Abstract
Objectives
(1) Detailed analysis of diffusion tensor imaging (DTI) parameters (fractional anisotropy and radial diffusivity) to evaluate white matter integrity in the corpus callosum (CC), and (2) examine correlations between DTI data and performance on multiple measures of cognitive functioning.Participants
Twelve individuals with a history of complicated mild, moderate, or severe traumatic brain injury (TBI) who were an average of 1.7 years postinjury and 12 control participants.Main measures
Standardized and experimental neuropsychological tests; detailed analysis of DTI parameters.Results
The TBI group demonstrated DTI values suggesting decreased white matter integrity and correlations with severity of injury. Both groups showed correlations between DTI parameters and cognitive measures, with more significant correlations observed for the TBI group. White matter changes in the CC were evident chronically and were related to severity of injury.Conclusions
Diffusion tensor imaging parameters suggesting disruptions in white matter in the CC may be implicated in impaired performance, both in terms of cognitive tasks and reaction time, after TBI.Free full text

Corpus Callosum Integrity and Neuropsychological Performance after Traumatic Brain Injury: A Diffusion Tensor Imaging Study
Abstract
Objective
To conduct a detailed analysis of DTI parameters [fractional anisotropy (FA) and radial diffusivity (RD)] to evaluate white matter integrity in the corpus callosum (CC), and to examine correlations between DTI data and performance on multiple measures of cognitive functioning.
Method
12 participants with a history of complicated mild, moderate, or severe TBI, and 12 control participants completed both standardized and experimental neuropsychological testing and an FMRI session, including DTI.
Measures
Detailed DTI analysis examined between-group and within-group comparisons of DTI parameters and demographic information, as well as measures of episodic memory and executive functioning.
Results
Differences were found between groups such that the TBI group demonstrated DTI values suggesting decreased white matter integrity, and correlations with injury severity. Both groups showed correlations between DTI parameters and cognitive measures, with more significant correlations observed for the TBI group. White matter changes in the CC were evident chronically, and were related to severity of injury.
Conclusions
DTI parameters suggesting disruptions in white matter may be implicated in impaired performance, both in terms of cognitive tasks and reaction time, after TBI.
Introduction
Traumatic brain injury (TBI) is a major public health concern in the U.S., with approximately 1.7 million injuries occurring each year.1 Of those injured yearly, approximately 275,000 individuals sustain moderate to severe injuries requiring significant medical care and often resulting in long-term functional impairment.2 While brain injuries are quite heterogeneous, it is generally accepted that the most common pathophysiological change persisting in the chronic phase of survival after TBI is diffuse axonal injury (DAI).3,4 DAI refers to the chronic disruption of axons as either the direct, or more typically indirect, effect of mechanical trauma5,3 and it has been implicated as a key issue related to persistent cognitive problems following TBI.6,7 Despite such significant impact on functional outcome after TBI, acute clinical scans are rarely able to demonstrate this type of injury definitively. The opportunity to evaluate white matter integrity after TBI in a more detailed manner currently requires the use of imaging techniques, such as diffusion tensor imaging (DTI), which are currently used primarily in research, and not yet widely used clinically.
DTI capitalizes upon the biophysical principle that the diffusion of water molecules in living tissue is anisotropic, meaning that the molecules have a tendency to move along the direction of the axon fibers in white matter tracts.8 The primary dependent variable resulting from DTI analysis is an index of fractional anisotropy (FA). Because diffusion occurs primarily in a linear direction along myelinated white matter tracts, as compared to areas that are less structurally organized or myelinated, higher FA values are used as an index indicating greater integrity of white matter tracts. Radial diffusivity (RD) is also often reported, and is an index of diffusivity perpendicular to the axon fiber direction. It is thought that higher RD values may indicate abnormalities in myelination along the axonal tracts.8
In choosing the focus of DTI studies after TBI, it is helpful to consider the areas of the brain most likely to be impacted by this type of injury. It is known that the gray-white matter junction and midline brain structures are particularly vulnerable to DAI, with the corpus callosum and dorsolateral midbrain reported to be the most frequently impacted.9,10 The corpus callosum (CC) is a thick tract of white matter known to be critical for interhemispheric communication. It is often grossly considered as having three major anatomic sections, with the anterior portion referred to as the “genu”, followed by the midsection (“body”) and the posterior part referred to as the “splenium”11 (though others propose further subdivision12). It has been asserted that injury to the CC may be the best single imaging correlate of injury severity13 and at least one volumetric study found a group of people with TBI to have a smaller mean callosal volume than matched controls.14 Together, these studies suggest that the CC might be a prime area on which to focus a study of chronic white matter changes after TBI.
Initial DTI studies of persons with TBI have focused on the presence and location of white matter findings and general measures of outcome. For example, Arfanakis and colleagues studied a group of 5 individuals who underwent DTI scanning within 24 hours after mild head injury.15 Their findings indicated reduced FA in areas vulnerable to axonal injury, including the CC, even when conventional MRI findings were normal. These findings were replicated and expanded in a larger cross-sectional sample of both acute and chronic participants with a diagnosis of mild TBI.16 Multiple studies utilizing DTI to study DAI after injury have now shown evidence of decreased FA in the CC of TBI groups as compared to controls.16,17,18,19 Ljungqvist and colleagues found changes in FA to be stable over time in a longitudinal study of eight patients with suspected DAI.20 When correlating with outcome measures, whole brain FA has been shown to be strongly related to initial severity of injury and length of posttraumatic amnesia.21 Sidaros and colleagues also found FA in the CC to be most strongly associated with functional outcome using the FIM and Glasgow Outcome Scale (GOS).22 These studies together suggest that DTI is a useful tool in evaluating injury to the CC, even when injury may not be apparent on clinical imaging, and may further suggest that these microscopic changes are related to injury severity and outcome.
Given that injury to the CC is common, the opportunity to study how injury to such a prominent white matter tract may impact cognition among persons that survive TBI is of high value. Recent DTI studies in the TBI population have included cognitive status, however, very few have directly studied the CC in relation to cognition. Salmond and colleagues studied persons with moderate and severe TBI in comparison to a group of matched healthy controls.23 Their findings demonstrated a relationship between reduced FA and measures of learning and memory. In a study that included participants with chronic mild, moderate or severe TBI diagnoses, FA was significantly related to measures of executive control and declarative memory.17 In addition, when specific white matter tracts were evaluated, reduced FA was noted in the TBI group in specified areas, including the CC. The authors also stressed that white matter changes were observed across the spectrum of injury severity. More recently, Warner and colleagues also found FA to be related to processing speed, learning, memory and executive functioning in an adult sample with DAI24. Another recent study evaluated 15 individuals with severe and diffuse TBI and a group of 16 matched controls.25 A group comparison indicated whole brain reductions in FA for the TBI group as compared to controls. Region of interest (ROI) analyses indicated positive correlations between performance on a working memory task and FA reductions in multiple areas, including the CC. Performance on a declarative memory task also correlated with FA values in the CC. Similar findings have been reported when the outcomes in question are more visuospatial in nature.26 Finally, correlations between DTI measures of the CC and outcomes have also been reported in studies describing pediatric TBI, including a test of inhibition and working memory and academic skills.27,28
In summary, the available DTI literature indicates that, after TBI, evidence of decreased white matter integrity consistent with injury has been noted both in whole brain analysis, as well as in specific areas known to be vulnerable to injury such as the CC. Further, these findings have been correlated with general outcome measures, and more recently, with cognitive measures. The purpose of our current study was to build on this literature by conducting a detailed analysis of DTI parameters (both FA and RD) in the CC, and to explore how these data correlated with both traditional neuropsychological tests, as well as a reaction time-based test of episodic memory. To our knowledge this is the first study which included specific analysis of reaction time-based memory measure, as well as more traditional neuropsychological testing, adding a unique perspective on functional outcome after injury. Based on the literature reviewed above, we expected to find differences between individuals with TBI and healthy controls, such that those with TBI would have decreased FA and increased RD in comparison with healthy controls. We expected DTI findings to correlate with neuropsychological testing results, such that DTI indices associated with disruptions in white matter integrity would be associated with reduced performance on cognitive measures. The overarching goal of this study was to gain new insights which may lead to improved treatments in the future, for persons with TBI.
Method
Participants
Participants consisted of 24 right-hand-dominant individuals who provided informed consent. The TBI group consisted of 2 women and 10 men who had sustained severe, moderate, or complicated mild TBI. Glasgow Coma Scale29 (GCS) scores were used as a measure of injury severity. For inclusion purposes, “complicated mild” was defined as injuries rating a GCS score of 13–15 with positive CT findings.30.31 Individuals with complicated mild injuries were included given that previous studies indicate that the cognitive and neuroimaging data for those with this level of injury are similar to those with moderate injury, as compared to those with uncomplicated concussion.30 The average best GCS score in the first 24 hours after injury for persons in the TBI group was 10.5 (SD = 4.2). Persons with TBI sustained injuries between 1 and 3 years prior to participation (average time post-injury = 1.7 years, SD = 0.58 years), with no reported history of previous TBI, and no disclosed litigation. The main cause of injury was motor vehicle accident (58.3%). Anatomic neuroimaging results from the TBI group included findings consistent with DAI, hemorrhagic contusions, subdural hematoma, and subarachnoid hemorrhage (see Table 1 for a description of demographic information). It is relevant to note that none of the persons with TBI were described in acute medical records as having a focal injury to the CC. To be included, persons with TBI needed to perform at least 1 SD below normative levels on at least one memory index from the California Verbal Learning Test - II (CVLT-II).32 The purpose of this criterion was to recruit participants with TBI who had a measurably lower performance on a test of episodic memory. This behavioral screening criterion was included to increase homogeneity in a population that will always be heterogeneous if compared solely on injury variables, by showing that the sample behaved in similar ways on our cognitive feature of interest. In comparison, to ensure that those in the control group did not have a measurable deficit in episodic memory, in order to be included, controls could perform no lower than 1 standard deviation below normative levels on any index from the CVLT-II. The uninjured control group consisted of 2 women and 10 men with no history of TBI. Persons in the control group were recruited via flyers and postings on the university’s clinical research website. They received the same screening questions and neuropsychological measures as persons in the TBI group. Controls were matched to persons with TBI for age, gender, and years of education. All participants were free from any other neurological history, psychiatric history or alcohol abuse.
Table 1
Demographic and Injury Information for Participants with TBI
| Gender | Age (Yrs) | Education (Yrs) | Time Post-Injury (Yrs) | Best in 24hr GCS | Injury Cause | Injury Type |
|---|---|---|---|---|---|---|
| M | 35.97 | 12 | 1.92 | 7 | MVC | DAI |
| M | 18.83 | 12 | 1.14 | 15 | Sports Injury | IPH & SDH |
| M | 31.28 | 15 | 2.17 | 7 | MVC | DAI & IVH |
| M | 24.61 | 10 | 1.23 | 7 | MVC | SAH, SDH, IPH |
| F | 20.66 | 15 | 2.36 | 15 | FALL | EDH & IPH |
| M | 23.80 | 14 | 2.23 | 11 | MVC | SAH |
| M | 54.94 | 16 | 1.06 | 7 | MVC | SAH, SDH, IPH, IVH |
| M | 41.37 | 18 | 1.11 | 11 | Falling Object | SAH, SDH, IPH |
| M | 30.26 | 16 | 2.02 | 15 | MVC | SAH, SDH, IVH |
| F | 24.12 | 16 | 1.08 | 14 | MVC | SAH, IPH, SDH |
| M | 28.23 | 16 | 1.38 | 3 | MVC (ped) | SDH, IPH, EDH |
| M | 53.07 | 16 | 2.61 | 14 | FALL | IPH & IVH |
Note. MVC = motor vehicle collision DAI= diffuse axonal injury; IPH=intraparenchymal hemorrhage; SDH=subdural hemorrhage; IVH=intraventricular hemorrhage; SAH=subarachnoid hemorrhage; EDH=epidural hemorrhage; ped = pedestrian
Materials and Procedure
Neuropsychological tests included several screening measures, as well as assessments of verbal learning and executive functioning. It was ensured that participants could comprehend and follow instructions using The Token Test.33,34 The Wechsler Test of Adult Reading (WTAR)35 was used to provide an estimate of general ability level. The Michigan Alcoholism Screening Test (MAST)36 was used to screen for current alcohol abuse. Finally, emotional status was screened using The Brief Symptom Inventory (BSI).37 No participant had to be excluded based on performance on these measures. The two groups did not show significant differences in responses on the Token Test, the MAST or the WTAR (all ps > 0.10). BSI performance, as reflected by the Global Severity Index, did not reach clinically significant levels for either group, though the groups were significantly different (GSI controls: M = 44.25, SD = 10.7; TBI: M = 58.83, SD = 12.33).
Episodic memory variables were assessed using the CVLT-II. This test has been shown to be valid and reliable, and has been well studied in the TBI population.38,39,40,41 Executive functioning was also examined in this study using the Wisconsin Card Sorting Task (WCST),42.43 Trail Making Test (TMT),44 Controlled Oral Word Association Test (COWAT),45 Similarities from the Wechsler Adult Intelligence Scale –III (WAIS-III),46 and the Stroop test.47
A behavioral task examining episodic memory was also administered as part of a larger functional magnetic resonance imaging (fMRI) study.48,49 Briefly, participants were presented with two dimensional pictures and words as well as control tasks, and asked to judge whether each individual object was pleasant or not based on their own criteria (encoding). Immediately after completion of each encoding block, participants were presented with a mixed block of previously seen objects along with new items, and asked to differentiate between “new” and “old” items (recognition). Reaction time in milliseconds was recorded for all responses during the encoding and recognition trials. Response accuracy was also recorded for the recognition trials.
Image acquisition
All participants completed a scanning session of approximately 75 minutes, comprised of structural, diffusion tensor imaging (DTI), and functional (fMRI) sequences during the episodic memory paradigm.48,49 DTI data were acquired on a Siemens 3T Allegra scanner using echo-planar imaging (EPI) sequences (matrix= 128×128×34, FOV 200, slice thickness 3mm) consisting of seven transaxial images collected in 6 gradient directions at a b-value of 850 with one b=0 weighted image. Structural scans acquired during these sessions included axial T2 weighted images (39 contiguous 3mm slices, TR = 6440 ms, TE = 73 ms, 256 × 256 matrix, FOV = 200mm, flip angle = 150°) and a sagittal 3-D MPRAGE sequence (224 contiguous 0.78 mm slices, TR = 1680ms, TE = 2.48ms, 256×256 matrix, FOV = 200mm, flip angle = 8°).
DTI data analysis
DTIStudio50 software was used for all analyses, following a standard processing stream which included eddy current correction. DTI parameters calculated included FA and RD. All DTI images were aligned to the mean B0 image to correct for movement using a 12 parameter affine, tri-linear alignment. The CC regions of interest were created by tracing the CC in the axial plane of the FA colormaps in ROIEditor (a part of the DTIStudio program). All tracings were completed by a single rater. Regional DTI data were also examined in the subregions of the CC, including the genu, body, and splenium. The genu was traced on 5–6 slices starting with the most inferior slice on which the CC was visible. The splenium was traced in a similar fashion on 5 planes. The body was traced on the superior 4 slices on which the CC was visible.
Results
Independent samples t-tests (two-tailed) were used in comparisons of demographic variables between the groups as well as performance on neuropsychological and experimental tests. Spearman’s correlations were used to relate DTI parameters to performance on these measures. Due to the small sample size and exploratory nature of this investigation, correction for multiple comparisons was not used.
Demographics
As expected due to our matching criteria, there were no significant differences between the TBI group and healthy control group on age (controls: M = 29.8, SD = 10.8, range = 19–50; TBI: M = 32.3, SD = 12.0, range = 18–54; t (22) = −0.52, p = 0.61) or number of years of education (controls: M = 16.2, SD = 3.1, range = 12–22; TBI: M = 14.7, SD = 2.3, range = 10–18; t (22) = 1.36, p = 0.19). DTI values in the corpus callosum were significantly different between groups, with the controls exhibiting higher FA and lower RD values than the TBI group (FA: controls: M = 0.62, SD = 0.03; TBI: M = 0.56, SD = 0.04; t(22) = 4.28, p < 0.001; RD: controls: M = 0.46, SD = 0.03; TBI: M = 0.56, SD = 0.08; t(22) = −4.35, p < 0.001).
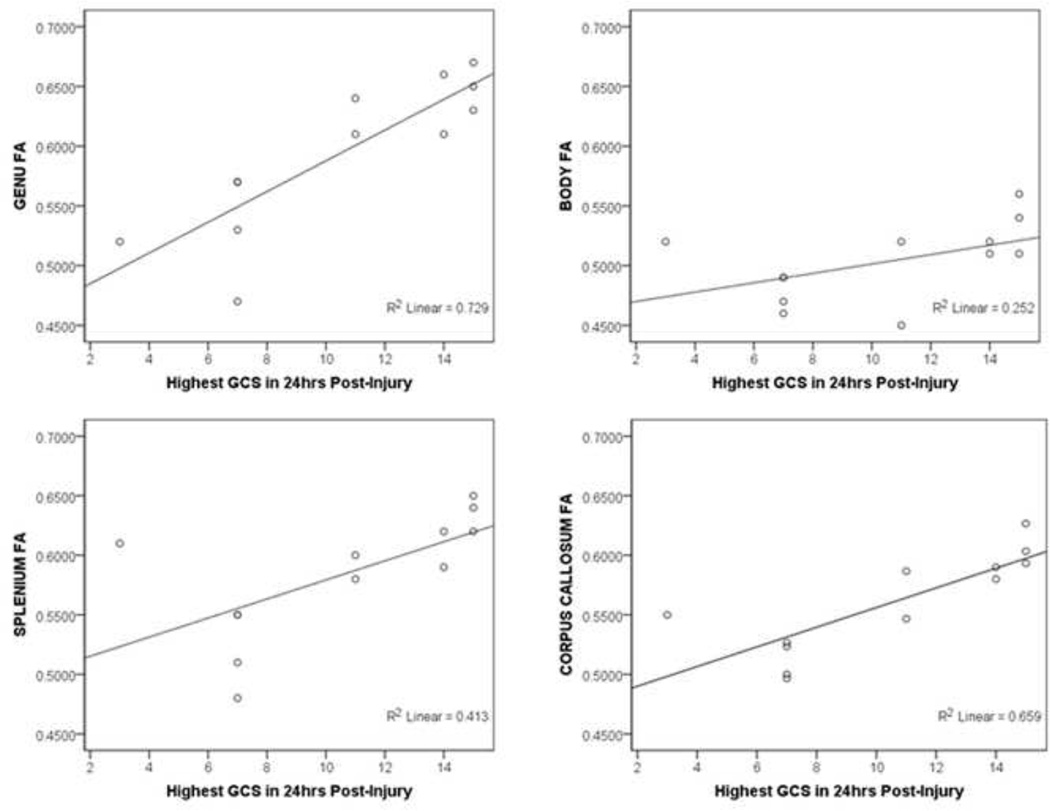
For the TBI group, injury severity, as measured by the highest GCS in the first 24 hours post-injury, was correlated with multiple DTI parameters. As shown in Figure 1, greater injury severity was associated with lower FA in the CC as a whole and specifically in the genu and splenium (FA rs(12) > 0.745, ps < 0.006). Injury severity was also correlated with higher RD in all regions of the CC (all rs(12) > −0.627, ps < 0.030). No significant correlations were found between time since injury and any DTI parameter (all ps > 0.381).
Correlations with CVLT-II variables
As mentioned above, the persons with TBI were screened to demonstrate at least a statistically below average episodic memory performance (i.e., at least 1 SD < normative mean) on at least one measure of the CVLT-II. The group profile confirmed these screening findings, revealing that the TBI group exhibited significantly lower performance than controls on nearly all scales of the CVLT-II, including Total Words (Trials 1–5), Short and Long Delay Free and Cued Recall, Repetition Errors, and Discriminability (all ps < 0.041). Recognition, False Positives, and Intrusions were not significantly different (all ps > 0.069) between groups.
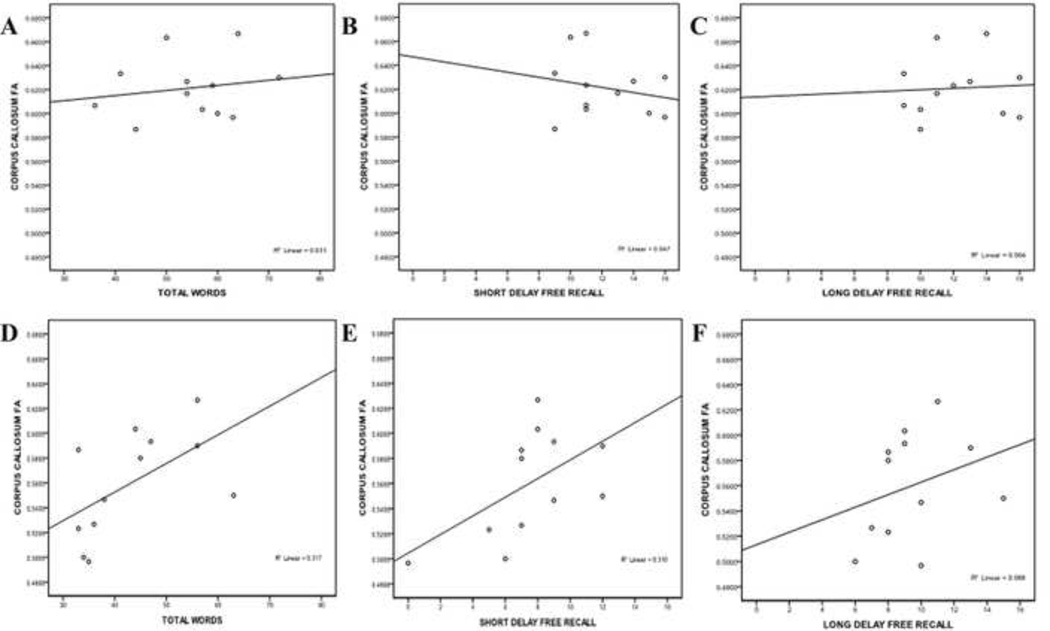
There were no significant correlations between CVLT-II variables and DTI parameters for the control group (all ps > 0.27) but significant correlations were found for the TBI group. FA in the CC was significantly positively correlated to total number of words (r(12) = 0.596, p = 0.041) and short delay free recall (r(12) = 0.627, p = 0.029). Figure 2 illustrates the differences between these factors for the two groups.
Correlations with executive measures
There were few differences found between group performance on the executive tests administered. Trails A or B time to complete, Stroop interference T score, and WCST perseverative errors T scores were not significantly different between groups (all ps>0.079). The control group, however, did significantly outperform the TBI group on verbal fluency on the COWAT CFL total score (t(22) = 3.147, p = 0.005), the Animals category score (t(22)=2.502,p=0.020), and the WAIS-III Similarities Scaled score (t(22) = 2.198, p = 0.039).
Despite a lack of group differences in performance on the Trails A or B, there was a significant correlation between performance on Trails B and DTI parameters in the TBI group but not in the control group. In the TBI group, time to complete Trails B was correlated with FA and RD in the CC (ps < 0.04). The TBI group also showed a negative relationship between time to complete Trails A and FA (r(12) = −0.81, p = 0.003), and a related positive correlation was shown between CC RD and time to complete Trails A in the TBI group (r(12) = 0.65, p = 0.03).
Associations between DTI parameters and performance in the control group on measures of executive function were less robust as compared to those observed for the TBI group. Higher FA in the CC was significantly correlated with faster time to complete Trails A (r(12) = −0.70, p = 0.04). No correlations for controls were found between either DTI measure and any other test of executive functioning.
Correlations with behavioral measures of episodic memory
There were no significant differences between groups on behavioral measures of episodic memory performance for either reaction time (RT) during encoding or accuracy and RT during recognition. No significant correlations were observed for either the TBI or control group between any DTI measure and either encoding RT or recognition ac curacy of pictures and words. Both groups did, however, exhibit correlations between both DTI parameters and RT during recognition of pictures. The TBI group also showed significant correlations between FA and RD and RT during recognition of words. In the TBI group, higher FA of the CC was significantly correlated with faster RT on recognition of words (r(12) = −0.70, p =0.01). In addition, RD in the CC was positively correlated with RT during recognition of words in the TBI group (r(12)=0.70, p=0.01). There were no significant correlations between word recognition RT and DTI parameters for the control group. In the control group, FA was negatively correlated with picture recognition RT in the CC (r(12)= −0.62,p=0.03). Picture recognition RT and CC RD were positively correlated (r(12)=0.74,p=0.006) in the control group. In comparison, for the TBI group, picture recognition RT was also negatively correlated with FA in the CC (r(12)= −0.61,p=0.047) and positively correlated with CC RD (r(12)=0.66, p=0.03).
Exploratory Regional Analyses
While the number of subjects enrolled in this study did not support extensive analysis of subregions of the CC, some exploration was undertaken for purposes of generation of future hypotheses in this area. In general, regional analyses for the control group revealed few significant correlations with cognitive measures. The TBI group, on the other hand, exhibited several significant correlations between cognitive measures and CC subregions, most notably the genu and splenium for the episodic memory tests. Splenium FA was correlated with Total Words on the CVLT-II (r(12) = 0.67, p = 0.02), and splenium RD was correlated with False Positives on the same test (r(12) = 0.61, p = 0.04). Response times for picture and word recognition in the TBI group exhibited similar results, with picture recognition being significantly correlated with splenium FA and RD, and word recognition significantly correlated with genu FA, and splenium FA and RD (all ps < 0.03).
Discussion
Due to the complex nature of TBI, multimodal paradigms provide an effective way to study the cognitive and structural changes associated with injury, and allow for the correlation of detailed neuroimaging analysis with multiple aspects of cognition. We believe that conducting studies in this manner will allow for optimal forward development of the literature, as the use of these methods allows us to gain new information, not only about changes in structure, brain activation, and function independently, but also about the interactions among these changes. In keeping with this aim, the DTI analyses presented here were conducted as part of a larger study including FMRI, DTI, and both standardized neuropsychological tests and experimental tests designed for measuring cognitive functioning as part of the fMRI paradigm.48,49 Our aims in the current study included analysis of DTI parameters within the CC, as well as the examination of how any changes observed may interact with not just cognition in general, but with distinct aspects of memory and executive functioning abilities.
Overall, the results of the current study suggested decreased white matter integrity to all regions of the CC as measured by DTI in persons with TBI in comparison to healthy controls. This was seen as a reduction in FA and an increase in RD values for all of the anatomical regions of the CC. Given that individuals in our TBI group were screened to have impairment of at least 1 SD below normative standards on an episodic memory task, these results suggest that reduction in white matter connectivity in the CC may be implicated in the mechanisms behind the reductions in cognitive functioning common after injury. DTI findings were not related to time since injury in our study. These findings are consistent with the literature indicating reduced FA in the CC both acutely15,16 and chronically16 after injury. Previous studies have also demonstrated a correlation between DTI measures and injury severity in the TBI population.21 In the TBI group of the current study, increased severity of injury was correlated with lower FA in the CC. Our results, paired with the previous literature, support suggestions that white matter changes in the CC are persistent chronically and are related to severity of injury.
The current study explored specific anatomical segments of the CC using DTI parameters, and found results consistent with another study indicating FA values in the splenium after TBI to be positively correlated with GCS.51 In addition to replicating these findings, we also observed positive correlations between FA and GCS in the genu in our TBI group
Region-specific changes in DTI parameters in relationship to cognitive performance in the TBI population have also been demonstrated in recent studies. One group observed reductions in FA of the splenium in relation to tasks involving declarative memory, while tasks involving working memory functioning were tied to DTI findings in both the genu and splenium.25 In the current study, participants completed both a standardized neuropsychological test of episodic memory (CVLT-II), as well as an experimental episodic memory task within the fMRI scanner. When DTI parameters were examined for both groups, there were no significant correlations between CVLT-II subtest scores and DTI for the control group. In comparison, for the TBI group, increased FA in the splenium was positively correlated with CVLT-II subtests for immediate recall (Total Words recalled for Trials 1 – 5), a finding which is consistent with the recent study.25 FA in the whole CC was also positively correlated with the total number of words recalled, as well as with the Short Delay Free Recall score. Increased false positives were also correlated with increased RD in the TBI group.
The experimental memory tests recorded during fMRI which were shown to be correlated with DTI measures include response times for picture recognition (both groups) and word recognition (TBI group only). Within those, both groups showed correlations between CC FA and RD and average response time (RT). Of note is that the DTI parameters associated with greater white matter disruption are associated with slower reaction times. This is consistent with reduced speed of processing, which is commonly encountered after moderate and severe TBI, and is likely to be a contributing factor to limitations in memory and other cognitive functioning.
Executive control is a complex construct, and there are many neuropsychological methods for assessing its components. In the current study, we included several measures examining how various aspects of executive control may relate to DTI parameters. Our results demonstrated differences between groups to be few, and mixed results were found among group correlations: The control group outperformed the TBI group only on tasks of both phonemic and categorical word list generation (COWAT Total, and Animals) and on a task requiring verbal abstraction (WAIS-III Similarities subtest). Despite a lack of difference in group performance on the task, within group analysis comparing DTI parameters to performance found DTI data that suggested decreased white matter integrity to be correlated with increased time to complete Trails A. Correlations between increased Trails B time to complete and DTI parameters suggesting decreased white matter integrity were observed in only the TBI group. Additionally, for the TBI group, significant correlations were not observed between DTI parameters and performance on COWAT Total, the WAIS-III Similarities Scaled Score, or Stroop Interference.. While somewhat difficult to fully interpret, these findings generally support the hypothesis that greater white matter integrity is associated with higher levels of performance on cognitive measures. In addition, we hypothesize that the differences between groups on executive measures may represent a divergence between more automatic executive control tasks and those which require greater effort. The latter, exemplified by the COWAT and semantic fluency, as well as WAIS-III Similarities, require more in the way of production and even a greater depth of processing (i.e., word generation and abstration) from the participants. Differences have been demonstrated in fMRI studies for automatic versus controlled processing in non-injured populations,52,53 suggesting a requirement for increased brain recruitment during controlled processing, which is likely to be supported by white matter integrity.
Although this project has had some interesting results, there are some limitations which should also be discussed. The data acquired in this study only utilized 6 gradient directions, the mimimum number necessary for measuring FA. This data was collected at a time when 6 directions was standard, while more current data collection methods favor higher numbers of directions in order to increase image quality. Our use of only 6 directions may have impacted our data and the conclusions we were able to draw from it. Although the number of directions was less than ideal, and may have impacted our data and the conclusions we were able to draw from it, we were still able to show correlations between measures of cognitive functioning and DTI parameters. In fact, a recent paper has suggested that six directions may be sufficient, though higher numbers of directions do provide advantages.54 Another limitation of the current study is the sample size, which, while comparable to other studies discussed in the introduction, is potentially a limiting factor in the sense that it may have obscured some differences between the groups. Finally, TBI is, by nature, heterogeneous and this is reflected in our group in terms of severity and mechanism of injury. However, we attempted to ameliorate this heterogeneity by doing behavioral screening to ensure that members of the TBI group exhibited similar performance on our main cognitive area of interest. While representative of a larger population of persons with TBI, however, this heterogeneity may have influenced our results. As we did find some correlations between DTI factors and injury severity, there is at least some reason to believe these factors are related. In future studies with larger sample sizes we plan to evaluate subgroups for further clarification of the relationships between those factors.
There may also be a limitation in using neuropsychological tests as the primary means of investigating cognitive functioning, as these do not generally provide highly specific measures of cognitive skills. In the current study, we did find similar correlations between DTI measures and both CVLT-II variables and reaction time on an experimental memory task. This converging evidence would suggest that the relationship between memory and DTI parameters in the CC is robust enough to be seen with different kinds of memory measures. Executive functioning, on the other hand, is really a term for a cluster of related abilities, so although we had multiple tests typically described as measuring “executive functioning,” we did not see similar results for all of these. More selective cognitive measures and/or multiple converging measures could make for future studies with clearer results.
Taken together, the results of the current study suggest that DTI parameters showing disruption in white matter integrity may be implicated in impaired performance, both in terms of cognitive tasks and reaction time, after TBI. Our exploratory analysis of DTI parameters in subsections of the CC further suggests that changes in CC white matter connectivity may differentially impact cognition after TBI. This unique combination of detailed analysis of DTI and cognitive measures in this study provides us with additional areas for further analysis and focus in future endeavors.
While this study only provided data for some of the relationships between DTI parameters and cognitive functioning, it appears that studying these relationships has merit. DTI has been widely cited as a method for revealing structural changes after TBI that cannot be visualized on other types of scans. With growth in the knowledge of how these changes can be related to changes in cognitive functioning, we come closer to predicting which persons with brain injury may come to exhibit cognitive deficits despite having normal acute scans. These predictions might then lead to earlier intervention and improved treatment, or might at least be integrated with existing evaluations and tests in making more accurate predictions. While we are still not at the point of being able to identify a specific marker that might suggest a unique treatment trajectory or a more focused diagnosis, such a goal may, one day, be plausible.
Acknowledgments
Sources of Funding: This research was supported in part by a grant (NIH-NINDS R01NS048178-01) awarded to Dr. Ricker. Dr. Russell was supported by 5T32HD040686 during the preparation of this manuscript.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/htr.0b013e318289ede5
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4918513?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/htr.0b013e318289ede5
Article citations
Utilization of clinical and radiological parameters to predict cognitive prognosis in patients with mild-to-moderate traumatic brain injury.
Front Neurosci, 17:1222541, 27 Jul 2023
Cited by: 0 articles | PMID: 37575301 | PMCID: PMC10412890
Microstructural Alterations in Tract Development in College Football and Volleyball Players: A Longitudinal Diffusion MRI Study.
Neurology, 101(9):e953-e965, 21 Jul 2023
Cited by: 0 articles | PMID: 37479529 | PMCID: PMC10501097
MRI factors associated with cognitive functioning after acute onset brain injury: Systematic review and meta-analysis.
Neuroimage Clin, 38:103415, 23 Apr 2023
Cited by: 3 articles | PMID: 37119695 | PMCID: PMC10165272
Review Free full text in Europe PMC
Angiotensin-(1-7) improves cognitive function and reduces inflammation in mice following mild traumatic brain injury.
Front Behav Neurosci, 16:903980, 04 Aug 2022
Cited by: 5 articles | PMID: 35990729 | PMCID: PMC9386567
Corpus callosum lesions are associated with worse cognitive performance in cerebral amyloid angiopathy.
Brain Commun, 4(3):fcac105, 26 Apr 2022
Cited by: 4 articles | PMID: 35611313 | PMCID: PMC9123849
Go to all (31) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Callosal Function in Pediatric Traumatic Brain Injury Linked to Disrupted White Matter Integrity.
J Neurosci, 35(28):10202-10211, 01 Jul 2015
Cited by: 52 articles | PMID: 26180196 | PMCID: PMC4502260
Whole brain and corpus callosum diffusion tensor metrics: How do they correlate with visual and verbal memory performance in chronic traumatic brain injury.
J Integr Neurosci, 18(2):95-105, 01 Jun 2019
Cited by: 5 articles | PMID: 31321950
Longitudinal changes in the corpus callosum following pediatric traumatic brain injury.
Dev Neurosci, 32(5-6):361-373, 14 Oct 2010
Cited by: 100 articles | PMID: 20948181 | PMCID: PMC3073757
Diffusion tensor imaging of traumatic brain injury review: implications for neurorehabilitation.
NeuroRehabilitation, 31(3):281-293, 01 Jan 2012
Cited by: 23 articles | PMID: 23093455
Review
Funding
Funders who supported this work.
NICHD NIH HHS (2)
Grant ID: 5T32HD040686
Grant ID: T32 HD040686
NINDS NIH HHS (2)
Grant ID: R01NS048178-01
Grant ID: R01 NS048178