling pathway kinases are subject to the rapid onset of resistance phenomena via compensatory pathways. In this regard, the approximately 10 y and 1 billion dollars required to bring an oncology therapy to Phase III (at which point the therapies often fail) raise questions regarding the value of the hundreds of potential new therapies we are accumulating through our ever-growing integrative capacity to distinguish driver from passenger genetic mutations. The development of additional molecular therapies could place an immense strain on our healthcare systems.
The wrong scenario: We are missing the target
In the 1970s, a new paradigm emerged with respect to the origin of epithelial malignancies. In a manner similar to hematological tumors, malignant stem cells, with their aberrant capacity for auto-renewal and differentiation, were implicated as the drivers of the origin, growth and metastatic dissemination of most epithelial carcinomas. Since then, an ever-growing number of studies have repeatedly suggested the existence of molecularly distinct subpopulations of cancer cells that have been termed tumor-initiating cells, tumor-propagating cells or CSCs. This subset of cancer cells are exclusively able to propagate tumor growth, and they have the ability of self-renewal and differentiate into multiple cell lineages, the two hallmark characteristics of normal stem cells (hereafter SCs), thus crucially contributing to the phenotypic and functional heterogeneity of a diverse array of cancer types.17-20 CSCs have been shown to be more resistant to a variety of conventional therapies with hormones, radiation, chemotherapeutic agents and also to molecularly targeted drugs; the differential behavior of CSCs in their responsiveness to treatment may largely explain the differences in the efficacy of therapy among cancer patients with comparable tumors by histopathological analysis. Together with their ability to colonize distant organs, CSCs have been therefore proposed to be ultimately responsible for the clinical failure of the majority of currently available oncology therapies and for metastases that appear after surgical treatment and/or systemic therapy of primary tumors.21-25
CSCs are also made not just born
Carcinogenesis involves the accumulation of numerous oncogenic events over long periods of time. In tumors that originate from tissues with high epithelial cellular turnover, only normal SCs, with their innate self-renewal capacity, remain in the tissue long enough to accumulate the oncogenic alterations necessary to support a complete transformation. Accordingly, adult SCs have been hypothesized to represent the cells-of-origin in cancer, because they can be directly targeted with CSC initiation events. Progenitor cells may similarly gain the ability to self-renew through oncogenic transformation. In this traditional view of a one-way CSC hierarchy, all of the cancer cells within a tumor arise from special self-renewing CSCs. However, although CSCs obviously exhibit the SC properties of self-renewal and differentiation, they do not necessarily originate from the transformation of normal tissue SCs or progenitor cells. Individual tumors generally harbor multiple phenotypically or genetically distinct CSCs because differentiated, either normal or non-SC tumor cells can gain CSC cellular properties, e.g. drug refractoriness via the activation of still partially known paths to stemness.26-31 The unstable CSC phenotype continuously evolves and can be switched on or off in response to cell-intrinsic or microenvironmental cues including therapeutics. The inherent plasticity of the CSC phenotype implies that eliminating CSCs alone may not effectively cure tumors, as they can be regenerated from non-CSCs, calling for dual targeting therapeutic regiments. Stochastic transitions of drug-responsive CSCs may also produce non-CSCs that lack the major CSC attributes and that can therefore remain hidden from drugs that are aimed to attack the CSC self-renewing machinery.
Targeting CSCs: We aim for hidden and moving targets
Although recent breakthroughs have highlighted the importance of some subsets of non-druggable transcription factors and epigenetic modifiers in the establishment, maintenance and reprogramming of CSC-like cellular identities, few anti-CSC drugs have been identified, and none have been approved for clinical use. Virtually all CSC-related drug discovery studies have genocentrically focused on ultimate causes, which generally involve the molecular mechanisms by which some cells elicit all of the unique properties that are commonly associated with the CSC cellular phenotype, such as immortality, self-renewal and cross-resistance to multiple treatments. However, because normal and non-CSC tumor cells can spontaneously convert to CSCs, i.e., CSCs are also made and not just born, an emerging consensus in the field is that the cellular state rather than the cellular phenotype is crucial to defining and investigating CSCs. This model indicates that, whereas CSCs can likewise differentiate into non-CSCs, non-CSCs may also be reprogrammed and converted to CSCs, suggesting the existence of a dynamic interconversion between CSCs and non-CSCs cellular states that is controlled by cell-autonomous and non-cell autonomous mechanisms (Fig. 2). The balance of the CSCs/non-CSCs interconversion could be shifted in one direction or another in response to intrinsic and/or microenvironmental signals, which may in part account for the frequency of CSC representation between different tumor types or within a tumor progressing through different stages. Such plasticity of the CSC cellular state is absent in the conventional depiction of the stem/progenitor cell hierarchy within a normal tissue being transformed into one-way CSCs hierarchy, and has revolutionary changed our current perception of CSCs biology. Finally, but still crucially important, a subset of CSCs appears to be exclusively responsible for metastatic spread, which is the final step in 90% of all fatal solid tumors. If an initial genetic defect in multi-potent adult SCs is not the sole mechanism for the generation of dynamically evolving CSC reservoirs, it follows that primary CSCs are not necessarily identical to metastatic CSCs.
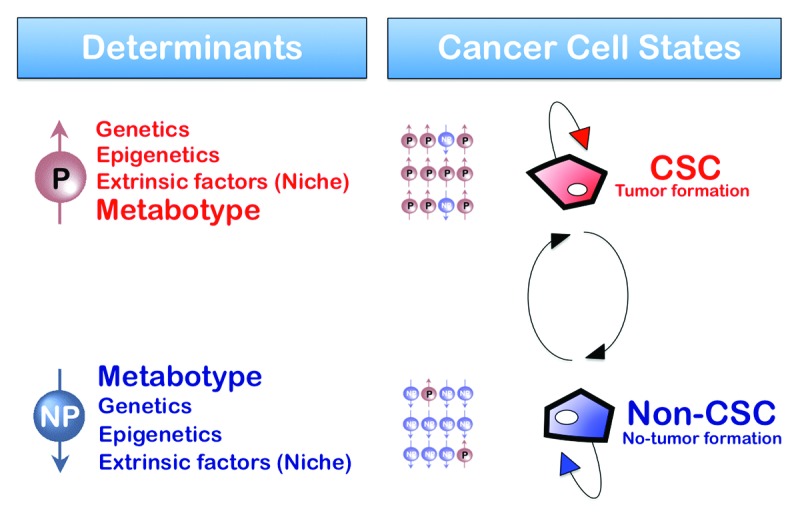
Figure 2. Metabolic reprogramming: A new parameter in the plasticity of the tumor cell differentiation-state that dictates the transition rates between non-CSC and CSC cellular states. Bidirectional interconversion exists between non-CSC and CSC cellular states. Any convergence toward phenotypic equilibrium in cancer cell state proportions likely occurs due to specific cancer cell state interconversion within tumors. Although the ultimate mechanisms stabilizing phenotypic proportions remain unclear, the transition rates between cell states, which vary across distinct cancer cell populations, have been suggested to largely depend on the intrinsic degree of differentiation-state plasticity dictated by genetic, epigenetic and/or microenvironmental (cell niche) parameters. Indeed, current descriptions of cancer cell states, i.e., the set of characteristics that are informative for the transition between two cell states of interest, consist mostly of the levels of gene expression or the levels of epigenetic methylation or acetylation of DNA or chromatin. The final dimension of the non-CSC/CSC transitions is therefore mostly “hard-wired” by the genome. Our current model hypothesizes that the metabotype is an additional characteristic that, in addition to genetic determinants, epigenetics and extrinsic factors (niche), causally determines either “permissive” (P) or “non-permissive” (NP) features that lastly determine the number of additional requirements to gain the self-renewal capacity, potency and tumorigenicity possessed by CSC cellular states.
A Metabolic Causation of CSC-Like Cellular States: A New Paradigm in Cancer Research
If normal, non-CSC tumor cells, CSCs and metastatic CSCs are flexible entities that can change state, how can we resolve the apparently impossible problem of discovering cancer drugs that specifically target CSC cellular states? Although it has been largely assumed that chemotherapy enriches residual cancer with CSCs, an accelerated production of new CSC populations from mature, differentiated cancer cell origins would also be concordant with the data. As self-renewal and differentiation capabilities are acquired traits, it might seem unwise to attack markers of the CSC phenotype either alone or in combination with debulking standard-of-care agents. Directly targeting the CSC machinery, wherever is located, could be a promising strategy. Counterintuitively, therefore, the existence of a unique, shared molecular hallmark that would be amenable to drug therapy among the CSCs of all carcinomas and/or the distinct sub-types of a single type of tumor should be investigated. In this regard, CSC-driven malignant progression might be envisioned as an evolving spatiotemporal heterogeneous structure that cannot be driven solely by irreversible genetic alterations;32-35 instead, CSC-driven malignant progression must be additionally determined by novel, non-genetic dimensions that operate as the proximate causes of the CSC cellular states (Fig. 2).
Under the assumption that the molecular biology of the stemness transformation itself would be the sole credible target in CSCs, we propose that Warburg’s thinking that reprogramming of the bioenergetic cancer cell state should be considered the metabolic switch defining the “root of cancer”36,37 almost a century ago might now clarify the immediate cause(s) through which the cells-of-origin in cancer appropriate the core machinery to support self-renewing cell divisions that are generally restricted to normal SCs and, as a result, become CSCs. Previously, scientists believed that metabolic changes were a mere consequence of the aberrant cancer cell growth. However, the most recent experimental approaches from our laboratory begin to indirectly suggest that metabolic reprogramming of CSCs may have cancer-causing activity.38-48 By viewing metabolic reprogramming of CSCs as an essential characteristic that allows dynamic, multidimensional and evolving cancer populations to compete successfully for their expansion on the organism, we now argue that CSCs bioenergetics might be another cancer hallmark.
Metabolic reprogramming and cancer: Chutes and ladders
The Canadian scientist Tak W. Mak, a world authority on the use of transgenic mice for cancer research, has recently stated that the “time has come to look at cancer in a different way, concentrating on processes that affect all tumor cells, or at least a majority, rather than an individual molecule […] treatments that target common features of cancer such as the metabolic abnormalities that have been known for nearly 80 years definitely hold the possibility of helping more patients less expensively”.49,50 To better understand Professor Mak’s assertions, we must revisit the advancements and setbacks that have occurred along the time path of cell metabolism and cancer investigation, which certainly resembles the ancient Indian board game Chutes and Ladders. If the arrival at the end of the board at the hundredth square means achieving a definitive understanding of the true molecular nature and clinical relevance of the unique CSCs’ metabolic infrastructure and functioning, the previous journey is full of scientific breakthroughs representative of a “ladder,” and bad deeds (ironically, just from a metabolic perspective!) representative of a “chute.” The game movements began almost 100 y ago.
The Otto Warburg hypothesis: Cancer is a metabolic disease
At the end of the 1920s, Otto Warburg first described the anomalous character of energy metabolism in tumor cells.36,37 Cancer cells were found to reprogram glucose metabolism and to display an intrinsic method of producing energy or ATP, by activating a bioenergetic state called aerobic glycolysis. In this process, the glycolytic pathway is favored over mitochondrial oxidative phosphorylation (OXPHOS) as the primary form of energetic metabolism even in the presence of oxygen. The Otto Warburg hypothesis, which states that the endogenous respiration defects of cancer cells, accompanied by the development of enhanced aerobic glycolysis during carcinogenesis, are a metabolic switch defining the “root of cancer” (Fig. 3), has persisted for many years (ladder!). However, when radioactive tracer techniques were employed to study the metabolism of radioactively labeled glucose into carbon dioxide in the 1950s, it became apparent that tumor tissues could also oxidize glucose at rates comparable with those observed in normal tissues. In addition, mutant mitochondrial proteins did not play pivotal functional roles, because most tumor tissues were found to contain functional respiratory enzymes and coenzymes, a functional citric acid cycle and a normal coupling of oxidation with ATP formation (chute!).
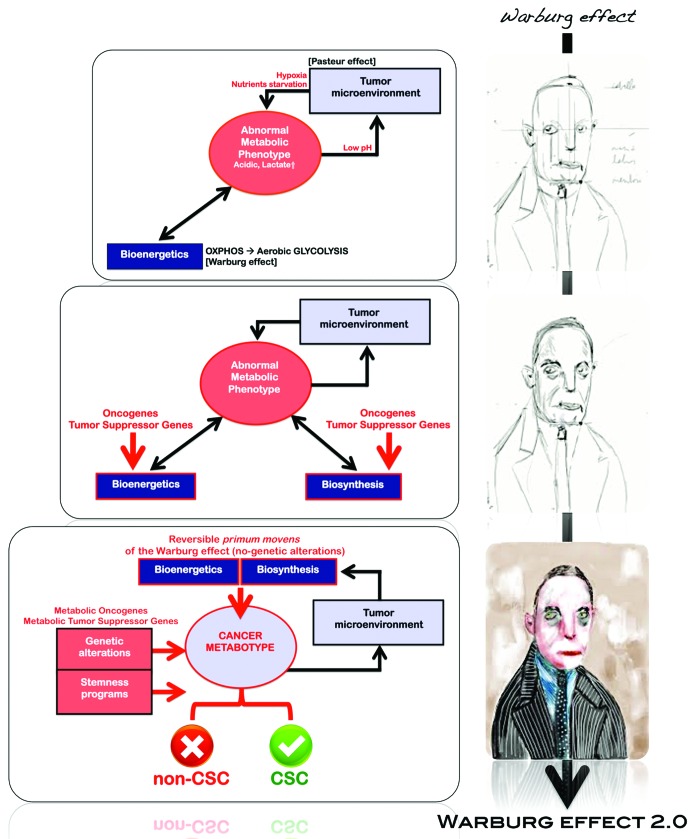
Figure 3. The Warburg effect: An evolutionary perspective. Exogenous microenvironmental factors (e.g., hypoxia, oxidative stress, extracellular matrix detachment and nutrient starvation) and intrinsic genetic determinants (e.g., oncogenes, tumor suppressor genes and epigenetics) converge to coordinately determine the induced and/or inherent activation status of the Warburg phenotype in tumor tissues. Once cancer cells reach the oxygen diffusion limit, they become hypoxic, and they can be directed either to cell death or adaptation to a glycolytic phenotype. In this state, tumor cells may adapt a Warburg phenotype as the need for complementary bioenergetic resources is satisfied by the ability of the glycolysis to generate not only energy but also building blocks for anabolic (biosynthetic) metabolism. As a consequence of glycolysis, lesions become acidotic and may require additional adaptation through resistance to apoptosis by upregulation of cellular anabolism (e.g., lipogenesis). The constitutive stimulation of regulatory cascades leading to the switch from mitochondrial OXPHOS to aerobic glycolysis (Warburg effect) can also be driven in response to early changes in certain metabolic oncogenes and tumor-suppressor genes (e.g., K-Ras, p53, PI3K, AKT, Myc), which have evolved to regulate cancer cell metabolism, as they ensure the activation and maintenance of the cancer-associated bioenergetics and biosynthetic metabolic pathways, including the Warburg effect. CSC cellular states emerging from these extrinsic/intrinsic convergent sequences should have a powerful growth and survival advantage, as the metabolic reprogramming of CSCs chronically exposed to an unstable oxic-hypoxic and nutrient- and growth factor-starved environment in pre-malignant and/or early stages of carcinogenesis is later maintained and likely enhanced in primary and metastatic malignancies with the appearance of the integrated circuits, in which aberrant metabolic activity reinforces the gene networks and transcriptional signatures, thereby driving a distinct bioenergetic/biosynthetic CSC cell state. We now propose that the Warburg effect is a/the “permitted” cell metabotype possessing the necessary plasticity to reprogram the tumor cell of origin so that it can acquire the cellular state of a CSC. Conversely, the intrinsic and extrinsic genetic/epigenetic factors that control the paths-to-CSC properties cannot operate in non-Warburg “protected” cell metabotypes. Previously, scientists believed that commonly observed Warburg-like metabolic changes were a mere consequence of the aberrant cancer cell growth within tumor tissues. We now propose for the first time that metabolic reprogramming of CSCs has cancer-causing activity. Such a new definition of the Warburg effect would make it possible to metabolically create non-permissive (or “hostile”) metabotypes to prevent the occurrence of cellular states with tumor- and metastasis-initiating capacities.
Hanahan and Weinberg’s cancer hallmarks: Moving metabolism from the cause of cancer to the consequence of cancer
The arrival of the molecular era in the 1990s rapidly emphasized that changes in energy metabolism, although universally recognizable in tumors from different tissue types, should no longer be regarded as the major root of cancer. In the former version of the 2000s landmark article in which Hanahan and Weinberg synthesized a large amount of information and condensed the molecular characteristics of a cancer cell into a single conceptual framework,51 the metabolic transformation that generally accompanies tumorigenesis was not among the six selected hallmarks of cancer. The tumor genetics and transduction pathway signatures were the exclusive parameters considered in producing their formal molecular-cancer disease model. Because Vogelstein’s multistep model of human tumor pathogenesis was used to define the cancer hallmark capabilities, all of the cancer cell attributes, i.e., self-sufficiency in growth signals, insensitivity to anti-growth signals, apoptosis evasion, limitless replicative potential, sustained angiogenesis and tissue invasion and metastasis, were thought to be necessary “in order for a cell to become cancerous.” A metabolic understanding of cancer was regarded as an out-of-date reductionist perspective of the one-size-fits-all approach; it was therefore rejected (chute!). Indeed, under the assumption that all aspects of tumor biology, from proliferative progression to invasion and metastasis, should be the result of a continuous optimization process governed by Darwinian dynamics (i.e., the Warburg effect must impart a selective advantage on the cells that possess such a phenotype), it may seem an evolutionary and bioenergetic conundrum to state that it is significantly advantageous for cancer cells to utilize glycolysis when oxygen is abundantly present. The use of glycolysis would not seem advantageous in these conditions, because aerobic glycolysis is 18-fold less efficient than mitochondrial OXPHOS in producing ATP.
Understanding cancer’s unorthodox metabolism within cancer orthodoxy
If cancer progression occurs via Darwinian somatic evolution, the inefficient, toxic glycolytic phenotype should be selected against after cancer cells secure access to blood vessels and normoxic environments. However, this result has not been observed; metastatic cancer cells are commonly found to express the glycolytic phenotype and to produce copious lactic acid irrespective of oxygen accessibility. Moreover, metabolic reprogramming occurs in a fully oxygenated state for leukemia. Renewed interest in Warburg’s metabolic model of cancer has occurred in the last decade due to the realization that the adoption of a less-efficient cancer metabotype by tumor cells is counterintuitive when solely explained in terms of ATP production. The elegant mathematical models and empirical observations of Gillies and Gatenby52-55 have established that tumor cells’ elevated glucose consumption is necessary, because carcinogenesis itself takes advantage of the elevated production of toxic glycolysis by-products, such as lactate. Increased glucose consumption in invasive and metastatic lesions is not used for substantial energy production (ATP) as is commonly assumed; rather, the glucose is used to produce acid, which gives the cancer cells a competitive advantage for invasion. Warburg aerobic glycolysis is elevated in tumors, because it invariably produces a toxic, acidic environment that, given that the acidic environment is more toxic to adjacent tissue compared than to the cancer cells themselves, represents an evolutionary advantage to cancer cells vis-à-vis the normal stroma into which they invade (ladder!). The recent milestone proposal by Vander Heiden, Cantley and Thompson emphatically rekindled interest in the Warburg effect in cancer.56 By answering the question “does aerobic glycolysis arise in cancer cells merely because it is the most favorable metabotype for all rapidly proliferating cells with high glucose uptake capacity?,” the authors illuminated how tumor cell metabolism is apparently adapted to facilitate the uptake and incorporation of nutrients into the biomass needed to produce a new cell. Because that cell division phenomenon requires substantial levels of cellular macromolecules, the bulk of the glucose cannot be committed to carbon metabolism for ATP production via mitochondrial OXPHOS. As most of the carbon, nitrogen, free energy and reducing equivalents required to support cell growth and division are supplied by only two molecules, glucose and glutamine, and because the synthesis of nucleotides, amino acids and lipids consumes more equivalents of carbon and NADPH (nicotinamide adenine dinucleotide phosphate, reduced) than ATP, the conversion of all of the glucose to CO2 via mitochondrial OXPHOS to maximize ATP production will drastically impair the needs of a proliferating cell.
A metabolic rewriting of the Hanahan and Weinberg cancer hallmarks
Cancer cells definitely possess requirements that extend beyond ATP. The Warburg effect, a necessary part of the glucose- and glutamine-addicted metabolism in tumor cells, can be correctly redefined in terms of the obligatory dependence of the tumor cells to rapidly produce other metabolic end products in an unrestricted manner; therefore, the cancer cells are self-autonomous within the generally starved microenvironmental conditions in growing solid tumors.57-60 The Warburg effect allows for the diversion of key metabolites into the major cellular biosynthetic pathways (amino acid synthesis, nucleic acid synthesis and de novo fatty acid biogenesis), thus maximizing the production of macromolecules and organelles in proliferating cancer cells.57-60 Considering that the Warburg effect involves a major replacement of the metabolic program that operates in most normal tissues to fuel the physiological operations of cancer cells, it might be tempting to suggest that aerobic glycolysis should gain the status of a cancer hallmark (ladder!). However, proteins that are somehow involved in programming the bona fide core hallmarks of cancer can also orchestrate the redirection of energy metabolism. As a result of oncogenic gain-of-function events or the loss of tumor suppressors primarily affecting the PI3K/AKT/mTOR/HIF axis and the p53 system, respectively, a stereotyped pattern of metabolic changes can be induced, including glucose transport, glycolysis, lactate production, reduced OXPHOS and lipid synthesis.61 Viewed in this way, the Warburg effect is simply an evolutionarily conserved target that is programmed by most common proliferation-inducing “metabolic oncogenes” and “metabolic tumor-suppressors,” thus raising the question of whether the deregulation of cellular energy metabolism is a core hallmark capability of cancer cells that is as fundamental as the six well-established core hallmarks (Fig. 3). When Hanahan and Weinberg revisited the hallmarks of cancer in 2011,62 they added the two following categories: “enabling characteristics,” which do not necessarily cause cancer but at least assist cells in acquiring cancer hallmarks in a transition from normal to malignant status (like tumor-promoting inflammation), and “emerging hallmarks,” which may not be pervasive in all cancers. Metabolism reprogramming was designated as an emerging hallmark to highlight both its evident importance and the unresolved issues surrounding its functional independence from the core hallmarks (chute!).
Metabolic reprogramming of CSCs: Engineering a new-dimensional cancer hallmark
To resolve the worst-case scenario in cancer biology research and oncology drug discovery, we must reconsider our current genocentric guidelines regarding the molecular classification and treatment of human carcinomas. In light of the recent cluster analyses of driver mutations showing that several of these mutations converge on metabolic pathways58,63 and considering the aforementioned findings revealing that bona fide cancer hallmarks can indeed impinge on cancer metabolism, Professor Mak’s statement is relevant in that, in cancer research, we are erroneously “putting the carts (driver gene mutations) before the horses (aberrant metabolism).” However, if we target specific alterations in cell metabolism as part of the mere development of the cancer phenotype, like radiation and chemotherapies do with the proliferative rate, should we not expect an analogous failure on the way to successfully treating cancer?
Beyond the increasingly recognized capability of cancer-associated metabolic reprogramming to contribute to the acquisition of the six bona fide cancer hallmarks, the a priori energetic infrastructure of the cells-of-origin in cancer may represent a key feature for optimal routing to CSC cellular states. The most recent studies begin to support a new developing paradigm in which the molecular logic behind the conversion of cells-of-origin into CSCs can be better understood in terms of the “metabolic facilitators” and “metabolic impediments” that operate as openings and roadblocks, respectively, for the transcriptional events and signal transduction programs that ultimately orchestrate the intrinsic and/or microenvironmental paths to CSC-like cellular states (Fig. 3). If this scenario proves true, we should be able to delineate the self-autonomous cell metabotype that is compatible with the operational properties exclusively owned by CSCs. Subsequently, we should be able to pharmacologically impose a normalized metabolic environment for the required pro-immortalizing metabotype that fuels the induction of cancer stemness, pluripotency and differentiation, thus endowing non-CSCs with an energetic infrastructure that is protected against the reprogramming events that lead to CSC cellular states.
Nobel Prize-winning iPSCs: The paradox and paradigm of cancer metabo-stemness
The most recent Nobel Prize in Medicine, which was awarded to Gurdon and Yamanaka for their genial contribution to somatic cell reprogramming to produce SCs, has underlined revolutionary consequences for regenerative medicine and provided alternative strategies in cancer research. Yamanaka-like reprogramming technologies have been developed to revert normal somatic differentiated cells into patient-specific iPSCs that can be differentiated into different lineages. Yamanaka’s method involves the transient expression of a set of four transcription factors (Oct4, Sox2, Klf4 and c-Myc) into cells; these factors have been demonstrated as critical for stemness and cell differentiation. Several of these reprogramming factors, which can “reset” the epigenetic status of the cells and allow them to adopt a new plethora of possible fates,64-66 were previously known for their oncogenic activity. Because a fundamental principle of cell biology is that the SCs with greater potentials for self-renewal and pluripotency will also have a higher probability of causing tumors, much research in the field has focused on the tumorigenic traits of iPSCs to facilitate the development of safe, tumorigenic-free iPSCs-based therapies.67-69 Indeed, due to the fact that remarkable similarities exist between the processes that lead to the acquisition of oncogenic CSC cellular states and the reprogramming of somatic cells to induced pluripotency,70,71 an improved understanding of the molecular details underlying the pluripotency reprogramming process has provided crucial insights into the mechanisms by which CSCs might arise.
Oncogenesis and iPSCs generation are closely related processes
During tumorigenesis, both the activation of cancer-promoting oncogenes and the loss of tumor suppressor genes destabilize the normal transcriptome of the target cell, which, coupled with epigenetic alterations that directly depend on oncogenic changes or secondary to tumor progression, leads to the partial or total loss of the cell’s original identity. One or several permissive/selective steps are also required to permit the cell to bypass the safety mechanisms that prevent survival in the presence of unfavorable conditions, such as DNA damage and uncontrolled growth. The generation of a CSC cellular state additionally requires the re-acquisition of self-renewal and asymmetric division capacities. Induced pluripotency similarly requires specific combinations of collaborating oncogenes/tumor suppressor genes that can efficiently produce a less-differentiated cell that can proliferate and self-renew indefinitely. The generation of iPSCs by the expression of stem cell-specific genes is negatively regulated by the activation of cell-intrinsic, cancer-protective programs such as p53- and p16INK4a-driven senescence, i.e., the major tumor-suppressive mechanism that must be also overcome during malignant transformation of normal epithelial cells.72-80 Escaping senescence or acquiring immortality is a crucial, rate-limiting step toward the establishment of iPSCs, further underscoring the similarities between tumorigenesis and induced pluripotency. Both oncogenesis and induced pluripotency are similarly more efficient when the cell of origin is closer to the SC state. When oncogenes, transcription factors or onco-microRNAs induce differentiated cells to reprogram into iPSCs, these cells spontaneously form teratocarcinomas when transplanted into nude mice due to the presence of an undifferentiated compartment of “teratoma-initiating” iPSCs.81-83 Thus, one of the primary assays of stemness is also a tumor assay, which clearly illustrates the strong link between CSCs, reprogramming and tumorigenicity.
Metabolic reprogramming enables the generation of induced pluripotency
iPSCs certainly offer a unique system for studying the molecular and cellular mechanisms underlying the process of de novo generation of CSC cellular states. This process is initiated when committed cells overcome certain cellular barriers, such as tumor suppressor pathways, e.g., oncogene-induced senescence (OIS), which also act as critical roadblocks against reprogramming, e.g., reprogramming-induced senescence (RIS), to acquire an indefinite self-renewal capacity and a certain degree of pluripotent plasticity. If CSC-driven tumorigenesis is closely associated with the acquisition of SC-like properties in induced pluripotency, determining the mechanisms that positively regulate the efficiency and kinetics of somatic reprogramming to iPSCs may provide a proof-of-concept validation for the novel self-renewing tumor-initiating mechanisms that regulate both the number and aberrant functionality of CSCs. Intriguingly, the latest discoveries in the field have revealed that iPSCs appear to share all of the core components of metabolic reprogramming that have been commonly observed in human tumors.
The enforced aerobic glycolysis (the Warburg effect) that typically accompanies the metabolic reprogramming of tumor tissues is a fundamental iPSC phenotypic trait
Somatic mitochondria within iPSCs drastically alter their morphology and functionality to acquire an immature organelle shape with underdeveloped cristae and low oxidative stress levels. These changes directly affect the cellular bioenergetic profile, which is shifted from OXPHOS to glycolysis upon reprogramming and returns to OXPHOS during the subsequent redirection of iPSC differentiation into defined lineages. Importantly, the glycolytic phenotype is necessary for and promotes the optimal routing of somatic cells to pluripotency; the stimulation of aerobic glycolysis favors, while the blockade of glycolytic enzymes blunts, reprogramming phenomena.84-90 These findings appear to establish cell bioenergetics as a novel pre-requisite of acquired stemness. Accordingly, an experimental model comparing oncogenic transformation and cellular reprogramming has recently confirmed that somatic cells must first acquire changes that lead to the downregulation of cell differentiation machinery combined with the upregulation of glycolysis and other glycolysis-related metabolic pathways.91 Only then do the oncogenic transformation/induced pluripotency pathways diverge, which depends on other factors, such as the activity of pluripotency genes.
iPSCs couple the Warburg effect to anabolic metabolism to fully mimic metabolic reprogramming in cancer cells
As above-mentioned, the Warburg effect has been correctly redefined in terms of the obligatory dependence of the tumor cells to maximize the production of macromolecules and organelles, because aerobic glycolysis, but not OXPHOS, allows for the rapid and efficient diversion of key metabolites into the major cellular biosynthetic pathways in proliferating cancer cells.56-59 Similar to biologically aggressive carcinomas and CSCs,92-95 we have recently demonstrated that iPSCs supercharge lipogenesis by triggering regulatory circuits that activate and provide substrates for the key lipogenic enzymes, fatty acid synthase (FASN),48 a highly NADPH-consuming metabolic strategy that facilitates the Warburg effect by rapidly generating NAD+ and avoiding low NAD+/NADH ratios that would eventually inhibit glycolysis. Like cancer cells, iPSCs may also evoke a high rate of lipid synthesis to overcome the detrimental effects of toxic free radical generation, because the de novo synthesized monounsaturated lipids are less susceptible to lipid peroxidation, thus cooperating with the suppression of the mitochondria-related oxidative stress pathway.
The a priori bioenergetics of somatic cells correlates with the reprogramming efficiencies
A bioenergetic shift from somatic oxidative mitochondria toward an alternative glycolytic phenotype maximizes the efficiency of iPSCs generation. Somatic cells that demonstrate oxidative:glycolytic energy production ratios that are closer to those of pluripotent SCs reprogram more rapidly and efficiently.88-90 Crucially, iPSCs glycolytic capacity relate to their intrinsic tumorigenic and differentiation potential. The inclusion of the c-Myc oncogene potentiates the pluripotent glycolytic behavior and the tumorigenic incidence of derived iPSCs;96 conversely, c-Myc removal decreases the tumorigenicity of iPSCs and facilitates OXPHOS-dependent lineage commitment and terminal differentiation.97
The reprogramming-competent metabotype of iPSCs involves the sole two-primum movens that account for OXPHOS inhibition and promote the acquisition of the Warburg effect in human carcinomas without requiring mutations in cancer genes
First, iPSCs notably downregulate the expression of the catalytic subunit of the AMP-activated protein kinase (AMPK),86,98 a master metabolic switch that senses and decodes intracellular changes in the energy status.99-101 iPSCs suppress the activation of AMPK to avoid anabolic inhibition and escape the growth-arresting effects of AMPK, which is similar to phenomena commonly observed in cancer cells. AMPK underexpression is frequently observed in human carcinomas, and AMPK inactivation promotes epithelial cell carcinogenesis. AMPK activity opposes tumor development, and the loss of AMPK activity fosters tumor progression, in part, by regulating the cellular metabolic pathways that support cell growth and proliferation. Crucially, AMPK has recently been shown to suppress tumor growth in vivo by negatively regulating the Warburg effect.102 Second, the H+-ATPase synthase-geared metabolism switch, a mitochondria-mediated energy adaptation that is sufficient to promote the acquisition of the Warburg effect in tumor cells,103-108 is employed during somatic reprogramming to limit the bioenergetic activity of mitochondria and shifting the metabotype to enhanced aerobic glycolysis. Somatic cell reprogramming involves a significant increase in the expression of the ATPase inhibitor factor 1 (IF1) accompanied by extremely low expression levels of the catalytic β1-F1-ATPase subunit.48 Mitochondrial H+-ATPase is repressed at both the activity and protein levels in human carcinomas. IF1, which limits the activity of the H+-ATPase, is negligibly expressed in normal tissues and highly overexpressed in numerous carcinomas. The cellular content of the H+-ATPase, which directly correlates with mitochondrial OXPHOS activity and inversely correlates with the rate of glucose utilization by aerobic glycolysis, is transcriptionally and translationally limited in cancer cells. In iPSCs, AMPK activity has been found to regulate the same mechanisms that limit the activity and level of the mitochondrial H+-ATPase in cancer cells,48 thus linking the two-primum movens that establish the Warburg effect in the absence of genetic alterations.
Pharmacological prevention of metabolic reprogramming eliminates tumorigenic traits in iPSCs
If the Warburg effect is a reversible trait, because it can be acquired without any genetic alteration, as repeatedly hypothesized for cancer cells, the cancer-like metabolic reprogramming of iPSCs could be pharmacologically targeted to impede the undesirable stemness-tumorigenicity coupling. Accordingly, direct and indirect AMPK activators have been shown to impose a normalized metabolic effect that differs from the required pro-immortalizing glycolysis that fuels the induction of stemness and pluripotency, endowing somatic cells with an energetic infrastructure that is protected against reprogramming.45 The metabolic barrier imposed by AMPK-activating strategies cannot be bypassed even through p53 deficiency, a fundamental mechanism that greatly improves the efficiency of stem cell-production. The activation of an anabolic metabolism like de novo fatty acid biogenesis is also an instrumental event that enables the reprogramming of somatic cells to iPSCs, because the pharmacological inhibition of FASN activity is sufficient to markedly decrease reprogramming efficiency.48 The activation of AMPK alone, which drastically impacts the OXPHOS:aerobic glycolysis ratio, fully reverses the high IF1/β-F1-ATPase ratio and suppresses the anabolic phenotype in iPSCs.48 Perhaps more importantly, AMPK activation is sufficient to fully recapitulate the ability of an increased dosage of tumor suppressors (like p53 and Ink4a/ARF) to prevent the occurrence or drastically reduce the size and weight of teratoma-like masses following the transplantation of iPSCs into immunodeficient mice.47,83 The pharmacological prevention of Warburg-like metabotypes therefore facilitates the specific elimination of teratoma-initiating pluripotent stem cells that are intermixed with the desired, non-tumorigenic iPSC derivatives that terminally differentiate into multiple lineages (Fig. 4).47,109 The close affinity of normal tissue SCs and CSCs can be problematic for the increased possibility of off-target effects of CSC-based anticancer therapies. Differentiating between the specific characteristics of normal tissue SCs and CSCs may be an essential requisite for assessing the best treatment targets for CSCs while minimizing sequelae. The specific, efficient elimination of the malignant iPSCs that are responsible for the teratocarcinoma growth strongly supports the proposal that the metabo-stemness hallmark is a previously unrecognized, indispensable component of the CSC machinery.
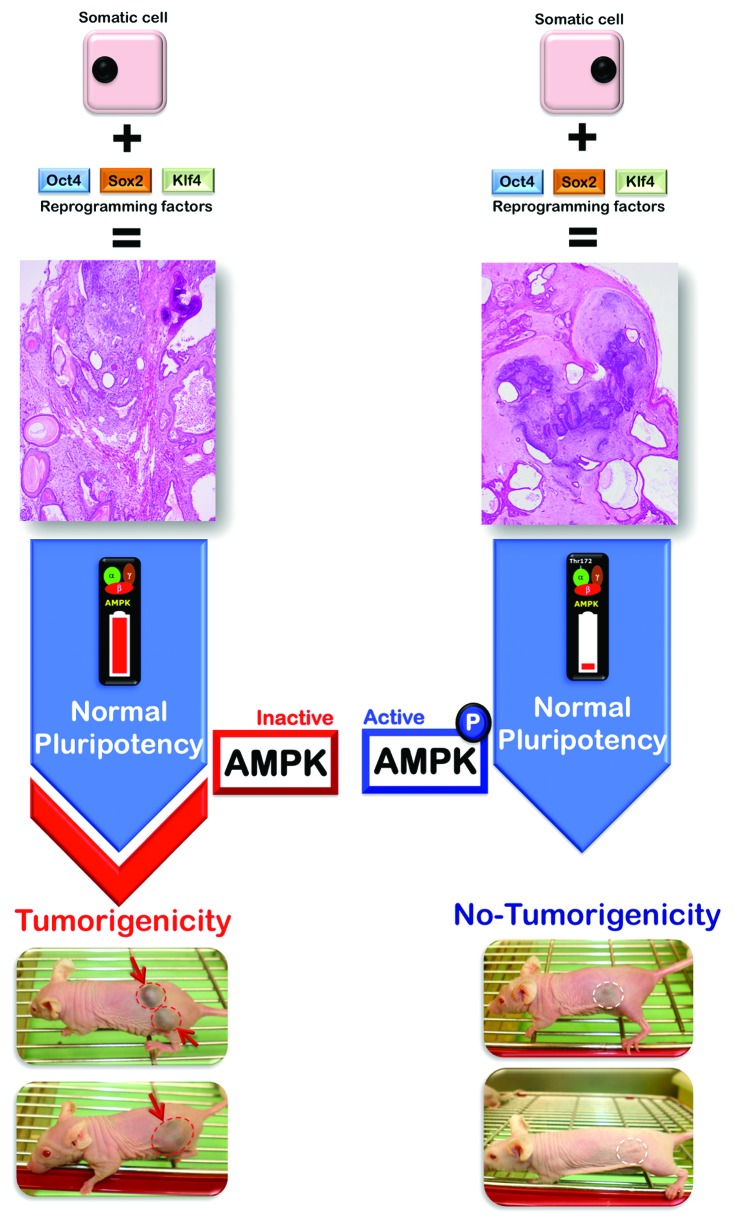
Figure 4. The metabo-CSC attribute might uncouple tumorigenesis from normal pluripotency. The close affinity of normal tissue SCs and CSCs is expected to be problematic for the increased possibility of off-target effects of CSC-based anticancer therapies. In this regard, the CSC metabotype (infrastructure and functioning) appears to show unique features that are pivotal for SC-related tumorigenesis but are dispensable for adult SC development in mature tissues. Our recent discovery of the specific, efficient elimination of the malignant teratoma-initiating iPSCs47 that are responsible for the teratocarcinoma growth strongly suggest that the metabo-CSC attribute is a previously unrecognized, indispensable component of the CSC machinery that differentiates between the specific characteristics of normal tissue SCs and CSCs, thus providing an essential requisite for assessing the best treatment targets for CSCs while minimizing undesirable sequelae.
Metabolic reprogramming of the CSC cellular states: A working model for a new cancer hallmark
Within an individual tumor, a bidirectional conversion exists between the non-CSC and CSC cellular states. Therefore, a convergence toward a phenotypic equilibrium, such as the heterogeneous tumor tissue that a pathologist would classify and an oncologist would treat, likely occurs due to highly dynamic cancer cell-state interconversions. The transition rates between the cell states, which can vary across distinct cancer cell populations, has been considered to largely depend on the degree of the differentiation-state plasticity dictated by genetic and epigenetic parameters. Cancer, cellular plasticity and cell fate reprogramming are tightly intertwined processes, because not all cancer cells possess the necessary plasticity to permit their reprogramming to CSC cellular states, and only some cancer genes possess, in the right cellular context, the required capacity to fully elicit the reprogramming process. Because the occurrence of parallel metabolic changes in oncogenesis and the induction of pluripotency support the notion that cell reprogramming is a naturally occurring phenomenon and not just a technology, it is reasonable to suggest that (as observed with iPSCs) cell bioenergetics can operate as the pivotal decision-making parameter during the reprogramming acquisition of SC properties in normal and non-CSC tumor cells. The individual ability of the cell of origin to enter reprogramming at different time points after reprogramming induction and the length of time required to complete the reprogramming sequence could be affected by the a priori bioenergetic and anabolic status. With the lack of an appropriate metabotype, fewer cells would undergo the required stochastic genetic and epigenetic events to proceed to a fully reprogrammed state of acquired self-renewal and pluripotency. The possession or intrinsic ability to develop a Warburg-like/anabolic metabotype represents a very early, crucial molecular event that imposes an a priori barrier on the path of transformation from somatic differentiated cells to CSCs. This process does not necessarily require the participation of oncogene-driven metabolic changes (Fig. 3). In this scenario, the remodelling of bioenergetic and biosynthetic metabolism is an active component (rather than a consequence) that defines cell fate, and both the intrinsic flexibility and reversibility may have revolutionary implications for the pharmacological manipulation of the self-renewal and pluripotent capabilities fuelling CSC-driven tumorigenesis.
Metabolic reprogramming of CSC cellular states: A therapeutic corollary
In the past, cancer genomics theories had pushed Warburg’s metabolic hypothesis of cancer into obscurity. We now propose the possibility of delineating a metabolic roadmap for malignant stemness that might not require pre-existing mutations or rearrangements of proto-oncogenes or tumor suppressor genes. Identifying the metabotypic infrastructure and the metabotype functioning of CSCs will add a novel dynamic dimension to well-recognized cancer hallmarks. In the clinic, the metabo-CSC attribute will be the basis to rapidly pursue unique therapeutic approaches that target the addiction of CSCs to certain metabolic infrastructures and metabolic fluxes, i.e., the CSC metabotype (Fig. 5). This novel approach might generate a shift in the theory of oncology, resulting in a new era of metabolo-genomic-personalized cancer medicine. The opportunities and challenges for targeting the metabolic infrastructure of CSCs might be rapidly achieved, because existing metabolic drugs may be easily repositioned from pre-clinical stages to clinical approaches. The drugs that would arise from the knowledge-based drug repositioning strategies selected to metabolically suppress the frequency and/or functionality of CSCs could silently operate as “cancer tissue sweepers” of dysfunctional cells capable of initiating and propagating tumors while sparing their normal counterparts.
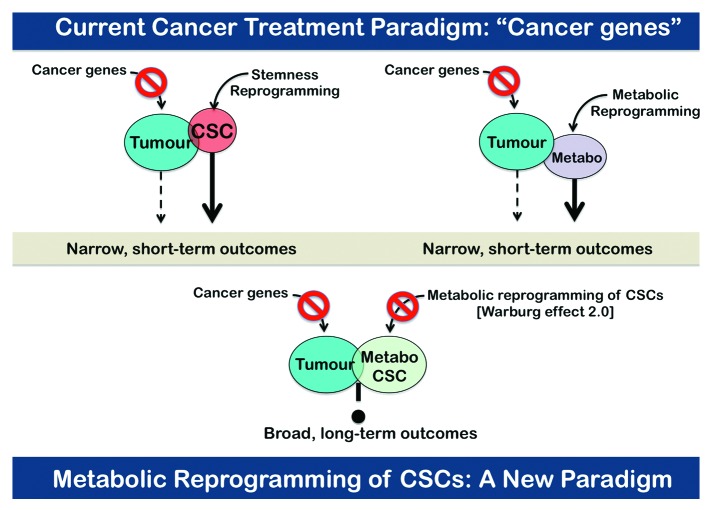
Figure 5. The metabo-CSC paradigm in cancer therapeutics. Identifying the metabotypic infrastructure of CSCs will add a novel dynamic dimension to well-recognized cancer hallmarks. In the clinic, the cancer metabo-CSC attribute will be the basis to rapidly pursue unique therapeutic approaches that target the addiction of CSCs to certain metabolic infrastructures and metabolic fluxes, i.e., the CSC metabotype. The opportunities and challenges for targeting the CSC metabolic infrastructure can be rapidly achieved, because existing metabolic drugs currently used for treatment of diabetes, obesity or cardiovascular diseases might be easily repositioned from the evolutionary pre-clinical stages to revolutionary clinical approaches in cancer.
In “The Prime Cause and Prevention of Cancer,” a lecture delivered at the 1966 annual meeting of Nobelists at Lindau, Germany, Otto Warburg stated, “Cancer, above all other diseases, has countless secondary causes. But, even for cancer, there is only one prime cause […]” “During the cancer development, the oxygen-respiration always falls, fermentation appears, and the highly differentiated cells are transformed to fermenting anaerobes, which have lost their body functions and retain only the now useless property of growth.” If Otto Warburg were alive today, he would be surely lecturing on “CSCs that turn back the clock, in metabolic terms, to share some properties with embryonic SCs, notably self-renewal and differentiation capacities, which are aberrantly utilized both in the acquisition of pluripotency and in tumorigenesis,” i.e., the Warburg effect version 2.0.
Acknowledgments
This work was financially supported by the Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain, grants CP05-00090, PI06-0778 and RD06-0020-0028), the Fundación Científica de la Asociación Española Contra el Cáncer (AECC, Spain) and the Ministerio de Ciencia e Innovación (SAF2012-38914, SAF2009-11579, Plan Nacional de I+D+ I, MICINN, Spain). Alejandro Vazquez-Martin received a Sara Borrell post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria -FIS-, Spain). Sílvia Cufí received a research fellowship (Formación de Personal Investigador, FPI) from the Ministerio de Ciencia e Innovación (MICINN, Spain).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24479
References
Articles from Cell Cycle are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.4161/cc.24479
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.4161/cc.24479?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4161/cc.24479
Article citations
Role of Autophagy and AMPK in Cancer Stem Cells: Therapeutic Opportunities and Obstacles in Cancer.
Int J Mol Sci, 25(16):8647, 08 Aug 2024
Cited by: 1 article | PMID: 39201332 | PMCID: PMC11354724
Review Free full text in Europe PMC
Mitochondrial Dysfunction, Its Oxidative Stress-Induced Pathologies and Redox Bioregulation through Low-Dose Medical Ozone: A Systematic Review.
Molecules, 29(12):2738, 08 Jun 2024
Cited by: 0 articles | PMID: 38930804
Review
Microglia and macrophage metabolism: a regulator of cerebral gliomas.
Cell Biosci, 14(1):49, 17 Apr 2024
Cited by: 2 articles | PMID: 38632627 | PMCID: PMC11022384
Review Free full text in Europe PMC
Histone lactylation bridges metabolic reprogramming and epigenetic rewiring in driving carcinogenesis: Oncometabolite fuels oncogenic transcription.
Clin Transl Med, 14(3):e1614, 01 Mar 2024
Cited by: 3 articles | PMID: 38456209
Review
Colorectal Cancer Stem Cell Biomarkers: Biological Traits and Prognostic Insights.
Curr Pharm Des, 30(18):1386-1397, 01 Jan 2024
Cited by: 0 articles | PMID: 38623972
Review
Go to all (111) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cancer Stem Cell-Associated Pathways in the Metabolic Reprogramming of Breast Cancer.
Int J Mol Sci, 21(23):E9125, 30 Nov 2020
Cited by: 21 articles | PMID: 33266219 | PMCID: PMC7730588
Review Free full text in Europe PMC
Reprogramming of central carbon metabolism in cancer stem cells.
Biochim Biophys Acta Mol Basis Dis, 1863(7):1728-1738, 11 May 2017
Cited by: 46 articles | PMID: 28502706
Review
Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway.
Cell Cycle, 12(18):3109-3124, 21 Aug 2013
Cited by: 71 articles | PMID: 23974095 | PMCID: PMC3875684
Basal/HER2 breast carcinomas: integrating molecular taxonomy with cancer stem cell dynamics to predict primary resistance to trastuzumab (Herceptin).
Cell Cycle, 12(2):225-245, 15 Jan 2012
Cited by: 40 articles | PMID: 23255137 | PMCID: PMC3575452





