Abstract
Free full text

Silencing Mutant ATXN3 Expression Resolves Molecular Phenotypes in SCA3 Transgenic Mice
Associated Data
Abstract
Spinocerebellar ataxia type 3 (SCA3) is a neurodegenerative disease caused by a polyglutamine expansion in the deubiquitinating enzyme, Ataxin-3. Currently, there are no effective treatments for this fatal disorder but studies support the hypothesis that reducing mutant Ataxin-3 protein levels might reverse or halt the progression of disease in SCA3. Here, we sought to modulate ATXN3 expression in vivo using RNA interference. We developed artificial microRNA mimics targeting the 3′-untranslated region (3′UTR) of human ATXN3 and then used recombinant adeno-associated virus to deliver them to the cerebellum of transgenic mice expressing the full human disease gene (SCA3/MJD84.2 mice). Anti-ATXN3 microRNA mimics effectively suppressed human ATXN3 expression in SCA3/MJD84.2 mice. Short-term treatment cleared the abnormal nuclear accumulation of mutant Ataxin-3 throughout the transduced SCA3/MJD84.2 cerebellum. Analysis also revealed changes in the steady-state levels of specific microRNAs in the cerebellum of SCA3/MJD84.2 mice, a previously uncharacterized molecular phenotype of SCA3 that appears to be dependent on mutant Ataxin-3 expression. Our findings support the preclinical development of molecular therapies aimed at halting the expression of ATXN3 as a viable approach to SCA3 and point to microRNA deregulation as a potential surrogate marker of SCA3 pathogenesis.
Introduction
Spinocerebellar ataxia type 3 (SCA3), also known as Machado–Joseph disease (MJD), is the most common form of dominantly inherited ataxia in the world.1 It is primarily characterized by neuronal dysfunction and degeneration in the cerebellum and functionally related brain regions.2 Although, some symptomatic relief can be provided, there are no effective treatments for this progressive and fatal disorder.
SCA3 belongs to the family of polyglutamine (polyQ) diseases, a group of neurodegenerative disorders in which the mutant protein contains a pathogenic glutamine repeat expansion.3 In SCA3, the polyQ expansion is encoded by a CAG trinucleotide repeat in exon-10 of ATXN3, a gene that encodes the deubiquitinating enzyme, Ataxin-3. Studies have established that disease pathogenesis and disease course of SCA3 and other polyQ-based diseases depend on the size of the polyQ expansion and the expression levels of the mutant polyQ protein.4,5,6 The polyQ expansion is thought to confer a toxic gain of function on the mutant polyQ protein that ultimately leads to the demise of specific neuronal populations. Thus, modulating levels of mutant polyQ protein in affected brain regions is predicted to be of significant therapeutic benefit.
Several studies suggest that Ataxin-3 recognizes and edits specific types of poly-ubiquitin linkages in a variety of cellular substrates.7,8 Ataxin-3, however, does not appear to be required for proper brain development or function as evidenced by the lack of an overt neurological phenotype in the Atxn3 null mouse.9 Adding to this observation, Alves et al.10 reported that RNAi-mediated suppression of endogenous rat ataxin-3 is well tolerated in the adult rat brain. They also noted that the loss of wild-type rat ataxin-3 activity in the adult rodent brain does not influence the neurotoxicity associated with a lentivirus-expressed mutant Ataxin-3.10 These data suggest that nonallele-specific suppression of Ataxin-3 in SCA3 might be a viable therapeutic approach. However, whether an RNAi-based strategy can affect phenotypic progression in a genetically accurate model of SCA3 remains to be investigated.
Here, we tested whether silencing mutant ATXN3 expression can resolve progressive molecular phenotypes observed in the cerebellum of SCA3/MJD84.2 transgenic mice expressing the full human ATXN3 disease gene.11 Optimized artificial microRNA (miRNA) mimics designed to target the 3′-untranslated (3′UTR) region of human ATXN3 mediated potent gene silencing of human ATXN3 in vitro and in vivo. Moreover, silencing mutant ATXN3 expression in the cerebellum of SCA3/MJD84.2 mice led to the clearance of mutant Ataxin-3 from neuronal nuclei throughout transduced regions. Finally, we provide evidence that changes in the levels of specific miRNAs in the cerebellum of SCA3/MJD84.2 mice are partially corrected following short-term RNAi-mediated suppression of mutant ATXN3. Together, our results lend support to the development of RNAi as a molecular therapy for SCA3.
Results
siRNAs targeting the 3′UTR of ATXN3 guide effective gene silencing
We scanned the 3′UTR of human ATXN3 in search of suitable siRNA target sites using an algorithm developed at the Whitehead Institute.12 Based on this analysis, five different siRNAs (Figure 1a) together with a scrambled-sequence (missense) negative control were delivered (10 nmol/l) twice during a 72-hour period into HEK293 cells, after which endogenous Ataxin-3 protein levels were analyzed (Figure 1b). Compared with untransfected cells (Null) or cells receiving the negative control (Mis), delivering any of the five siRNAs (148, 149, 316, 501, and 673) led to a robust reduction in Ataxin-3 levels (Figure 1b, si148: 77.1 ±
± 5%, si149: 80
5%, si149: 80 ±
± 4.1%, si316: 78.3
4.1%, si316: 78.3 ±
± 7.9%, si501: 76.7
7.9%, si501: 76.7 ±
± 4.5%, si673: 73.5
4.5%, si673: 73.5 ±
± 10.7%, P < 0.0001 for all siRNAs compared with “Null” control). Thus, targeting the 3′UTR of ATXN3 with siRNAs is an effective method by which to reduce Ataxin-3 levels in human cells.
10.7%, P < 0.0001 for all siRNAs compared with “Null” control). Thus, targeting the 3′UTR of ATXN3 with siRNAs is an effective method by which to reduce Ataxin-3 levels in human cells.
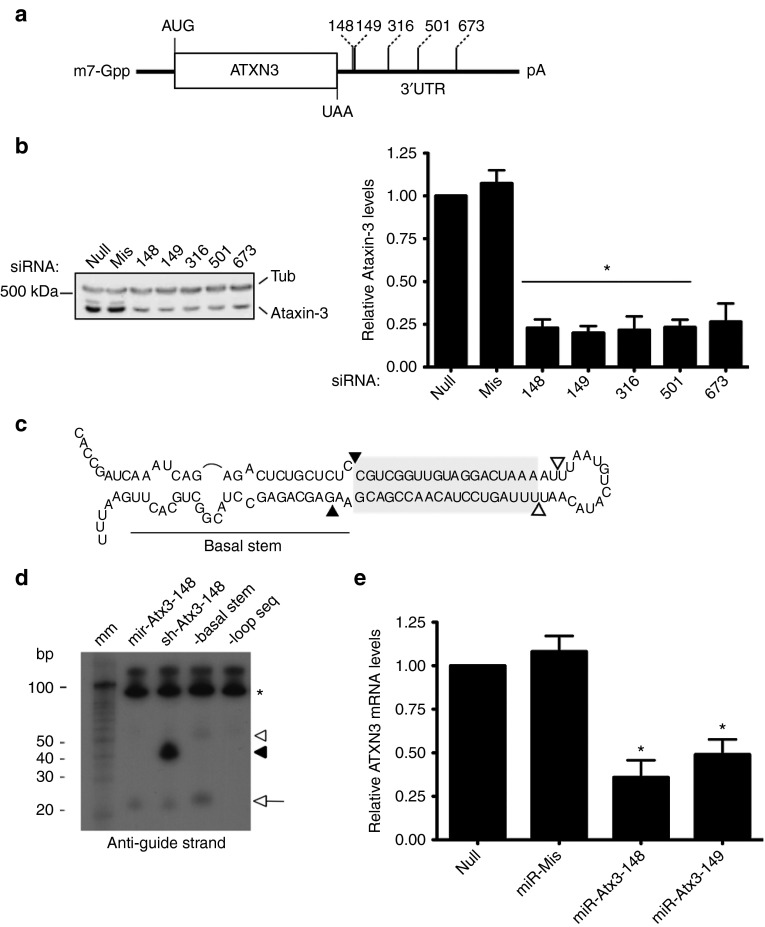
Targeting human ATXN3 expression with RNA interference. (a) The 3′UTR of ATXN3 was scanned for siRNA target sites and the top five candidates, numbered by their position with respect to the ATXN3 UAA stop codon (RefSeq: NM_004993), were experimentally validated. (b) A representative western blot analysis of endogenous Ataxin-3 expression following delivery of siRNAs into HEK293 cells. Compared with untreated (null) cells or those receiving a scramble siRNA control (miss), ectopic delivery of anti-ATXN3 siRNAs (148, 149, 316, 501, and 673) led to a marked reduction in the levels of Ataxin-3 protein 72-hours post-transfection. The results from four biological replicates are graphed to the right of the blot. Ataxin-3 protein levels were normalized to those of α-tubulin (Tub). (c) Scheme of miR-Atx3-148 miRNA mimic. siRNA sequences were embedded (grayscale-shaded region) in a miRNA-like RNA scaffold (black nucleotides) that mimics the human miRNA-124. The U6 snRNA promoter was used to drive intracellular expression of the miRNA mimics. Closed and opened triangles indicate the predicted Drosha and Dicer cleavage sites, respectively. (d) Northern blot analysis was used to compare the intracellular production and processing of the anti-ATXN3 miRNA mimic (miR-Atx3-148) to that of a “first-generation” short-hairpin RNA design (sh-Atx3-148), a miR-Atx3-148 construct lacking the basal stem sequence (-basal stem) and a miR-Atx3-148 construct lacking the loop sequence (-loop seq). Accumulated precursor RNA molecules were detected in the sh-Atx3-148 (black arrowhead) and -basal stem (white arrowhead) lanes using a probe against the guide strand sequence. Approximately equal levels of fully processed guide-strand were detected (bottom arrow) in all samples but the -loop seq sample. RNA levels were normalized to those of the 5S small noncoding RNA (asterisk). The molecular marker (mm) is shown in the first lane. (e) Analysis ofATXN3 mRNA levels following transient expression of anti-ATXN3 miRNA mimics in HEK293 cells. miR-Atx3-148 and miR-Atx3-149 mediated effective gene silencing of endogenous human ATXN3 mRNA when compared with untreated (null) or scramble-control (miss) treated cells. Error bars: ±SD.
Anti-ATXN3 miRNA mimics lead to sustained gene silencing
RNAi-based therapies for SCA3 and other polyglutamine diseases will likely require long-term intracellular production of therapeutic RNAi molecules. We previously used the human miRNA-124 primary sequence as a platform from which to generate siRNAs inside the cell.13 Based on this design, we produced anti-ATXN3 miRNA constructs (miR-Atx3-148 and miR-Atx3-149) under the regulation of the U6 noncoding RNA promoter (Figure 1c). siRNA sequences 148 and 149 were chosen for subsequent experiments because they outperformed all other siRNAs in dose-response experiments (data not shown).
We characterized the processing of the miRNA-124-based siRNA delivery shuttle (miR-Atx3-148) by generating two additional shuttle variants, one lacking the basal stem sequence (-basal stem) and one lacking the loop sequence (-loop seq) (Supplementary Figure S1, online). The processing of miR-Atx3-148 was also compared with a first-generation short-hairpin RNA vector carrying the same siRNA targeting sequence (sh-Atx3-148). In agreement with our previous data,13 the mir-Atx3-148 shuttle was effectively processed in HEK293 cells resulting in the release of a mature ~21-nt long guide-strand and nearly undetectable levels of precursor RNA hairpins (Figure 1d). In contrast, the other three shuttles were either not processed (-loop seq), processed ineffectively leading to the accumulation of precursors (sh-Atx3-148 and -basal stem), or processed to release a mature miRNA of a larger-than-expected size (-basal stem).
Sequence analysis using a 3′-rapid amplification of cDNA ends (3′RACE) approach revealed that ~80% of mature guide-strands released from the miR-Atx3-148 construct carried the expected anti-ATXN3 siRNA targeting sequence (Table 1). To confirm that the processed anti-ATXN3 artificial miRNAs suppressed ATXN3 expression by directly binding to the targeted 3′UTR sequence, we cloned the entire ATXN3 3′UTR sequence downstream of a luciferase reporter plasmid. As expected, luciferase expression from this reporter plasmid was suppressed only after co-expression with the U6-driven miR-Atx3 constructs (Supplementary Figure S2, online). Importantly, intracellular expression of miR-Atx3-148 or miR-Atx3-149 led to sustained endogenous ATXN3 gene silencing in HEK293 cells (Figure 1e, 64 ± 9.9% and 49 ±
± 8.6% reduction in Atx3 mRNA levels respectively, P < 0.0001 for miR-Atx3-148 and miR-Atx3-149 compared with “null” control). Thus, anti-ATXN3 miRNA mimics are effectively processed to release predicted RNAi effector molecules that can mediate gene silencing of endogenous ATXN3 expression.
8.6% reduction in Atx3 mRNA levels respectively, P < 0.0001 for miR-Atx3-148 and miR-Atx3-149 compared with “null” control). Thus, anti-ATXN3 miRNA mimics are effectively processed to release predicted RNAi effector molecules that can mediate gene silencing of endogenous ATXN3 expression.
Table 1

rAAV1-mediated delivery of miR-Atx3-148 to the cerebellum of SCA3 transgenic mice
We tested the activity of miR-Atx3-148 against human ATXN3 expression in the cerebellum of SCA3/MJD84.2 mice. SCA3/MJD84.2 mice carry two copies of a yeast artificial chromosome containing a full mutant ATXN3 gene locus with an 84 CAG repeat expansion.11 These mice express full-length mutant ATXN3 transcripts (including the 3′UTR region) under the regulation of the human ATXN3 promoter at levels close to and in a similar pattern to that observed in human SCA3 brain. Moreover, they develop a mild and progressive neurological phenotype characterized by pathologic (i.e., neuronal intranuclear aggregates) and behavioral abnormalities (i.e., gait abnormalities) akin to human SCA3.11
As depicted in Figure 2a, we produced recombinant adeno-associated virus (rAAV-2/1) containing the miRNA expression cassette upstream of a CMV-driven humanized-Renilla green fluorescent protein (hrGFP) gene (rAAV-miR-Atx3-148). We also generated two control rAAV viruses, one containing a scrambled miRNA sequence (rAAV-miR-Mis) and one lacking the U6-driven miRNA cassette (rAAV-CMV-hrGFP).
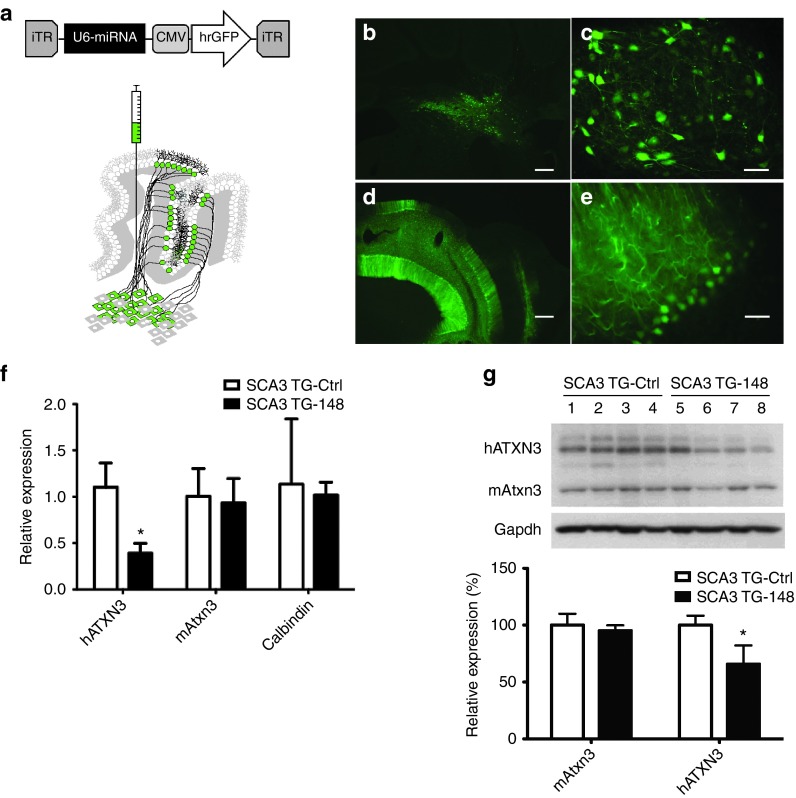
rAAV2/1-mediated delivery of miR-Atx3-148 to the cerebellum of SCA3/MJD84.2 mice leads to gene silencing of human mutant Ataxin-3. (a) The U6-miRNA expression cassette was cloned into a recombinant AAV expression vector upstream of a CMV-driven hrGFP expression cassette. rAAV2/1 virus was delivered into the deep cerebellar nuclei (DCN) of SCA3/MJD84.2 transgenic mice. Purkinje neurons in the cerebellar cortex, which project their axons to the DCN, are also transduced by DCN-delivered rAAV2/1 via retrograde transport. (b–e) Cerebellar coronal sections were obtained from injected mice 8 weeks post-surgery. Confocal microscopy analysis revealed strong hrGFP expression throughout neurons in the DCN (b, c) and in cerebellar Purkinje neurons (d, e).Scale bar in (b) and (d) = 200 µm. Scale bar in (c) and (e) = 50 µm. (f) Mutant human ATXN3 mRNA levels were suppressed following delivery of the rAAV2/1 miR-Atx3-148 virus. In contrast, the steady state levels of mouse Atxn3 and Calbindin-1 mRNAs remained unchanged in the presence of rAAV-miR-Atx3-148 virus. (g) Cerebellar mutant Ataxin-3 protein expression was confirmed by western blot using the 1H9 antibody. This antibody recognizes human mutant Ataxin-3 (top band) and endogenous mouse ataxin-3 (lower band). Four control-injected (lanes 1–4) and four rAAV-miR-148-injected (lanes 5–8) SCA3/84.2 mice were compared. As a group, SCA3/84.2 mice injected with rAAV-miR-148 showed a significant reduction (~34%) in mutant Ataxin-3 levels when compared to control injected mice. Gapdh levels were used to normalize for protein loading.*P < 0.05, unpaired t test.
RNAi and control rAAVs were delivered into the deep cerebellar nuclei (DCN) of 2-months old SCA3/MJD84.2 mice (rAAV-miR-Atx3-148, n = 9; rAAV-miR-Mis, n = 6; rAAV-CMV-hrGFP, n = 6). This delivery strategy has been shown to result in the transduction of DCN neurons and of cerebellar Purkinje neurons via retrograde transport of the virus (Figure 2a).14 Two months post-surgery (4 months of age) we analyzed hrGFP expression and confirmed widespread transduction of DCN and Purkinje neurons in the cerebellum of injected SCA3/MJD84.2 mice (Figure 2b–ee). We failed to detect hrGFP expression in other cerebellar resident cell types (i.e., Granule cells, Basket cells, Golgi cells, and Stellate cells) following rAAV2/1 delivery into the mouse DCN.
To assess the in vivo gene silencing potency and specificity of miR-Atx3-148, RNA was extracted from hrGFP-positive and anatomically matched hrGFP-negative sections, reverse-transcribed and subjected to real-time quantitative PCR. As shown in Figure 2f, human ATXN3 (hATXN3) mRNA levels were suppressed 71.2 ±
± 10.6% in the cerebellum of SCA3/MJD84.2 mice expressing miR-Atx3-148 when compared with control-injected SCA3/MJD84.2 mice (P < 0.05 Bonferroni post hoc test comparison). In contrast, the levels of mouse ataxin-3, which is not targeted by miR-Atx3-148, were unaltered. Levels of Calbindin-1 mRNA, a surrogate marker of cerebellar Purkinje cell function, were also unchanged between control and miR-Atx3-148 treated transgenic mice. Western blot analyses of whole cerebellar lysates obtained from miR-Atx3-148 and control-injected SCA3/84.2 mice revealed varying degrees of mutant human Ataxin-3 suppression (Figure 2g).The levels of mouse ataxin-3 protein were unchanged, in agreement with the mRNA data. As a group, SCA3/84.2 mice injected with rAAV-miR-Atx3-148 had a 34% reduction in mutant Ataxin-3 levels when compared with control-injected mice.
10.6% in the cerebellum of SCA3/MJD84.2 mice expressing miR-Atx3-148 when compared with control-injected SCA3/MJD84.2 mice (P < 0.05 Bonferroni post hoc test comparison). In contrast, the levels of mouse ataxin-3, which is not targeted by miR-Atx3-148, were unaltered. Levels of Calbindin-1 mRNA, a surrogate marker of cerebellar Purkinje cell function, were also unchanged between control and miR-Atx3-148 treated transgenic mice. Western blot analyses of whole cerebellar lysates obtained from miR-Atx3-148 and control-injected SCA3/84.2 mice revealed varying degrees of mutant human Ataxin-3 suppression (Figure 2g).The levels of mouse ataxin-3 protein were unchanged, in agreement with the mRNA data. As a group, SCA3/84.2 mice injected with rAAV-miR-Atx3-148 had a 34% reduction in mutant Ataxin-3 levels when compared with control-injected mice.
To assess the “off-targeting” potential of miR-Atx3-148, we identified and analyzed by quantitative PCR the expression of a number of transcripts containing potential “miRNA-like” binding seeds for miR-Atx3-148 on their 3′UTR sequence. We failed to detect significant changes in the expression of these transcripts following delivery of rAAV-miR-Atx3-148 when comparing untreated to treated SCA3/84.2 transgenic mice (Supplementary Figure S3, online). Finally, we measured the levels of Iba-1 and Gfap transcripts by quantitative PCR to address the possibility of an immune response to rAAV-mediated miR-Atx3-148 expression. We could not detect any significant differences in the levels of Iba-1 or Gfap mRNA between untreated, control treated and miR-Atx3-148 treated SCA3/84.2 mice (Supplementary Figure S3, online). Together, these data indicate that rAAV-miR-Atx3-148 leads to a specific and well-tolerated suppression of mutant Ataxin-3 expression in SCA3/84.2 mouse cerebellum.
miR-Atx3-148 activity reduces nuclear accumulation of mutant Ataxin-3 in SCA3 mice
In 2-month old littermate control mice, wild-type Ataxin-3 is typically diffusely localized to the nucleus and the soma of DCN and cerebellar Purkinje neurons (Supplementary Figure S4, online). However, in 2-month old SCA3/MJD84.2 mice, mutant Ataxin-3 is primarily found in the nucleus of DCN neurons and many other neuronal populations (Supplementary Figure S4, online). This aberrant nuclear accumulation of mutant Ataxin-3 is also observed in human SCA3 brain and is a well-established marker of disease pathogenesis.15
Immunohistochemical analysis of cerebellar sections obtained from SCA3/MJD84.2 mice injected with rAAV-miR-Atx3-148 showed a marked reduction in the levels of nuclear mutant Ataxin-3 signal (Figure 3a, cc, ee) when compared with the uninjected contralateral DCN (Figure 3b, dd, ff).Quantitative analysis of sections immunohistochemically stained with the anti-Ataxin-3 1H9 antibody revealed a greater than 80% loss in nuclear mutant human Ataxin-3 signal (Supplementary Figure S5, online). This reduction was specific to the expression of miR-Atx3-148, as it was not observed following expression of a control rAAV-CMV-hrGFP (Figure 3g, hh, ii). Moreover, miR-Atx3-148 activity in the DCN did not alter the levels of E4B, a well-known Ataxin-3 interacting protein (Supplementary Figure S4, online), nor did it lead to gross changes in the morphology of DCN neurons, consistent with a lack of rAAV-miR-Atx3-148-mediated toxicity.
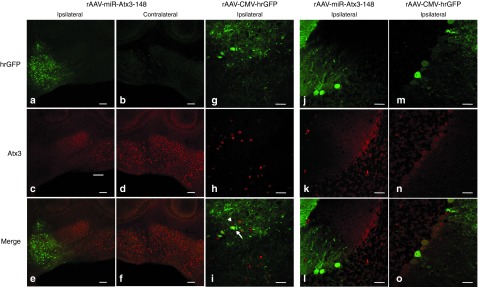
miR-Atx3-148 activity resolves neuronal nuclear accumulation of mutant Atx3 in SCA3/84.2 cerebellum. (a) Mutant human Ataxin-3 expression was analyzed immunohistochemically using the 1H9 monoclonal antibody against human Ataxin-3 (red color). hrGFP expression was evident in neurons throughout the ipsilateral DCN (a) but absent in the contralateral, uninjected DCN where only a few hrGFP-positive tracks could be observed (b). As expected, hrGFP-positive areasin the rAAV2/1-miR-Atx3-148 injected DCN of SCA3/MJD84.2 mice were almost completely devoid of mutant Ataxin-3 signal (c, ipsilateral) whereas intense neuronal nuclear mutant Ataxin-3 staining was detected throughout the contralateral DCN of SCA3/MJD84.2 mice (d, contralateral). This mutant Ataxin-3 “exclusion zone” is highlighted in the merged image panels (e) and (f). (g–i) In contrast, mutant Ataxin-3 was still clearly present inthe nuclei of DCN neurons expressing the control hrGFP-only vector. Arrow in (i) points to DCN neurons transduced with rAAV-CMV-hrGFP control vector (hrGFP-positive) while the arrowhead points to a neighboring hrGFP-negative DCN neuron. Notice the similar levels of nuclear mutant Ataxin-3 expression between neurons transduced with the control vector and untransduced DCN neurons, as seen in the merged image (i). (j–o) hrGFP signal in the cerebellar cortex of injected SCA3/MJD84.2 mice predominantly localized to Purkinje neurons. The anti-Ataxin-31H9 signal (red) was absent in Purkinje neurons expressing rAAV-miR-Atx3-148 (j–l, left side of lobule) but easily detected in the nuclei and processes of untransduced Purkinje neurons (j–l, right side of lobule). In comparison, nuclear mutant Ataxin-3 signal was observed in Purkinje neurons expressing the control rAAV2/1-CMV-hrGFP vector (m–o). Scale bar in (a–f) = 200 µm. Scale bar in (g–o) = 50 µm.
We also examined the Purkinje cell layer in SCA3/84.2 mice injected with rAAV-miR-Atx3-148 and control rAAV vectors. We failed to detect aberrant nuclear accumulation of mutant Ataxin-3 in Purkinje neurons expressing miR-Atx3-148 (Figure 3j–ll) when compared with Purkinje neurons expressing the control rAAV-CMV-hrGFP vector (Figure 3m–oo). For both DCN and Purkinje neurons, suppression of mutant Ataxin-3 was largely confined to transduced cells. In some cases, however, hrGFP-negative neurons immediately adjacent to hrGFP-positive regions also displayed reduced mutant Ataxin-3 expression. This “penumbra” effect could reflect low level, but effective, miR-Atx3-148 activity in the context of nearly undetectable hrGFP protein levels. Alternatively, intercellular transfer of miR-Atx3-148 molecules or some other, as yet undetermined, mechanism could underlie the observed effect. Nevertheless, these data suggest that miR-Atx3-148 expression reduces levels of human Ataxin-3 protein, leading to the clearance of mutant Ataxin-3 from the nuclei of cerebellar neurons in SCA3/84.2 mice.
Silencing mutant Ataxin-3 expression partially restores miRNA steady-state levels in SCA3/MJD84.2 mice
A potential drawback of long-term viral-based RNAi therapy is the possibility of disrupting endogenous miRNA processing by coopting the RNAi machinery. We investigated if sustained expression of the anti-ATXN3 miRNA mimic in SCA3/84.2 mice cerebella disrupted miRNA biogenesis. First, we characterized the miRNA expression profile of 2- and 6-month old untreated SCA3/84.2 and wild-type littermate control mice (n = 3 per group). SCA3/84.2 is minimally symptomatic at both of these time points (Ref. 16 and unpublished results). In these samples, the expression of 346 endogenous mouse miRNAs was reliably detected using a TaqMan low-density array quantitative-PCR platform (see “Materials and Methods”). Differential expression analysis identified 10 out of 346 endogenous mouse miRNAs with significantly different expression profiles between SCA3/84.2 and littermate control mice at 2-months of age (Figure 4a, ≥1.2-fold change, P < 0.05). Steady-state levels of the mature strands for miR-101a (-1.2-fold, P = 0.0043), -146b (-1.3-fold, P = 0.0274), -324-3p (-1.2-fold, P = 0.0249), -361 (-1.3-fold, P = 0.0282), -365 (-1.2-fold, P = 0.0379) and -674 (-1.6 fold, P = 0.0495) were significantly lower in the cerebellum of SCA3/84.2 mice. In contrast, the steady-state levels of miR-15b (1.5-fold, P = 0.0021), -181a (1.7-fold, P = 0.0275), -342-5p (1.2-fold, P = 0.0251) and miR-467a (1.2-fold, P = 0.0188) were upregulated in the cerebellum of SCA3/84.2 mice. Intriguingly, at 6-months of age, only miR-181a levels were significantly different between SCA3/84.2 and littermate control mice (Figure 4b, 2.01-fold, P = 0.0484). Bioinformatics analysis identified, among numerous candidates, the presence of a conserved miR-181a binding site in the 3′UTR of human ATXN3. These data suggest that only a small number of miRNAs display altered steady-state levels in the cerebellum of SCA3/84.2 mice.
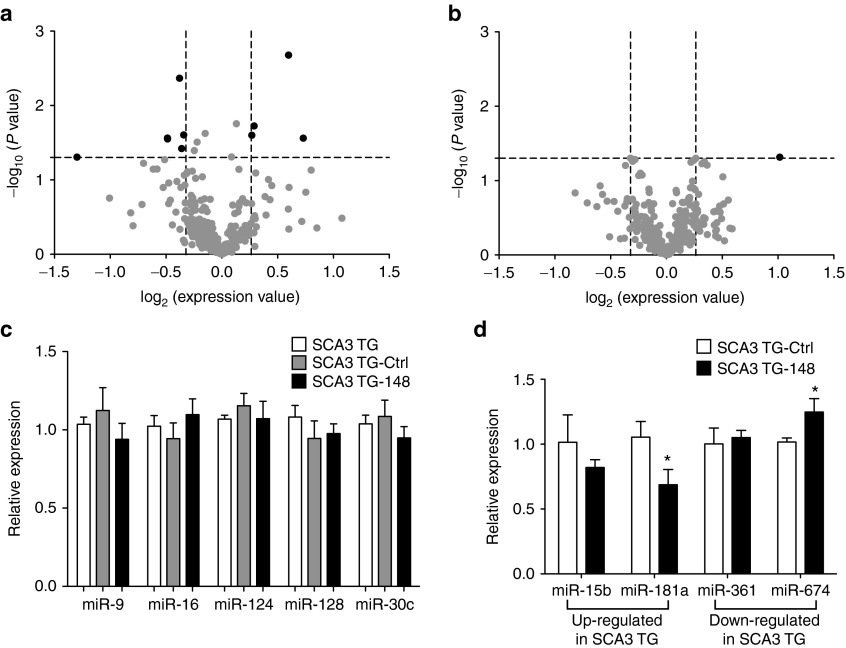
Silencing mutant Ataxin-3 leads to a partial normalization of endogenous miRNA expression in SCA3/84.2 mice. Global miRNA expression profiles for 2-month (a) and 6-month (b) old SCA3/84.2 and littermate control mice (n = 3 per group). The volcano plot shows the magnitude (x-axis, log2 fold change) and the statistical significance (y-axis, -log10 P value) of the calculated difference in miRNA expression. miRNAs with a significant (P < 0.05, above horizontal dash line) fold increase (≥1.2, upper right quadrant) or decrease (≥1.2, upper left quadrant) in steady-state levels in SCA3/84.2 mice relative to age-matched littermate controls are represented by black filled circles. Grey-filled circles represent expressed cerebellar miRNAs with similar expression levels in SCA3/84.2 relative to age-matched littermate controls. At 2-months of age, 10 different miRNAs (listed in the “Results” section) significantly differed between SCA3/84.2 and littermate control mice. By 6-months of age, only the steady-state levels of miR-181a were significantly altered between transgenic and control mice. (c) A subset of miRNAs (miR-9, -16, -124, -128 and -30c) that displayed similar steady-state levels in untreated SCA3/84.2 mice relative to littermate controls (e.g., grey-filled circles) was used to detect potential adverse effects of sustained miR-Atx3-148 expression on the endogenous mouse miRNA pathway. Following 8 weeks of miR-Atx3-148 expression in SCA3/84.2 cerebellar neurons, there was no significant difference in the levels of miR-9, -16, -124, -128, or -30c in transduced SCA3/84.2 mice compared with untreated (SCA3 TG) or control injected (SCA3 TG-Ctrl) transgenic mice. (d) Delivery of miR-Atx3-148 expression, however, appeared to partially normalize steady-state levels of miR-181a and miR-674, two of the miRNAs with altered steady-state levels in the cerebella of untreated SCA3/84.2 mice. The graph shows steady-state levels of each miRNA in SCA3/84.2 mice expressing miR-Atx3-148 (SCA3 TG-148, black bars) relative to control treated SCA3/84.2 mice (SCA3 TG-Ctrl, white bars). Since miR-181a levels were increased by ~twofold in untreated SCA3/84.2 mouse cerebellum (see panels a and b), a reduction in its levels following suppression of human ATXN3 suggests a partial restoration to control levels. *P < 0.05, unpaired t test.
Next, we quantified the expression level of a subset of miRNAs following delivery of miR-Atx3-148 into the cerebellum of SCA3/84.2 mice. For this analysis, we queried five miRNAs that were reliably detected and showed normal expression in untreated SCA3/84.2 mouse cerebella when compared with littermate controls. SCA3/84.2 mice (4-months old) received AAV-miR-Ctrl (n = 4) or AAV-miR-Atx3-148 (n = 4). As shown in Figure 4c, 8 weeks postdelivery we did not detect any significant differences in the steady-state levels of miR-9, -16, -124, -128 and -30c when comparing untreated SCA3/84.2 mice with those treated with control or anti-ATXN3 miRNAs. Thus, sustained expression of AAV-delivered miRNA mimics in the cerebellum of SCA3/84.2 does not adversely affect the expression of at least a subset of endogenous mouse miRNAs.
Finally, we asked whether suppression of mutant Ataxin-3 expression by miR-Atx3-148 normalized the steady-state levels of the miRNAs that displayed altered expression in the SCA3/84.2 cerebellum. We chose to compare the two most upregulated (miR-15b and miR-181a) and the two most down-regulated (miR-361 and miR-674) miRNAs identified in the TaqMan array studies. Compared with untreated or AAV-control treated SCA3/84.2 mice, we observed significantly reduced miR-181a levels and significantly upregulated miR-674 levels in the cerebella of 6-month old SCA3/84.2 mice expressing miR-Atx3-148 (Figure 4d, P < 0.05). Since miR-181a levels were upregulated (~twofold, Figure 4b) and miR-674 levels were down-regulated (~1.6-fold, Figure 4b) in untreated SCA3/84.2 mouse cerebellum, these findings suggest that suppressing mutant Ataxin-3 expression with AAV-miR-Atx3-148 leads to a partial normalization of some of the altered miRNAs in SCA3/84.2 mouse cerebella.
Discussion
SCA3 is the most common form of dominantly inherited ataxia in the world.1 Although the pathogenic mechanisms underlying SCA3 remain unclear, it is well established that expression of mutant Ataxin-3 carrying an expanded polyQ domain of at least 55 glutamine residues in length is necessary to cause MJD.2,4,10 Thus, strategies aimed at curtailing mutant Ataxin-3 levels in the SCA3 brain may be of significant therapeutic value. Here, we developed an AAV-based RNAi strategy that effectively suppressed mutant Ataxin-3 expression in a genetically accurate mouse model of the disease.
We identified several siRNA sequences that target the 3′UTR of human ATXN3 and mediate significant suppression of human Ataxin-3 expression. Several reasons led us to target the 3′UTR of ATXN3. First, the human ATXN3 transcript undergoes alternative splicing along its coding sequence, upstream of the 3′UTR region, to generate at least 31 different ATXN3 splice-isoforms (RefSeq database, GRCh37/hg19 assembly). We reasoned that siRNAs targeting the 3′UTR of ATXN3 would guide Argonaute 2 (Ago2)-mediated cleavage of most ATXN3 isoforms leading to a robust reduction in Ataxin-3 levels. Indeed, all five of the siRNAs tested mediated greater than a 70% loss in Ataxin-3 protein in human cells. Second, most mammalian microRNAs interfere with the expression of protein-coding genes by binding to target sites in the 3′UTR of mRNAs.17 Although this biological restriction remains poorly understood, it has been hypothesized that the RNAi machinery can more easily access the 3′UTR of mRNAs due to limited translational activity in this region of the transcript.18 The human ATXN3 3′UTR is predicted to be a biological target for several endogenously expressed miRNAs.19,20,21,22 We hypothesize that this natural “bio-accessibility” might in part explain the potency of our 3′UTR-targeted siRNAs. Supporting this hypothesis is the fact that siRNA target site accessibility has been shown to be a strong determinant of siRNA potency.23,24 Finally, Ataxin-3's cellular function as a deubiquitinating enzyme appears to be redundant since knockout of the ATXN3 homologues in C. elegans and M. musculus does not lead to overt phenotypes.9,25 Thus, nonallele-specific targeting of ATXN3 through its 3′UTR should be tolerated in mammalian systems.
The efficacy (i.e., potency and specificity) of human ATXN3 3′UTR-targeted RNAi molecules was characterized in the SCA3/84.2 transgenic mouse. This genetic model of SCA3 develops a mild phenotype that mirrors some aspects of the human disease, such as neuronal intranuclear aggregates, physiological abnormalities, and motor deficits.11 Nuclear accumulation of polyQ-expanded proteins and/or their fragments is thought to be a hallmark event in the pathogenicity of polyQ diseases.2,26,27 In fact, abnormal nuclear accumulation of mutant polyQ proteins is thought to play a role in the early deregulation of neuronal transcriptional programs observed in this family of neurodegenerative disorders. AAV-mediated delivery of miR-Atx3-148 led to a significant, sustained suppression of mutant Ataxin-3 and to a marked reduction of mutant Ataxin-3 nuclear accumulation intransduced regions of the SCA3/84.2 cerebellum. However, as described in an accompanying paper in this issue,28 in spite of effective RNAi-mediated suppression of mutant Ataxin-3, long-term cerebellar expression of miR-Atx3-148 did not significantly alter the progression of motor deficits in homozygote SCA3/84.2 mice.
Consistent with our previous findings13 artificial miRNA mimics based on the human miRNA-124 primary sequence were effectively processed by the RNAi machinery, resulting in the release of potent effectors of human ATXN3 gene silencing. Sustained expression of therapeutic RNAi molecules could disrupt endogenous miRNA expression or processing which is detrimental to neuronal homeostasis.29 In fact, deregulated expression of miRNAs and their downstream targets has now been implicated in the pathogenesis of several neurodegenerative diseases.30 Endogenous miRNA expression profile analysis showed that the miRNA-like molecule used in these studies did not significantly alter the expression of miRNAs in SCA3/84.2 mice. This result is in agreement with previous studies.31,32
Global miRNA profiling surprisingly revealed a limited number of changes in the steady-state levels of mature miRNAs in untreated SCA3/84.2 mice when compared with age-matched littermate controls. Ten different miRNAs displayed altered steady-state levels in 2-months old SCA3/84.2 mice while only miR-181a levels were significantly changed at 6-months of age. We speculate this difference might reflect compensatory changes that occur in the cerebellum of SCA3/84.2 mice following the initial insult from mutant Ataxin-3 expression. It is worth noting that miR-181a is a dopamine-responsive miRNA implicated in neuronal homeostasis.33,34 Importantly, the 3′UTR of ATXN3 itself is likely under the regulation of miR-181a as it displays multiple conserved binding sites for this miRNA. From a therapeutic perspective, our studies suggest the intriguing possibility of regulating Ataxin-3 levels in the brain using endogenous miRNA mimics such as miR-181a.
In conclusion, we have successfully employed an AAV-based RNAi strategy to effectively suppress Ataxin-3 expression in a transgenic mouse model of SCA3 expressing the full human disease gene. Several observations support targeting ATXN3 mRNA as a therapeutic approach to SCA3. First, expression of a mutant form of Ataxin-3 is required for the onset and progression of SCA3. Second, Ataxin-3's function appears to be redundant in the mammalian brain. Thirdly, the ATXN3 mRNA carrying an expanded CAG repeat by itself can be toxic, even in the absence of translation.35 Thus, therapeutic compounds that target Ataxin-3 at the protein level might prove only partially beneficial. More preclinical work is needed; however, to determine the brain regions in SCA3 that must be targeted for effective prevention or amelioration of disease features. As discussed in an accompanying article in this issue,28 the therapeutic success of molecules engineered to suppress ATXN3 expression may hinge upon our ability to broadly distribute them throughout the cerebellum and other areas of the posterior fossa.
Materials and Methods
siRNA synthesis and miRNA vectors. RNAi targeting sequences against human ATXN3 3′UTR were obtained using the Whitehead Institute siRNA selection algorithm (http://jura.wi.mit.edu/bioc/siRNAext/). Oligonucleotide sequences are listed in Supplementary Table S1, online. SiRNA duplexes were synthesized as previously described.13 Artificial miRNA mimics were built by embedding anti-ATXN3 targeting sequences in a miRNA backbone composed of the basal stem (52 nucleotides) and loop (17 nucleotides) sequences of the human miR124-1 miRNA (http://rfam.sanger.ac.uk/family?entry=RF00239). The resulting PCR products were cloned into the pENTR/U6 vector following the manufacturer's recommendations (Invitrogen, Carslbad, CA).
Transfections. Human HEK293 cells were maintained in high-glucose DMEM media supplemented with 10% fetal bovine serum at 37 °C in 5%-CO2 atmosphere. Lipofectamine 2000 reagent was used to introduce anti-ATXN3 siRNAs into cultured cells twice within the span of a 72-hour incubation period. U6-driven miRNA mimic expression vectors were delivered into HEK293 cells once, 72-hours before performing protein and RNA analyses.
Protein and RNA analyses. Protein extractions from transfected HEK293 cells were performed as previously reported.13 Human Ataxin-3 protein expression was detected in western blots using the 1H9 monoclonal antibody (1:2,000 dilution; Millipore, Billerica, MA). Tubulin levels were detected using a monoclonal antibody against α-tubulin (1:10,000; Sigma, St Louis, MO) and the signal used to normalize the levels of Ataxin-3. In all cases, following primary antibody incubation, membranes were incubated with peroxidase-conjugated anti-mouse secondary antibodies (1:20,000 dilution; Jackson Immuno Research Laboratories, West Grove, PA) followed by treatment with the ECL-plus reagent (Western Lighting, PerkinElmer, Waltham, MA) as previously described.13 Band intensities were quantified using the Quantity One Software analysis tool.
Total RNA from HEK293 cells was extracted using the Trizol Reagent (Invitrogen) as previously reported.36 Total RNA (1 µg) was reverse-transcribed (iScript cDNA Synthesis Kit, BioRad) and the resulting cDNA analyzed using quantitative PCR with TaqMan assays for the genes analyzed. Northern blot analysis of small RNA pools was performed as previously described.13
RNA was extracted from mouse brain using standard procedures. Briefly, control or miR-Atx3-148 treated mice were anesthetized and intracardialy perfused with sterile saline solution before the brain was quickly dissected and placed on a cold rodent brain matrix (1 mm thick channels). A total of five 1 mm thick coronal cerebellar sections were obtained and placed under a dissecting scope fitted with a UV-lamp. hrGFP-positive sections were identified and the ipsilateral (hrGFP-positive) and contralateral (hrGFP-negative) hemispheres separated and used for extractions. Total RNA from these sections was obtained using the Trizol Reagent as previously reported.36 RNA (2 µg) was reverse transcribed using the High-Capacity Reverse Transcription Kit (Applied Biosystems, CA, USA) following the manufacturer's recommendations. Resulting cDNA was analyzed using TaqMan assays (Applied Biosystems) specific for each of the reported gene transcripts. Results were normalized using two endogenous controls, c-myc and b-actin, and, for each treatment, expressed relative to the mRNA levels present in the corresponding hrGFP-negative sections.
Protein lysates were prepared from whole cerebella in RIPA buffer containing protease inhibitors (Complete, Roche Diagnostics), followed by sonication and centrifugation. The supernatants were collected and stored at −80 °C. Ataxin-3 levels were detected by western blotting using the 1H9 antibody as mentioned above and normalized for Gapdh levels assessed using a monoclonal anti-GAPDH antibody (1:3,000 dilution; Millipore).
3′RACE. Small RNAs (1 μg) were extracted, polyA-tailed (Poly(A) Polymerase Tailing Kit, Epicentre Biotechnologies), and reverse-transcribed (RLM-RACE Kit (Ambion)) as previously described.13 We used 148-guide strand or 148-passenger strand specific primers and 3′RACE inner or outer primers to amplify the cDNA library. The resultant PCR products were separated on a 2% agarose gel and specific bands of ~100 bp in size were excised, purified and subcloned. The small cDNA library was sequenced at The University of Michigan's DNA Sequencing Core using the Sanger sequencing method.
bp in size were excised, purified and subcloned. The small cDNA library was sequenced at The University of Michigan's DNA Sequencing Core using the Sanger sequencing method.
Virus production. rAAV serotype 2/1 (AAV-2 inverted terminal repeats; AAV-1 viral capsid) vectors were made at the University of Iowa Gene Transfer Vector Core facility using an insect cell (Sf9)/baculovirus-based system as previously described.37 A two-step purification process consisting of an iodexanol gradient (15–60% wt/vol) followed by ion exchange (Mustang Q Acrodisc membranes, Pall, East Hills, NY) purification was used to generate purified, high-titer rAAV virus. rAAV titers (viral genomes/ml) were determined by quantitative PCR and range from 1012 to 1013 viral genomes/ml.
Mouse surgery. Stereotaxic administration of rAAV2/1 vectors was performed on 2 to 4-month-old wild type or SCA3/MJD84.2 transgenic mice placed under anesthesia using a mixture of O2 and isoflurane. Mice received intracerebellar injections (two-sites/hemisphere) of rAAV2/1 virus diluted in Lactated Ringer's solution. For each injection, a 1 µl volume was delivered to the medial (site 1) or lateral (site 2) deep cerebellar nucleus at an infusion rate of 0.25 µl/min using a 10-µl Hamilton syringe retrofitted with a glass micropipette. One minute after the infusion was completed the micropipette was retracted 0.3 mm and allowed to remain in place for an additional 4 minutes prior to its complete removal from the mouse brain. Anterior-posterior and medial-lateral coordinates were calculated from bregma and the dorsal-ventral coordinates were calculated from the dural surface. These measurements were made on an experimentally determined flat skull.
mm and allowed to remain in place for an additional 4 minutes prior to its complete removal from the mouse brain. Anterior-posterior and medial-lateral coordinates were calculated from bregma and the dorsal-ventral coordinates were calculated from the dural surface. These measurements were made on an experimentally determined flat skull.
Immunohistochemistry. Mice were perfused transcardially with 0.1 mol/l PBS, pH 7.4 followed by 4% paraformaldehyde, pH 7.4. The brains were postfixed overnight in 4% PFA, pH 7.4 and cryoprotected by immersion in 30% sucrose/0.1
mol/l PBS, pH 7.4 followed by 4% paraformaldehyde, pH 7.4. The brains were postfixed overnight in 4% PFA, pH 7.4 and cryoprotected by immersion in 30% sucrose/0.1 mol/l PBS for 48-hours at 4 °C. The brains were cut frozen on a sliding microtome at 30 μm (coronal) and collected in 12-well plates containing 30% sucrose-30% ethylene glycol in 0.1
mol/l PBS for 48-hours at 4 °C. The brains were cut frozen on a sliding microtome at 30 μm (coronal) and collected in 12-well plates containing 30% sucrose-30% ethylene glycol in 0.1 mol/l PBS. Prior to histological processing, sections were washed in 0.1
mol/l PBS. Prior to histological processing, sections were washed in 0.1 mol/l PBS overnight. For antigen retrieval the sections were incubated for 25 minutes in a 10 mmol/l sodium citrate buffer (pH 8.5) preheated to and maintained at 80 °C in a dry bath incubator, cooled down to room temperature, followed by 30 minutes incubation in 2% nonfat dry milk containing 0.3% Triton X-100–0.01% sodium azide.
mol/l PBS overnight. For antigen retrieval the sections were incubated for 25 minutes in a 10 mmol/l sodium citrate buffer (pH 8.5) preheated to and maintained at 80 °C in a dry bath incubator, cooled down to room temperature, followed by 30 minutes incubation in 2% nonfat dry milk containing 0.3% Triton X-100–0.01% sodium azide.
miRNA expression analysis. The global miRNA expression profiling of SCA3/84.2 cerebellum was performed using the TaqMan Array Mouse MicroRNA panel v2.0 (Applied Biosystems) which includes cards A and B in a 384-well format. In brief, total cerebellar RNA (800 ng) from SCA3/84.2 or littermate control mice was reverse-transcribed with the miRNA Multiplex RT pool set using the High-Capacity cDNA kit and following the manufacturer's recommendations. The products were then amplified in a 384-well plate that contained sequence-specific primers for endogenous mouse miRNAs and endogenous mouse control RNAs using the 7900-HT Real-Time PCR system (Applied Biosystems). The data were collected and processed using the DATA 3.0 software. For each miRNA, a Ct value of 32 was used as a cutoff for expression. Expression level was determined using the 2−ΔΔCt method. miRNAs with a ≥1.2-fold change and a P < 0.05 significance were reported.
ng) from SCA3/84.2 or littermate control mice was reverse-transcribed with the miRNA Multiplex RT pool set using the High-Capacity cDNA kit and following the manufacturer's recommendations. The products were then amplified in a 384-well plate that contained sequence-specific primers for endogenous mouse miRNAs and endogenous mouse control RNAs using the 7900-HT Real-Time PCR system (Applied Biosystems). The data were collected and processed using the DATA 3.0 software. For each miRNA, a Ct value of 32 was used as a cutoff for expression. Expression level was determined using the 2−ΔΔCt method. miRNAs with a ≥1.2-fold change and a P < 0.05 significance were reported.
Single miRNA expression analysis was performed using the TaqMan Reverse Transcription Kit and miRNA-specific TaqMan probes following the manufacturer's recommendations.
Statistical analysis. Quantifications are expressed as mean ± SD or SEM as noted in figure legends. Statistical analysis involved ANOVAs followed by Bonferroni post-tests or Student's t tests as stated in figure legends. P values obtained from the TaqMan Array Mouse MicroRNA analysis were adjusted using a Benjamini-Hochberg False Discovery Rate. Significance thresholds were set at P < 0.05.
SUPPLEMENTARY MATERIAL
Figure S1. RNAi expression cassettes.
Figure S2. miR-Atx3-148 suppression of a Luciferase/ATXN3-3'UTR fusion construct.
Figure S3. Off-target potential analysis for miR-Atx3-148.
Figure S4. Immunohistochemical analysis of SCA3/84.2 mice.
Figure S5. Reduced nuclear mutant Ataxin-3 levels following miRAtx3-148 expression in the cerebellum of SCA3/84.2 mice.
Table S1. Oligonucleotide sequences used in this study.
Acknowledgments
This work was supported by the National Institutes of Health (NS50210 and NS067111 to ER and HLP), the Fauver Family Ataxia fund (HLP) and the Mateus research fund (HLP).
References
- Costa Mdo C, Paulson HL. Toward understanding Machado-Joseph disease. Prog Neurobiol. 2012;97:239–257. [Europe PMC free article] [Abstract] [Google Scholar]
- Matos CA, de Macedo-Ribeiro S, Carvalho AL. Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog Neurobiol. 2011;95:26–48. [Abstract] [Google Scholar]
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. [Abstract] [Google Scholar]
- Boy J, Schmidt T, Wolburg H, Mack A, Nuber S, Böttcher M, et al. Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Hum Mol Genet. 2009;18:4282–4295. [Abstract] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. [Abstract] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–8861. [Europe PMC free article] [Abstract] [Google Scholar]
- Winborn BJ, Travis SM, Todi SV, Scaglione KM, Xu P, Williams AJ, et al. The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J Biol Chem. 2008;283:26436–26443. [Europe PMC free article] [Abstract] [Google Scholar]
- Todi SV, Scaglione KM, Blount JR, Basrur V, Conlon KP, Pastore A, et al. Activity and cellular functions of the deubiquitinating enzyme and polyglutamine disease protein ataxin-3 are regulated by ubiquitination at lysine 117. J Biol Chem. 2010;285:39303–39313. [Europe PMC free article] [Abstract] [Google Scholar]
- Schmitt I, Linden M, Khazneh H, Evert BO, Breuer P, Klockgether T, et al. Inactivation of the mouse Atxn3 (ataxin-3) gene increases protein ubiquitination. Biochem Biophys Res Commun. 2007;362:734–739. [Abstract] [Google Scholar]
- Alves S, Nascimento-Ferreira I, Dufour N, Hassig R, Auregan G, Nóbrega C, et al. Silencing ataxin-3 mitigates degeneration in a rat model of Machado-Joseph disease: no role for wild-type ataxin-3. Hum Mol Genet. 2010;19:2380–2394. [Abstract] [Google Scholar]
- Cemal CK, Carroll CJ, Lawrence L, Lowrie MB, Ruddle P, Al-Mahdawi S, et al. YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum Mol Genet. 2002;11:1075–1094. [Abstract] [Google Scholar]
- Yuan B, Latek R, Hossbach M, Tuschl T, Lewitter F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 2004;32 Web Server issue:W130–W134. [Europe PMC free article] [Abstract] [Google Scholar]
- Tsou WL, Soong BW, Paulson HL, Rodríguez-Lebrón E. Splice isoform-specific suppression of the Cav2.1 variant underlying spinocerebellar ataxia type 6. Neurobiol Dis. 2011;43:533–542. [Europe PMC free article] [Abstract] [Google Scholar]
- Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. [Abstract] [Google Scholar]
- Paulson HL, Perez MK, Trottier Y, Trojanowski JQ, Subramony SH, Das SS, et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron. 1997;19:333–344. [Abstract] [Google Scholar]
- Shakkottai VG, do Carmo Costa M, Dell'Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31:13002–13014. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim YK, Heo I, Kim VN. Modifications of small RNAs and their associated proteins. Cell. 2010;143:703–709. [Abstract] [Google Scholar]
- Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3' untranslated region in mammalian mRNAs. Nat Struct Mol Biol. 2009;16:144–150. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. [Abstract] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. [Europe PMC free article] [Abstract] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. [Europe PMC free article] [Abstract] [Google Scholar]
- Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18:1139–1146. [Europe PMC free article] [Abstract] [Google Scholar]
- Brown KM, Chu CY, Rana TM. Target accessibility dictates the potency of human RISC. Nat Struct Mol Biol. 2005;12:469–470. [Abstract] [Google Scholar]
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. [Abstract] [Google Scholar]
- Rodrigues AJ, Coppola G, Santos C, Costa Mdo C, Ailion M, Sequeiros J, et al. Functional genomics and biochemical characterization of the C. elegans orthologue of the Machado-Joseph disease protein ataxin-3. FASEB J. 2007;21:1126–1136. [Abstract] [Google Scholar]
- Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D, et al. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480:543–546. [Abstract] [Google Scholar]
- Walsh R, Storey E, Stefani D, Kelly L, Turnbull V. The roles of proteolysis and nuclear localisation in the toxicity of the polyglutamine diseases. A review. Neurotox Res. 2005;7:43–57. [Abstract] [Google Scholar]
- do Carmo Costa M, Luna-Cancalon K, Fischer S, Ashraf NS, Ouyang M, Dharia RM, et al. 2013Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease. Mol Ther10.1038/mt.2013.144). [Europe PMC free article] [Abstract]
- McNeill E, Van Vactor D. MicroRNAs shape the neuronal landscape. Neuron. 2012;75:363–379. [Europe PMC free article] [Abstract] [Google Scholar]
- Hébert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32:199–206. [Abstract] [Google Scholar]
- Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17:169–175. [Europe PMC free article] [Abstract] [Google Scholar]
- McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci USA. 2008;105:5868–5873. [Europe PMC free article] [Abstract] [Google Scholar]
- Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci. 2009;42:350–362. [Abstract] [Google Scholar]
- Saba R, Störchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, et al. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol Cell Biol. 2012;32:619–632. [Europe PMC free article] [Abstract] [Google Scholar]
- Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008;453:1107–1111. [Europe PMC free article] [Abstract] [Google Scholar]
- Rodríguez-Lebrón E, Gouvion CM, Moore SA, Davidson BL, Paulson HL. Allele-specific RNAi mitigates phenotypic progression in a transgenic model of Alzheimer's disease. Mol Ther. 2009;17:1563–1573. [Europe PMC free article] [Abstract] [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. [Abstract] [Google Scholar]
Articles from Molecular Therapy are provided here courtesy of The American Society of Gene & Cell Therapy
Full text links
Read article at publisher's site: https://doi.org/10.1038/mt.2013.152
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1525001616322572/pdf
Citations & impact
Impact metrics
Article citations
Quantification of Solid Embryonic Cerebellar Graft Volume in a Degenerative Ataxia Model.
Cerebellum, 23(5):1811-1823, 02 Mar 2024
Cited by: 0 articles | PMID: 38430389
Hereditary Ataxias: From Bench to Clinic, Where Do We Stand?
Cells, 13(4):319, 09 Feb 2024
Cited by: 1 article | PMID: 38391932 | PMCID: PMC10886822
Review Free full text in Europe PMC
Blood levels of neurofilament light are associated with disease progression in a mouse model of spinocerebellar ataxia type 3.
Dis Model Mech, 16(9):dmm050144, 04 Sep 2023
Cited by: 0 articles | PMID: 37664882 | PMCID: PMC10499033
Therapeutic effects of engineered exosome-based miR-25 and miR-181a treatment in spinocerebellar ataxia type 3 mice by silencing ATXN3.
Mol Med, 29(1):96, 12 Jul 2023
Cited by: 3 articles | PMID: 37438701 | PMCID: PMC10337053
Tissue-Specific Vulnerability to Apoptosis in Machado-Joseph Disease.
Cells, 12(10):1404, 17 May 2023
Cited by: 3 articles | PMID: 37408238 | PMCID: PMC10216795
Go to all (69) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
RefSeq - NCBI Reference Sequence Database
- (1 citation) RefSeq - NM_004993
Rfam - Collection of RNA families
- (1 citation) Rfam - RF00239
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A new humanized ataxin-3 knock-in mouse model combines the genetic features, pathogenesis of neurons and glia and late disease onset of SCA3/MJD.
Neurobiol Dis, 73:174-188, 07 Oct 2014
Cited by: 37 articles | PMID: 25301414
Upregulation of miR-25 and miR-181 Family Members Correlates with Reduced Expression of ATXN3 in Lymphocytes from SCA3 Patients.
Microrna, 8(1):76-85, 01 Jan 2019
Cited by: 8 articles | PMID: 30147021
Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease.
Mol Ther, 21(10):1898-1908, 14 Jun 2013
Cited by: 70 articles | PMID: 23765441 | PMCID: PMC3808129
Toward understanding Machado-Joseph disease.
Prog Neurobiol, 97(2):239-257, 23 Nov 2011
Cited by: 157 articles | PMID: 22133674 | PMCID: PMC3306771
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: T32 HL007121
NIEHS NIH HHS (1)
Grant ID: P30 ES005605
NINDS NIH HHS (6)
Grant ID: R21 NS067111
Grant ID: R21 NS072229
Grant ID: R01 NS038712
Grant ID: P01 NS050210
Grant ID: NS067111
Grant ID: NS50210





