Abstract
Free full text

Neuro-invasion of Chandipura virus mediates pathogenesis in experimentally infected mice
Abstract
Neuro-tropism is a major feature in many viral infections. Chandipura virus produces neurological symptoms in naturally infected young children and experimentally infected suckling mice. This study was undertaken to find out the neuro-invasive behaviour of Chandipura virus in suckling mice. The suckling mice were infected with the virus via footpad injection. Different tissues were collected at 24-h intervals up to 96-h post infection and processed for virus quantification and histological study. Further confirming the virus predilection to nerves tissues, the adult mice were inoculated with the virus via different routes. The suckling mice experimental results revealed a progressive replication of virus in spinal cord and brain. The progressive-virus replication was not observed in the other tissues like kidney, spleen, liver etc. Histo-pathological lesions noticed in the spinal cord and brain tissues suggested the extensive damages in these tissues. In adult mice experiment, the virus replication observed only in the brain of the mice infected via intra-cerebral route. From this study, we conclude that nervous tissues are predilection sites for Chandipura virus replication in suckling and adult mice. In suckling mice, virus might transmit through nervous tissues for dissemination. In contrast, the adult mice the nervous terminal might not pick up the virus through footpad infection. The pathogenesis in mice might be due to the virus replication mediated damage in the central nervous system.
Introduction
Chandipura virus (CHPV) belonging to the family Rhabdoviridae, genus vesiculovirus, has been associated with acute and fatal encephalitis of young children in several parts of India [1,2]. Children under 15 years of age are vulnerable to natural infection whereas adults are refractory. The retrospective analysis of clinical data from Andhra Pradesh outbreaks suggested that death in children might be due to brain stem encephalitis [3]. These observations suggest that the CHPV has strong neuro-tropic behaviour.
Neuro-tropic viruses such as Rhabdoviurses, Reoviruses, Bunyaviruses, Alphaviruses and Flaviviruses produces pathogenesis in young animals [4-9]. Most of the studies on Semiliki forest virus, Sindbis virus, Japanese encephalitis virus [10] and Reo virus [11] concluded that the neuronal maturation is a critical factor for resistance to viral infection in adult animals. Neuronal maturation correlates not only with resistance to viral replication but also is accompanied by increased cellular resistance to virus-induced apoptosis [12,13]. Similar susceptibility studies in mice have shown that CHPV is lethal to young mice but adults are only susceptible through the intra-cerebral route of infection [14].
Central Nervous System (CNS) infection may occur in part via haematogenous spread or axonal transport from infected peripheral neurons. In Rabies virus infection, the virus spreads by retrograde axonal transport from the peripheral nerves to the neuronal cell body [15]. Neuro-invasion by Herpes viruses requires entry into nerve endings in the periphery and trans-neuronal viral spread [16]. The axonal transport was also reported in some other viruses like West Nile, Herpes simplex virus, Human immunodeficiency virus and Theiler’s disease [17-20]. In Chandipura virus infection, neuro-invasion and axonal transmission of the virus have not been demonstrated. The possible experimental approaches, to prove neuro-invasion, are axonal ligation or degeneration [17], in vitro injection of the virus into squid neuron [21] or engineered virus expressing fluorescent tags [22]. In the present study, we attempted a new approach to study the neuro-invasion of CHPV. In which the viral RNA was quantified in different nervous tissues at different time points after footpad injection. The result was correlated with histo-pathological lesions in different nervous tissues. We hypothesize that if the virus transmits through nervous tissues, the nervous tissue proximal to the inoculation site will have viral RNA at earlier time points and have more viral RNA than those tissues at distal site. Similarly, the histo-pathological lesions start at earlier time points in the nervous tissues close to the inoculation site than other tissues.
In the present study, we inoculated the suckling mice with the live Chandipura virus via right hind footpad. Different tissues were collected at different time points and processed for virus quantification and histology. The findings establish that the virus predilection to the nervous tissues and possible route of dissemination.
Material and method
Cells and virus
The Vero E6 cell line was obtained from the National Centre for Cell Science, Pune, India. The cells were cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal calf serum (FCS, Gibco) and antibiotics. The Chandipura virus strain (034267) was originally isolated from the CHPV outbreak in Andhra Pradesh in 2003 [1]. The virus was propagated and the virus concentration was determined by end point dilution assay. The titer was calculated by the method of Reed and Muench 1937 [23]. The virus titer was expressed as 50% tissue culture infective dose per mL (TCID50/mL).
Suckling mice experimentation
Swiss albino mice were used for the study. Suckling mice (13-days-old) were used in this experiment. The experimental procedure was followed as per the recommendations of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCESA) India and the protocol was approved by the Institute Animal Ethics Committee (IAEC), National Institute of Virology, Pune. The mice were infected with 25 μL (105 TCID50/mL) of CHPV via right footpad injection. Blood was collected through intra-orbital route before the sacrifice and the sera were separated. Mice were perfused through trans-cardial route with 20 mL of PBS and different tissues including brain, sciatic nerve, lower spinal cord (below lumbar region), upper spinal cord (above lumbar region), liver, spleen, thymus, lungs, heart, tail, and legs were collected at 0, 24, 48, 72, and 96 h PI and frozen immediately at -80 °C. In some experiments, the tissues were preserved in 10% formalin in saline. Before use, the frozen brain, liver, spleen, thymus, lungs, heart, kidney was freeze thawed and 10% homogenate was prepared in PBS. The suspension was clarified at 1000 x g for 10 min and the virus in the supernatant was titrated. Pools of sciatic, lower spinal cord, upper spinal cord, mid brain, cerebellum, and cerebrum tissues were prepared separately from three mice and directly homogenized in RNA lysis buffer supplied along with PureLink RNA mini kit (Invitrogen).
Quantification of viral RNA copies by real time RT-PCR
The real time RT-PCR assay was carried out by the method as described earlier [24] with slight modification. Briefly, the viral RNA was isolated from 10-100 mg of tissue using PureLink RNA mini kit (Invitrogen). The RNA was eluted in 50 μL volumes and 5 μL was used for RT-PCR. The real time RT-PCR assay was performed by using the SuperScript III Platinum One-Step Quantitative RT-PCR System (Invitrogen) in Rotor-Gene Q 5-plex real time PCR machine (Qiagen). The viral RNA copy of the sample was calculated by the standard curve drawn against the standard viral RNA and expressed in copies per mL.
Histology
Tissues were fixed in formalin for 24–48 h and then embedded in paraffin. Sections were cut at 3 μm and stained with hematoxylin and eosin. The sections were examined under microscope (Axioskop 40 FL, Zeiss) and the lesions were documented.
TUNEL assay
The TUNEL assay was performed on 3 μm paraffin section. The deparaffinised and rehydrated sections were pre-digested with 0.02 mg/ml of proteinase K (NEB) for 20 minutes at room temperature (RT). Optimal conditions for digestion were previously determined by titration experiments. Tissue sections were analyzed for in situ apoptotic DNA fragmentation using the terminal transferase TdT-mediated dUTP-biotin end-labelling (TUNEL) assay using Dead End Colorimetric TUNEL system as per the manufacturer’s protocol (Promega). Negative controls consisted of sections in which the terminal transferase was omitted. For positive controls, some tissue sections were treated with DNase I (10 U/section) supplied along with the kit. The sections were then counterstained with Mayer’s hematoxylin and mounted in glycerol.
Adult mice experiment
Swiss albino mice (60-days-old) were divided into four groups (n = 15 per group). The group I mice was infected with 25 μL (105 TCID50/mL) of CHPV via intra-cerebral route. The group II, III, and IV mice received virus (25 μL) via intra-venous, intra-peritoneal, and footpad at right hind foot route respectively. Brain was collected and processed for virus titration. The mortality pattern was also recorded in these mice.
Result
CHPV infect the nervous tissues and produce pathogenesis
The suckling mice were infected with the virus via right footpad injection. Real time RT-PCR method was employed to quantify the viral RNA form nervous tissues and endpoint dilution method was used to quantify the virus titre from other tissues. Based on the availability on amount of tissues, endpoint dilution or real time RT-PCR technique was decided. A time dependent increase in viral RNA was observed in all nervous tissues tested in this study (Table 1). At 24-h PI, no detectable level of viral RNA was noticed in the cerebrum but detectable level was noticed in the spinal cord tissues. At 96-h PI, all the nervous tissues were positive for viral RNA and the level was less in left sciatic nerve compared to other nervous tissues. All other tissues, other than nervous tissues, the virus titre was peaking at 24-h PI and then declined in a time dependent manner (Figure 1). In the periphery, detectable level of viral RNA was noticed only in legs at 96-h PI. Viral RNA was not detected in the tail and skin (Table 2). Progressive-replication of virus only noticed in the nervous tissues.
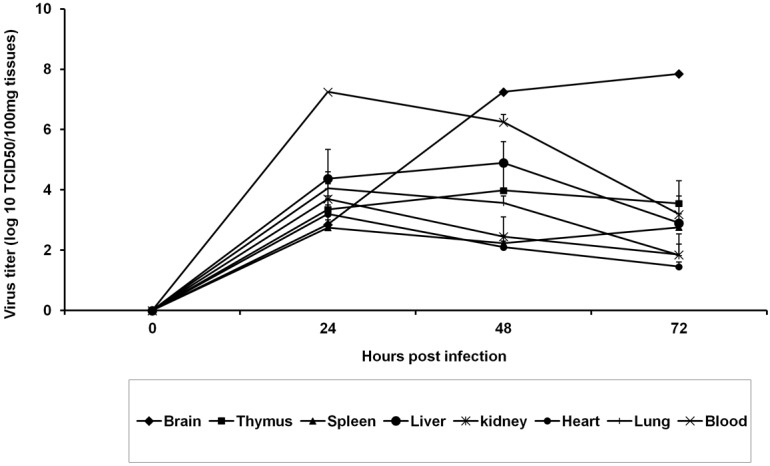
Time course of virus replication in suckling mice inoculated via right food pad region. Data point represents virus titre (mean+SE) of the log10 TCID50%/100 mg of tissue from individual animals (three animals per pool) at 24, 48 and, 72 h post infections.
Table 1
Mean viral RNA copies in different nervous tissues collected from CHPV infected suckling mice at different hours post infection
| HPI | LSN | RSN | LSC | USC | MB | CBM | CRM |
|---|---|---|---|---|---|---|---|
| 24 | ND | ND | 103.91 | 104.41 | 104.43 | 104.93 | ND |
| 48 | ND | ND | 105.78 | 106.69 | 105.77 | 104.92 | 105.95 |
| 72 | ND | 103.56 | 107.9 | 107.84 | 107.62 | 108.00 | 107.88 |
| 96 | 103.32 | 105.83 | 108.20 | 107.61 | 107.99 | 107.31 | 107.70 |
Note: The values are viral RNA copies per mL from a pool of tissues from three mice and the experiment was repeated in twice. ND, not detected; HPI, hours post infection; LSN, left sciatic nerve; RSN, right sciatic nerve; LSC, lower spinal cord; USC, upper spinal cord; MB, mid brain; CBM, cerebellum; CRM, cerebrum.
Table 2
Mean viral RNA copies in different tissues collected from CHPV infected suckling mice at different hours post infection
| HPI | LHL | RHL | LFL | RFL | Tail | Skin |
|---|---|---|---|---|---|---|
| 24 | 101.71 | 104.87 | 102.38 | 104.41 | ND | ND |
| 48 | 102.54 | 104.35 | 102.35 | 102.10 | ND | ND |
| 72 | 103.83 | 104.85 | 102.45 | 102.03 | ND | ND |
| 96 | 101.69 | 104.57 | 103.57 | 104.36 | ND | ND |
Note: The values are viral RNA copies per mL from a pool of tissues from three mice and the experiment was repeated in twice. ND, not detected; LHL, left hind leg; RHL, right hind leg; LFL, left foreleg; RFL, right foreleg.
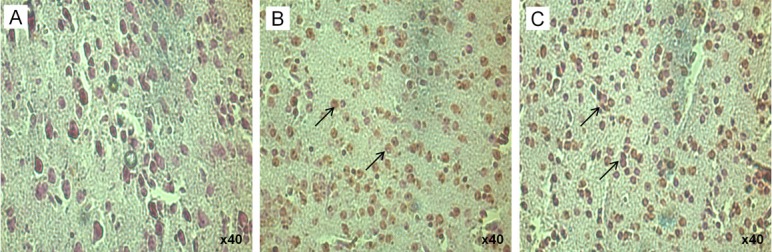
Histological lesions were consistently observed in all nervous tissues including lower and upper spinal cord, and brain. In lower spinal cord, the lesions started at 48-h PI onwards and the lesions like perivascular cuffing, congestion and degenerative and necrotic changes were noticed (Figure 2). At 72-h PI, moderate to high vacuolar degeneration and condensation was noticed in the lower spinal cord. In the brain, the lesions are visible from 72-h PI onwards and the lesion includes perivascular cuffing and congestion (Figure 3). At 96-h PI, lower spinal cord showed perivascular changes with oedematous changes and central chromatolysis. Severe neuronal vascular degeneration and mild gliosis were observed in the upper spinal cord. Oedema, crowding of glial cells and vacuolation observed in the brain (Figure 4). Mild haemorrhage was present in the heart. TUNEL positive nuclei were noticed in the cortex of the brain as early as 24-h PI (Figure 5). Gross neurological symptoms like hemiplegic or paraplegia of the hind limb, circling movement, eye lesions, difficult to suck milk from the mother were noticed in infected suckling mice.
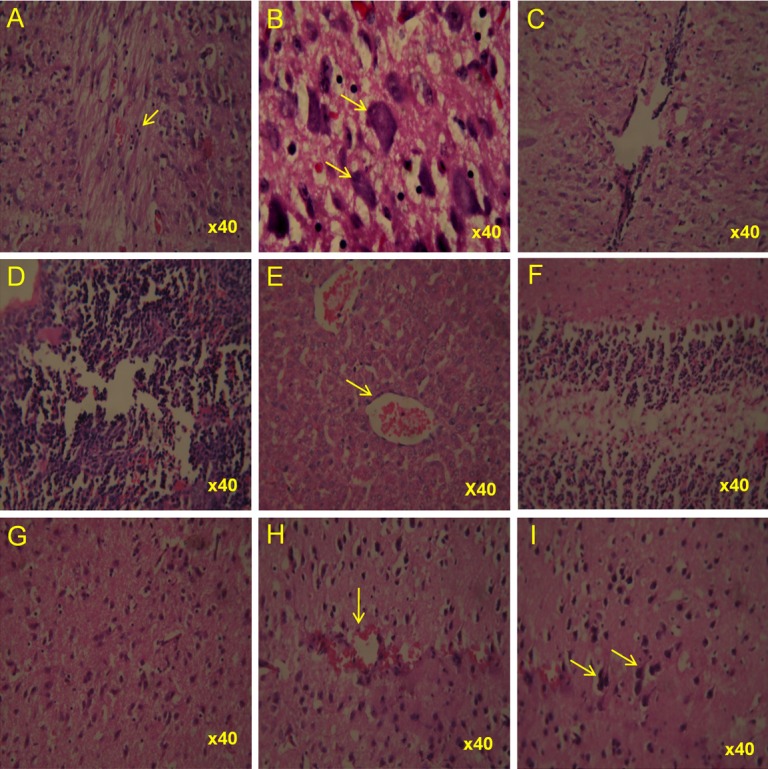
Haematoxylin & Eosin stained tissues from CHPV infected suckling mice at 48 hours post-infection. Representative photomicrographs showing lesions in selected tissues. Lower spinal cord showing (A) moderate perivascular cuffing (B) a cluster of neurons exhibiting early stage of degenerative and necrotic changes including condensation and central chromatolysis (C) mild congestion as well as perivascular cuffing (D) thymus showing mild degenerative changes in the form of mild depletion of its constituent cells (E) liver showing mild congestion, Brain showing (F) mild disturbance in the cellular constituents in the cortical region of cerebellum (G) moderate gliosis (H) mild congestion as well as glial cell proliferation (I) few degenerating neurons with basophilic nuclei indicating early degeneration.
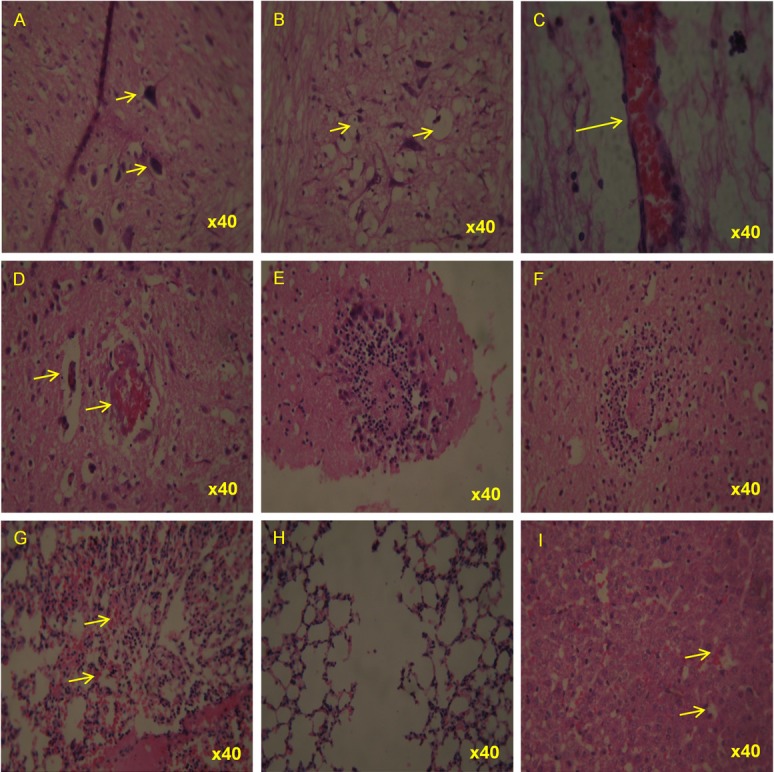
Haematoxylin & Eosin stained tissues from CHPV infected suckling mice at 72 hours post-infection. Representative photomicrographs showing lesions in selected tissues. Lower spinal cord showing (A) varying degree of neuronal degeneration including condensation and vacuolation (B) massive vacuolar degenerative changes upper spinal cord showing (C) severe congestion (D) moderate congestion and perivascular cuffing at adjacent smaller vessels (E) infiltration of mononuclear cells and aggregation of glial cells around the necrotic area (F) focal necrosis surrounded by granulocytic and stray glial cells lung showing (G) moderate congestion and inter alveolar haemorrhage (H) emphysematous changes of alveoli resulting in bullae formation (I) liver showing mild vacuolar degeneration of hepatocytes and sinusoidal congestion.
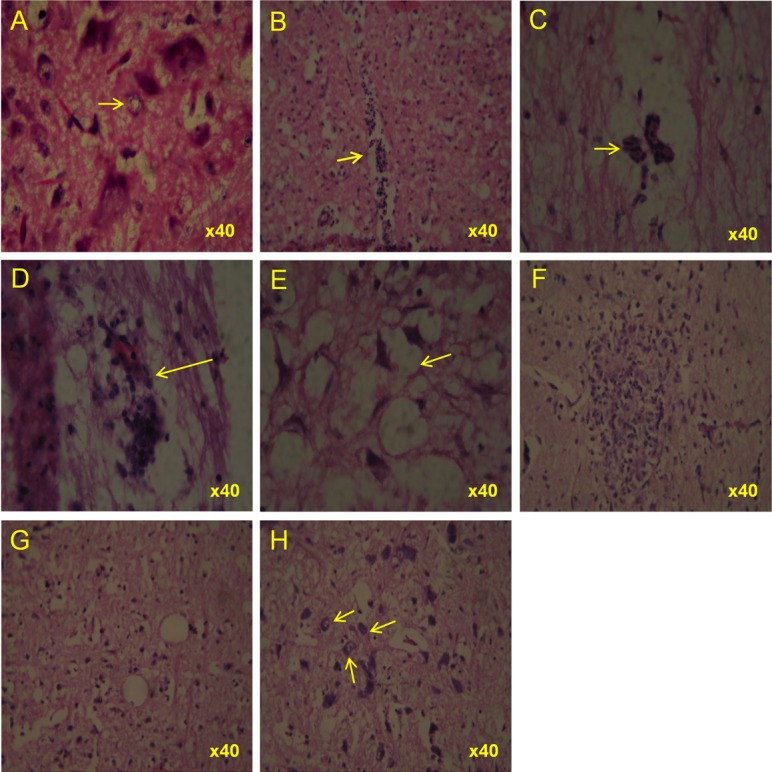
Haematoxylin & Eosin stained tissues from CHPV infected suckling mice at 96 hours post-infection. Representative photomicrographs showing lesions in selected tissues. Lower spinal cord showing (A) characteristically the central chromatolysis of neuron (pale in colour) (B) moderate perivascular cuffing with vacuolar degeneration (C) perivascular changes with edematous changes. Upper spinal cord showing (D) moderate perivascular cuffing (E) severe neuronal vacuolar degeneration and mild gliosis. Brain showing (F) neuronophagia in which necrotic neuron is surrounded by a cluster of glial cells (G) edema and vacuolation of neurons with moderate crowding of glial cells (H) variable necrotic changes of neurons.
CNS tissues are predilection sites for CHPV replication
Adult mice were used to prove the predilection of virus to nervous tissues. The adult mice were infected via different routes. The brain was collected at defined time points and processed for virus titration. In brain, progressive virus replication was observed in group of mice which was infected through intra-cranial route and the virus titre reached to 105 TCID50/100 μL at 72-h PI (Figure 6). Most of the mice in these groups died at 96-h PI. Gross neurological symptoms like hemiplegic or paraplegia of the hind limb, circling movement, eye lesions were noticed in infected adult mice. Undetectable level of virus was observed in the brain of mice infected via footpad, intra- peritoneal and intra-venous route. No mortality was recorded from these groups.

Time course of virus replication in brain of adult mice inoculated via intra cranial, intra-venous, intra-peritoneal and footpad. Data point represents virus titre (mean+SE) of the log10 TCID50%/100 mg of brain tissues from individual animals (three animals per pool) at 24, 48 and, 72 h post infection.
Discussion
Neuro-tropism is a major feature of many viruses. The CHPV infected children had shown the symptoms similar to brain stem encephalitis [3]. Experimental CHPV infection in suckling mice also confirmed the CNS infection [25]. In the present study, we have demonstrated that the CHPV infected and replicated in the spinal cord and brain, and produced tissue damage which led to pathogenesis. We also confirmed that progressive viral replication occurred almost exclusively in the nervous tissues. Although the route of dissemination is not fully explored in this study, there is some indication that the virus may use nervous tissues for transmission and dissemination.
Nervous tissues are said to be a immunologically privilege sites which may not accessible to the immune system. The intact central nervous system is non permeable to circulatory antibodies and immune cells. This could be the reason for progressive replication of virus in the nervous tissues. However, the rapid production of CHPV specific IgM in blood clears the virus from circulation and other tissues [25]. In our earlier study we reported the passive immunization in mice an hour after virus infection protected less than 50% of mice. In addition, the mice treated with the same antibody 24-h post infection fail to protect the mice from pathogenesis [25]. The present and the earlier passive immunization study results suggest that the virus might hide in the nervous tissues immediately after experimental virus inoculation. In suckling mice, the CNS may act as an epicentre for virus replication and disseminate virus into the other tissues. In other tissues, the free virus might be removed by the CHPV specific IgM in the circulation. This may be the reason behind the reduction of the virus titre in these tissues.
The lower spinal cord is very close to the virus inoculation site. Histo-pathological lesions in the lower spinal cord which was observed as early as 48-h PI suggested the virus may be reached and replicated in these tissues earlier than other nervous tissues tested in this study. This observation compels us to believe the chance of axonal transmission of CHPV during infection. The presence of viral RNA in the left sciatic nerve (opposite to inoculation site) and all four legs (extremities) also suggested the virus might be disseminated through nervous tissues.
Virus replication in the adult mice brain further confirmed the CHPV predilection to the nervous tissues. This observation also indicate that like JE, Reo, Semiliki virus the neuronal maturation does not have any impact on virus replication in adult mice. The undetectable level of virus in the brain of adult mice infected through footpad, intra-peritoneal, intra-venous route indicated that the nerve terminals at the inoculation site might not pick up and transmitted the virus.
Overall, from these results we conclude that CNS is a predilection site for CHPV replication. Virus replication mediated damages in nervous tissues might lead to pathogenesis in mice. There is a possibility that the virus might transmit through nervous tissues in suckling mice. However, detail study is necessary to prove the axonal transmission of Chandipura virus in mice.
Acknowledgements
We thank Director, National Institute of Virology for arranging in house grant for entire research. The author acknowledge to Drs. K. Alagarasu and Cecilia D for critical review of manuscript.
References
Articles from International Journal of Clinical and Experimental Pathology are provided here courtesy of e-Century Publishing Corporation
Citations & impact
Impact metrics
Citations of article over time
Article citations
Re-emerging <i>Chandipura</i> <i>vesiculovirus</i>: A cause of concern for global health.
Virusdisease, 35(3):385-399, 01 Sep 2024
Cited by: 0 articles | PMID: 39464728
Review
Chandipura Virus Forms Cytoplasmic Inclusion Bodies through Phase Separation and Proviral Association of Cellular Protein Kinase R and Stress Granule Protein TIA-1.
Viruses, 16(7):1027, 26 Jun 2024
Cited by: 1 article | PMID: 39066190 | PMCID: PMC11281494
Chandipura viral glycoprotein (CNV-G) promotes Gectosome generation and enables delivery of intracellular therapeutics.
Mol Ther, 32(7):2264-2285, 03 May 2024
Cited by: 0 articles | PMID: 38702887
Chandipura virus dysregulates the expression of hsa-miR-21-5p to activate NF-κB in human microglial cells.
J Biomed Sci, 28(1):52, 07 Jul 2021
Cited by: 5 articles | PMID: 34233673 | PMCID: PMC8265105
Analysis of the dark proteome of Chandipura virus reveals maximum propensity for intrinsic disorder in phosphoprotein.
Sci Rep, 11(1):13253, 24 Jun 2021
Cited by: 9 articles | PMID: 34168211 | PMCID: PMC8225862
Go to all (6) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Immune response during acute Chandipura viral infection in experimentally infected susceptible mice.
Virol J, 5:121, 20 Oct 2008
Cited by: 18 articles | PMID: 18937835 | PMCID: PMC2577095
Role of cell surface vimentin in Chandipura virus replication in Neuro-2a cells.
Virus Res, 285:198014, 08 May 2020
Cited by: 8 articles | PMID: 32418904 | PMCID: PMC7270567
Pathogenesis of experimental vesicular stomatitis virus (New Jersey serotype) infection in the deer mouse (Peromyscus maniculatus).
Vet Pathol, 38(4):396-406, 01 Jul 2001
Cited by: 32 articles | PMID: 11467473
Reviewing Chandipura: a vesiculovirus in human epidemics.
Biosci Rep, 27(4-5):275-298, 01 Oct 2007
Cited by: 39 articles | PMID: 17610154 | PMCID: PMC7087735
Review Free full text in Europe PMC