Abstract
Free full text

Gambogic acid moderates cardiac responses to chronic hypoxia likely by acting on the proteasome and NF-κB pathway
Abstract
Gambogic acid (GA) is the principal active ingredient of gamboges. GA was reported to exert anti-tumor and anti-inflammatory effects both in vitro and in vivo. Previously, we have shown that GA is a more tissue-specific proteasome inhibitor than bortezomib and it is less toxic to peripheral white blood cells compared to bortezomib. Ubiquitous proteasome inhibition was shown by some reports, but not by others, to prevent cardiac remodeling in response to pressure overload by blocking the NF-κB pathway; however, whether GA modulates the development of chronic hypoxia-induced right ventricular hypertrophy has not been investigated yet. Here we report that GA can significantly attenuate right ventricular hypertrophy induced by chronic hypoxia, reduce cardiac fibrosis, and remarkably block the reactivation of bona fide fetal genes in the cardiac tissue. Furthermore, we also investigated the potential molecular targets of GA on right ventricular hypertrophy. The results showed that GA could accumulate the IκB levels associated with decreased proteasomal activity, block the translocation of NF-κB from the cytoplasm to the nucleus, decrease NF-κB DNA-binding activity, and reduce IL-2 levels. In conclusion, GA is capable of preventing the development of chronic hypoxia-induced right ventricular hypertrophy. GA has great potential to be developed into an effective anti-hypertrophy agent.
Introduction
Right ventricular hypertrophy occurs in a variety of clinical situations including pulmonary arterial hypertension (PAH), congenital or valvular heart disease, etc., which eventually often results in right-sided heart failure. Right-sided heart failure is the leading cause of death in the majority of severe PAH patients [1]. Therefore, it is clinically highly significant to intervene the development of right ventricular hypertrophy. Although many anti-hypertrophy agents, such as angiotensin converting enzyme inhibitors (ACEIs), Ca2+ channel antagonist, and β-receptor blockers, have been reported [2], the effects are still not satisfactory in decreasing cell size because of the existence of overwhelmingly redundant prohypertrophic signaling pathways [3], which highlights an urgent need for novel therapeutic strategies and agents.
The ubiquitin-proteasome system (UPS) is the main non-lysosomal protein degradation pathway, responsible for the degradation of most intracellular proteins, normal and abnormal [4,5]. Current research data have shown that the functional alteration of the UPS is closely related to the development of cardiac hypertrophy. The increase of myocardial proteasome expression and activity in the heart with pressure overloaded left ventricular hypertrophy has been shown. However, there is a controversy regarding the effect of proteasome inhibition on pressure overloaded hearts [6]. Several reports showed that proteasome inhibition by proteasome inhibitors, such as epoxomicin, MG132 (Z-Leu-Leu-Leu-al), bortezomib and PS519, could prevent the cardiac remodeling in response to pressure overload [7-11]. Several signal pathways have been reported to be relevant to cardiac hypertrophy such as Akt, calcineurin/NFAT, MAPK and TGF-β1/Smad [7-11]. However, Tang et al has reported that proteasome inhibition activates the calcineurin-NFAT pathway in cardiomyocytes and promotes maladaptive remodeling of stressed mouse hearts [12]. More recently, Herrmann et al showed in a large animal model that intermittent ubiquitous primary proteasome inhibition is sufficient to cause cardiac functional and structural abnormalities that are consistent with hypertrophic-restrictive cardiomyopathy phenotype [6,13]. However, virtually all these previous studies on the effect of proteasome inhibition on cardiac hypertrophy and remodeling have been centered on the left ventricle and primarily left heart failure. One recent study has reported UPS alterations in right ventricle pressure overload hypertrophy and heart failure, which suggests proteasome functional impairment contributes to the development of cardiac hypertrophy and heart failure due to pressure overload to the right ventricle [14]. It remains unclear whether and how proteasome inhibition modulates cardiac responses to chronic exposure to hypoxia.
It also should be noted that the proteasome inhibitors used by virtually all previous studies are non-tissue-specific and are non-selective for proteasome-subtypes. It has been demonstrated that there is differential susceptibility of cardiac proteasome subtypes to proteasome inhibitors [15]. Therefore, we propose that a search for more effective and tissue-selective proteasome inhibitors may help resolve the debate on the effect of proteasome inhibition on cardiac hypertrophy and remodeling.
Numerous studies have suggested that gambogic acid (GA), a main active ingredient of gamboges, has many kinds of biological activities such as anti-tumor, anti-inflammatory, and so on [16-18]. The anti-tumor effect of GA has been extensively studied and GA has been approved by Chinese FDA for clinical trial in cancer therapy. We and others have most recently reported that the proteasome is the most important molecular target of GA [19,20]. Our previous study showed that GA is a more tissue-specific proteasome inhibitor with lower toxicity, compared with bortezomib [20]. Additionally, it has been found that GA could also directly target the upstream molecule of NF-κB, thus inhibiting NF-κB activation [21]. Although proteasome inhibitors such as bortezomib have been extensively studied and some of the studies showed its effectiveness in animal models of cardiac hypertrophy and myocardial ischemia/reperfusion [22-24], the non-anticancer effects of GA have not been reported.
NF-κB is an ubiquitously inducible transcription factor, which can activate expression of many genes, including the genes associated with immune, inflammatory responses, cell survival, and apoptosis [25]. The phosphorylation and degradation of IκB protein is a decisive step in NF-κB activation, which allows NF-κB to rapidly translocate into the nucleus and stimulate the expression of multiple target genes. Hence, specific inhibition of IκB ubiquitination or non-specific inhibition of the proteasome can effectively block NF-κB activation. Previous research has also showed that the NF-κB pathway is closely associated with cardiac hypertrophy and pathological remodeling, and the activation of NF-κB pathway is essential in the process of the development of cardiac hypertrophy [26]. Genetic manipulation of a muscle-specific ubiquitin E3 ligase has been shown to modulate cardiac hypertrophy via impacting on NF-κB signaling in the heart [27].
Therefore, we put forward the hypothesis: gambogic acid can inhibit proteasome function, block the activation of the NF-κB pathway and thereby prevent the development of right ventricular hypertrophy in response to chronic hypoxia. In the present study, we have demonstrated that gambogic acid protects against right ventricular hypertrophy caused by chronic hypoxia. And our data suggest that proteasome inhibition and blocking NF-κB activation are among the potential underlying mechanisms.
Materials and methods
Animal models of cardiac hypertrophy
All animal protocols were approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University (Guangzhou, China). The male Sprague-Dawley rats aged 6 weeks old were obtained from Guangdong Laboratory Animal Monitoring Institute. All animals were allowed free access to drinking water and a standard laboratory diet.
Right ventricular hypertrophy caused by chronic hypoxia
The animal models were performed as described previously [28]. All animals were randomly divided into 4 groups (5 rats per group): vehicle-treated normoxia group (Normoxia+Vehicle); GA-treated normoxia group (Normoxia+GA); vehicle-treated hypoxia group (Hypoxia+Vehicle); GA-treated hypoxia group (Hypoxia+GA). The hypoxic rats were placed to a hypobaric chamber (oxygen concentration controlled at about 10%) for 21 consecutive days, and the normoxic rats were kept under normoxic conditions. GA or vehicle was administered via daily intraperitoneal injections (0.75 mg/kg) for 20 consecutive days.
Histological analysis
Rats were sacrificed by cervical dislocation at designated times. Hearts were rapidly removed from the body, washed with cold PBS, then weighed; some heart samples were fixed with 4% paraformaldehyde, others were frozen in liquid nitrogen and then stored at -80°C. Fixed heart samples were used for paraffin section, the thickness of tissue section is 5 μm. Tissue sections were stained with hematoxylin-eosin (HE) and Masson’s trichrome. The latter was used to examine extracellular matrix or collagen [9].
Real-time PCR
Frozen heart samples were homogenized in TRIzol reagent (Invitrogen, USA) using an electrical tissue homogenizer and the isolation of RNA was performed according to the manufacturer’s protocol. The ratio of the absorbance at 260 nm and 280 nm was used to assess the purity of RNA. The conversion of RNA to cDNA and the amplification of RNA were performed according to PrimeScript® RT Master Mix Perfect Real Time assay kit (Tekara, Japan). The targeted genes included α-skeletal-actin (α-SK-actin), brain natriuretic peptide (BNP), and hypoxanthine phosphoribosyltransferase (HPRT). The results of the fetal genes are presented as relative expression to HPRT using the 2-ΔΔCt method. Real-time PCR was performed using an ABI 7500 instrument (Applied Biosystems, USA). Primer sequences were as follows: α-SK-actin-forward: 5’-TCA CTT CCT ACC CTC GGC AC-3’; α-SK-actin-reverse: 5’-AGG CCA GAG CCG TTG TCA CA-3’; BNP-forward: 5’-GGT CTC AAG ACA GCC CTT C-3’; BNP-reverse: 5’-ACA ACC TCA GCC CGT CAC AG-3’; HPRT-forward: 5’-GTA ATG ATC AGT CAA CGG GGG AC-3’; HPRT-reverse: 5’-CCA GCA AGC TTG CAA CCT TAA CCA-3’.
Chymotrypsin (CT)-like peptidase activity assay
CT-like peptidase assay was performed as described previously with the synthetic fluorogenic peptide substrate (Suc-LLVY-AMC) (Calbiochem, USA) [20]. Briefly, Cardiac tissues were homogenized in assay buffer (20 mM Tris-HCl, pH 7.5) to extract proteins, centrifuged at 12000 rpm for 10 min at 4°C, and then total protein concentrations of the supernatants were determined using BCA protein assay kit (Bio-rad, USA), followed by co-incubation with Suc-LLVY-AMC for 2.5 hours at 37°C. Luminescence was detected with a luminescence microplate reader (Varioskan Flash 3001, Thermo, USA) with 355 nm excitation and 460 nm emission wavelengths.
Western blot analysis
Western blot was performed as described previously [7,9]. Briefly, total protein was extracted from heart tissue, an equal amount of total protein was separated by 12% SDS-PAGE, and then transferred to PVDF membrane. Primary antibodies and horseradish peroxidase (HRP)-conjugated appropriate secondary antibodies (Santa Cruz, USA) were used to detect the designated proteins. The immune complex on the PVDF membrane was detected with a enhanced chemiluminescence (ECL) system, and exposed to X-ray films (Kodak, Japan). The x-ray film was scanned and digitalized using a high-resolution scanner.
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Cardiac tissues were homogenized in a cold phosphate-buffered saline, and then nuclear extracts were prepared using nuclear and cytoplasmic protein extraction kit (Kaiji, China) according to the manufacturer’s instructions. Electrophoretic mobility shift assay was performed as described previously [29]. In brief, the oligonucleotide probe was end-labeled with DIG-dUTP and 0.4 ng/μl labeled probes were prepared using DIG Gel Shift kit (Roche, Germany). Nuclear proteins were incubated for 20 min at room temperature with 20 μl binding buffer (0.5 μg poly (dI-dC), 0.1 μg poly L-lysine, 0.8 μg labeled oligonucleotide). The formed DNA-protein complexes were resolved in a 8% native polyacrylamide gel in a TBE buffer (890 mM Tris; 890 mM boric acid; 20 mM EDTA pH 8.0) at 100 V for 1.5-2 h, transferred to positively charged nylon membrane for 30 min with 400 mA, and then cross-linked for 3 min with 120 mJ using a UV cross-linker. Thereafter the membrane was washed, blocked, incubated, and detected in different solutions according to the manufacturer's instructions. Finally, the membrane was exposed to X-ray films (Kodak) and the x-ray films were scanned using a high-resolution scanner.
Statistical analyses
All data are expressed as mean ± SE. one-way ANOVA was used to test for differences among groups using SPSS 11.0 software (SPSS Inc., USA), P value of less than 0.05 was considered statistically significant. GraphPad Prism 5.0 software (GraphPad Inc., USA) was used to generate graphs.
Results
Changes in cardiac gravimetric parameters
The rats of different experimental groups were weighed daily to assess the effect of GA on body weight (Figure 1A). Compared with the normoxic groups, hypoxia clearly slowed down the increase in rat body weight in both vehicle-treated and GA-treated groups. The changes of body weight in Hypoxia+vehicle and Hypoxia+GA groups were similar, which provides indirect evidence that the toxicity of GA was low. Compared with Normoxia+vehicle group, the ratio of heart weight (mg) (HW) to body weight (g) (BW) was significantly increased in Hypoxia+vehicle group (P < 0.05). Compared with the hypoxia+vehicle group, the ratio of HW/BW was significantly decreased in hypoxia+GA group (P < 0.05) (Figure 1B).
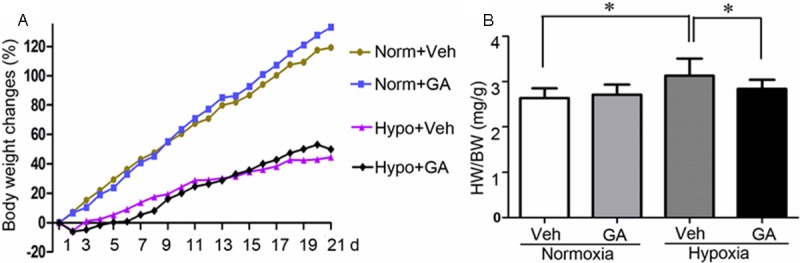
Changes in gravimetric parameters. A: Temporal changes in the body weight of rats exposed to chronic hypoxia. Rats undergoing hypoxia (Hypo) or control normoxia (Norm) receiving gambogic acid (GA) or vehicle control (Veh) treatment were weighed daily. B: the changes in the heart weight (mg) to body weight (g) ratio (HW/BW) at the terminal experiments. N = 5 rats/group, *p < 0.05.
Histopathological analysis
To assess the cardiomyocyte hypertrophy in rats exposed to chronic hypoxia, HE staining was performed. It was found that the arrangement of myocardial cells are normal and no obvious hyperplasia were observed in interstitial tissue in both normoxia groups, whereas obvious cardiomyocyte thickening (a sign of cardiac hypertrophy) and myofiber disarray were present in the Hypoxia+Vehicle group. Cardiac hypertrophy and myofiber disarray were attenuated by administration of GA (Figure 2A). The hypertrophic response of cardiomyocytes to chronic hypoxia often involves fibrosis, thus Masson’s trichrome was performed. Remarkable increases in interstitial fibrosis (areas stained blue by trichrome staining) were detected in rat hearts of the Hypoxia+Vehicle group but not those of the Hypoxia+GA group (Figure 3B). The results indicated increased myocardial interstitial fibrosis in rats exposed to chronic hypoxia is largely prevented by GA treatment.
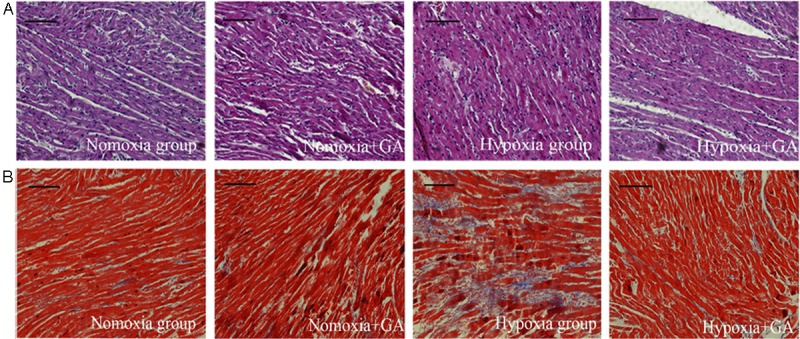
Histopathological changes. Myocardial tissue collected form the indicated groups were fixed and processed for collection of paraffin-embedded tissue sections. A: representative micrographs of H-E stained myocardial sections. Increases of myofiber thickness and myofiber disarray in rats exposed to chronic hypoxia were attenuated by gambogic acid (GA) treatment. B: representative micrographs of myocardial sections with Masson’s trichrome staining. Areas with blue staining are interstitial fibrotic tissues. Cardiac fibrosis remarkably increased in rats exposed to chronic hypoxia but this increase was considerably reduced by GA treatment. Scale bar = 100 μm.
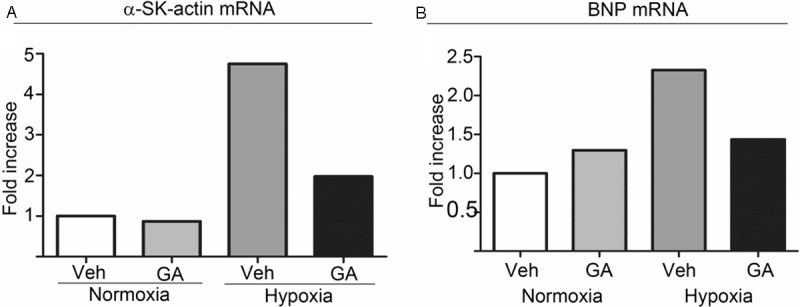
The relative mRNA expression levels of α-SK-actin and BNP in the cardiac tissue. The relative mRNA expression levels of α-SK-actin (A) and BNP (B) in the cardiac tissue of rats exposed to chronic hypoxia were measured using by real-time PCR. Results of a representative cohort out of 3 sets are shown. GA, gambogic acid; Veh, vehicle.
GA down-regulated the relative expression levels of α-skeletal-actin and BNP mRNA in the cardiac tissues
In general, cardiac pathology including pathological cardiac hypertrophy is often associated with the reactivation of the fetal gene program consisting of genes that are highly expressed in the fetal hearts but down-regulated in adults, such as α-skeletal actin (α-SK-actin) and BNP [30]. The relative mRNA expression levels of α-SK-actin (Figure 3A) and BNP (Figure 3B) in the cardiac tissue of rats exposed to chronic hypoxia increased significantly and the increase was markedly attenuated by GA treatment.
GA treatment led to reduced proteasome peptidase activity and increased levels of IκBα in cardiac tissue
Because NF-κB activation can be regulated by UPS-mediated degradation of its inhibitor IκB and GA has been shown to inhibit proteasome in a relatively tissue-specific manner, it is necessary to examine myocardial proteasome function status in the control and GA-treated groups. We therefore performed the proteasomal CT-like peptidase assay to provide the direct evidence for GA inhibiting cardiac proteasomes. As shown in Figure 4A, although there is no statistically significant difference in myocardial CT-like activity between the Normoxia+Vehicle and the Nomroxia+GA groups myocardial CT-like peptidase activity in the Hypoxia+GA group was significantly lower than that in the Hypoxia+Vehicle group (p < 0.01). These results suggest that GA treatment shows no significant effect on proteasome activity in normoxic myocardium but significantly inhibits proteasome activity in hypoxic myocardium, which is consistent with the previous report [19].
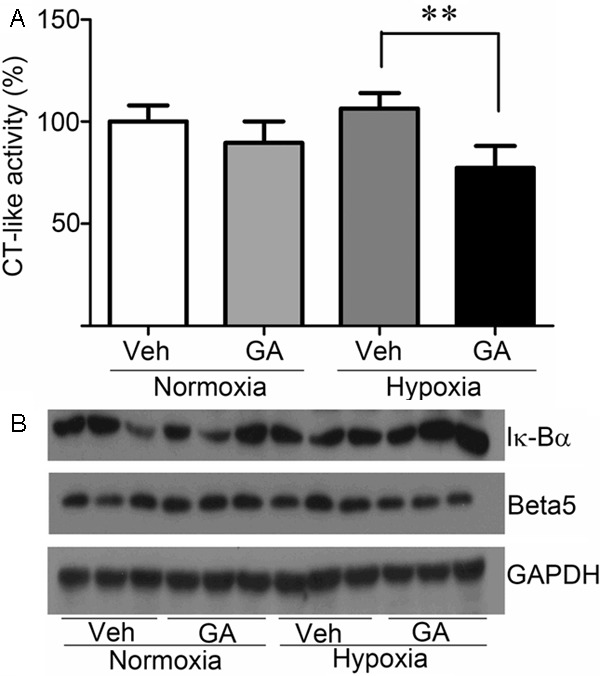
Gambogic acid (GA) treatment inhibited the proteasome activity and increased IκBα accumulation in cardiac tissues of rats with chronic hypoxic exposure. A: changes of myocardial proteasomal chymotrypsin-like (CT-like) peptidase activity in right ventricular hypertrophy induced by chronic hypoxia. **p < 0.01. B: western blot images for the protein levels of β5 proteasome subunit and IκBα in right ventricular hypertrophy induced by chronic hypoxia. GAPDH was probed as a loading control.
To investigate further the proteasome function and the levels of IκBα in cardiac tissues, the protein levels of the β5 subunit of the 20 S proteasome and IκBα were examined by western blot analyses using whole myocardial tissue extracts. As shown in Figure 4B, compared with the Normoxia+Vehicle group, β5 subunit levels were modestly increased in the cardiac tissues of the Hypoxia+Vehicle group but more importantly β5 subunit levels were considerably lower in the Hypoxia+GA group than the Hypoixa+Vehicle group. This is consistent with the results of CT-like peptidase activity assay. Conversely, the levels of IκBα were obviously higher in the Hypoxia+GA group than the Hypoixa+Vehicle group. Taken together, these results suggest that the proteasome inhibition by GA may have contributed to the increase in the levels of IκBα, possibly involving in inhibiting the activation of NF-κB pathway.
GA could inhibit NF-κB translocation from the cytoplasm to the nucleus in the heart of rats exposed to chronic hypoxia
The NF-κB pathway plays an important role in cardiac remodeling under pathological conditions and has proven to be an essential factor in the process of the development of cardiac hypertrophy in vivo [26]. NF-κB translocation from the cytoplasm to the nucleus, where it binds to specific sequences of target genes, is an important process in the activation of the NF-κB pathway. As shown in Figure 5A, the nuclear levels of the p65 subunit of NF-κB were remarkably increased in the cardiac tissues of rats exposed to chronic hypoxia. Moreover, the increase in nuclear p65 was significantly inhibited by GA treatment. As shown in Figure 5B, the protein levels of IL2, an NF-κB target gene, in the cardiac tissues of rats exposed to chronic hypoxia were increased but this increase was significantly attenuated by GA treatment. Indeed, as shown in Figure 5C, the increased NF-κB DNA-binding activity in hypoxic hearts was obviously suppressed by GA treatment. These data provide consistent evidence that GA could inhibit NF-κB translocation from the cytoplasm to the nucleus in cardiac tissues of rats undergoing chronic hypoxia. This is in line with the notion that inflammatory mediators contribute to cardiac remodeling [30], and the activation of the NF-κB pathway is involved by promoting the release of inflammatory mediators [31].
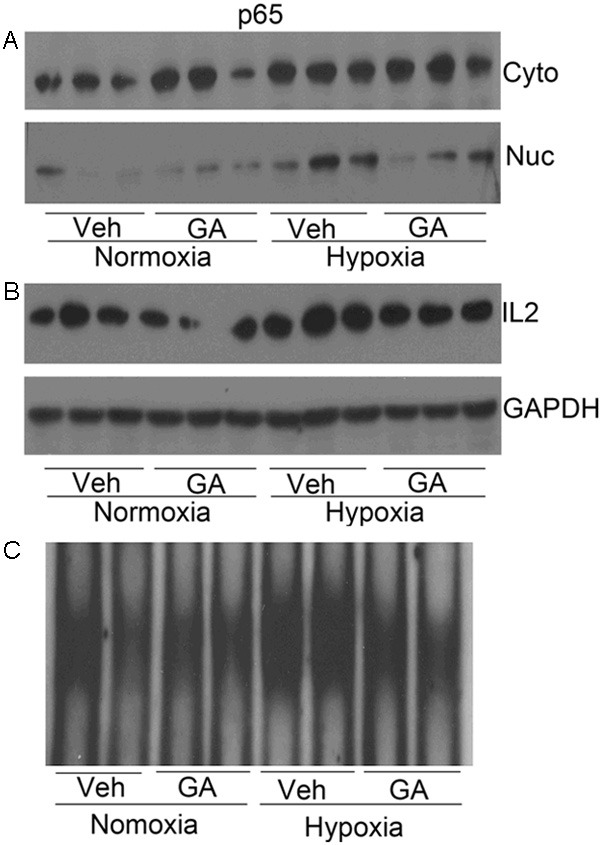
The effect of gambogic acid (GA) on the NF-κB signaling. A: western blot analyses for the p65 subunit of NF-κB in the cytoplasmic fraction and the nuclear fraction of myocardial protein extracts. Hypoxia increased nuclear translocation of NF-κB, which was attenuated by GA treatment. B: western blot analysis for myocardial levels of IL2 proteins. C: NF-κB DNA-binding activity examined by EMSA.
Discussion
Many studies have suggested that right ventricular hypertrophy contributes to the cardiovascular morbidity and mortality in PAH patients. However, effective therapies targeted to right ventricular hypertrophy are still lacking. It was recently reported that bortezomib treatment could significantly attenuate hypoxia-induced or pharmacologically induced PAH and associated right ventricular hypertrophy possibly through inhibition of pulmonary vascular remodeling and correction of endothelial dysfunction [32], suggesting that the proteasome inhibitors can prevent the right ventricular hypertrophy in response to chronic hypoxia. In the current study we employed a rat right ventricular hypertrophy model induced by chronic hypoxia to explore the effect of GA on cardiac responses to chronic global hypoxia. Our data show that GA, as a potent proteasome inhibitor with low toxicity, protects against cardiac hypertrophy induced by chronic hypoxia in rats likely through inhibition of NF-κB signaling by acting on the proteasome. The proposed mechanism by which GA effects on right ventricular hypertrophy is illustrated in Figure 6.
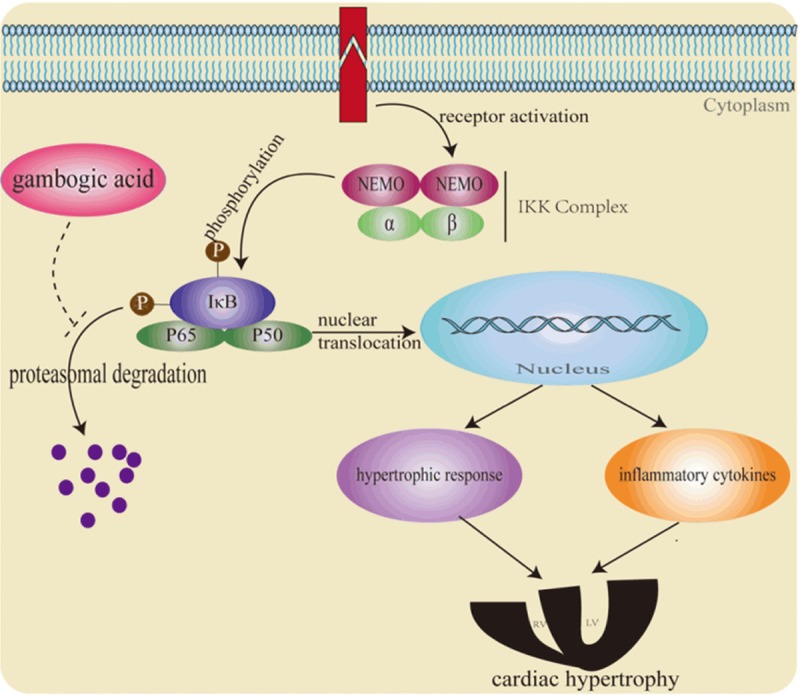
A schematic illustration of a proposed mechanism by which gambogic acid suppresses cardiac responses to chronic hypoxia. Gambogic acid could increase the IκBα levels by the inhibition of proteasome activity, which blocks NF-κB translocation from the cytoplasm to the nucleus, thereby attenuating cardiac hypertrophy by decreasing hypertrophic gene expression and the levels of inflammatory cytokines (e.g. IL-2).
Several previous studies have shown that the activation of cardiac proteasome plays a role in cardiac hypertrophy and remodeling induced by pressure overload, and the mechanism of which remains elusive. We and others have reported that ATP decline to some extent contributes to the activation of the proteasome [33,34], therefore, the decrease of ATP generation in cardiac hypertrophy will conceivably activate the proteasome. The proteasome can both degrade the inhibitors of cardiac hypertrophy (the inducible cyclic adenosine monophosphate early repressor) [35], and recycle the denatured/misfolded proteins because of increased peptide turnover [36]. More importantly, the proteasome can be involved in the activation of signal pathway related to cardiac hypertrophy, such as it can activate the NF-κB pathway by the degradation of IκBα [26]. Accordingly, we show in this report that the NF-κB signaling is remarkably activated in the heart under chronic hypoxia and GA, likely via suppression of the proteasome, inhibits NF-κB signaling and significantly attenuates cardiac pathological responses to chronic hypoxia.
Several lines of evidence based on in vivo and in vitro studies suggest that the activation of NF-κB plays an important role in the development of cardiac hypertrophy, and the inhibition of NF-κB can promote the regression of cardiac hypertrophy [37,38]. Our data suggest that the proteasome inhibition by GA could inhibit the activation of NF-κB pathway by increasing the levels of IκBα in the cytoplasm (Figure 4B), as further supported by the increased nuclear levels of the p65 subunit of NF-κB in the cardiac tissues of rats exposed to chronic hypoxia, which was significantly inhibited by GA treatment (Figure 5A). In addition, NF-κB DNA-binding activity was also obviously decreased by GA treatment according to the EMSA assay (Figure 5C). The release of inflammatory mediators can be mediated by the activation of the NF-κB pathway. IL-2 levels in the cardiac tissues of rats exposed to chronic hypoxia were increased, which were significantly inhibited by GA, consistent with the inhibition of NF-κB by GA (Figure 5B). All these results suggest that GA could block the process of NF-κB nucleus translocation via inhibition of proteasome-mediated IκBα degradation.
Myocardial fibrosis is mainly one of the histopathological characteristics of cardiac remodeling following chronic hypoxia [28], the major constituent of which is the accumulated collagen. The accumulation of collagen will affect the cardiac geometry and wall stress and in turn increase the cardiac stiffness, eventually lead to heart failure [39]. Epoxomicin, a proteasome inhibitor, could prevent the transcription of genes encoding collagen isoforms via inhibition of proteasome-mediated NF-κB pathway [9], and NF-κB was reported to play an important role in cardiac fibroblast survival under hypoxic stress [40]. Therefore, inhibition of NF-κB will lead to hypoxia-induced cell death in cardiac fibroblasts, reducing collagen deposition. Our results of Masson’s trichrome staining show that increased interstitial fibrosis in rats exposed to chronic hypoxia, which was also nearly completely prevented by GA (Figure 2B). Another characteristic believed to signify cardiac pathology/hypertrophy is up-regulated expression of fetal genes including α-skeletal actin and BNP. The activation of NF-κB pathway was confirmed to play a key role in the increased BNP gene expression in cardiac hypertrophy in vivo [41]. Our data also show that the relative expression levels of α-SK-actin and BNP mRNA in the cardiac tissue of rats exposed to chronic hypoxia increased significantly and GA could considerably curtail the increased fetal gene expression (Figure 3). Obviously, the effect of GA treatment on fetal gene expression can also be explained by its inhibition of proteasome-mediated NF-κB pathway. It should be pointed out that our data fail to show a significant increase in myocardial proteasome activity in the vehicle-treated hypoxia rats and the proteasome CT-like activity in hypoxic rat hearts was only modestly reduced by GA treatment, raising the possibility that a proteasome-inhibition action of GA might not be fully responsible for the remarkable inhibitory effect of GA on the activation of the NF-κB pathway in the heart of rats exposed to chronic hypoxia. Additional studies are required to address this issue.
Since GA was administered systemically it can act potentially on all cells and tissue types in the body although its tissue-specific property may limit its actions to certain cell types [20]. The attenuation of cardiac responses by GA may result from its direct action on cardiomyocytes, pulmonary artery, intramyocardial vasculature, or remote organs beyond the heart and lungs. For example, reduction of pulmonary hypertension could explain most of the observed effects. In agreement with this possibility, Rajagopalan et al reported recently that epoxomicin and MG132 failed to inhibit right ventricle hypertrophy in a surgically produced pulmonary artery constriction model [14], whereas proteasome inhibition by bortezomib treatment was shown to significantly attenuate hypoxia-induced or pharmacologically induced PAH [32].
In conclusion, the present study provides new evidence that GA can protect against cardiac pathological responses to chronic hypoxia and inhibition of the proteasome and suppression of the NF-κB pathway are likely underlying mechanisms. However, the previous studies show that GA, as a potent proteasome inhibitor, might have multiple cellular targets to achieve the biological effects, and consequently, other underlying mechanisms by GA treatment on right ventricular hypertrophy are required to be further investigated. Our data suggest that GA has the great potential to be developed into an anti-hypertrophy agent besides an anticancer drug.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (Project 2006AA02Z4B5), NSFC (81272451/H1609, 81070033/H0108, 30770835), Key Projects (9251018201002, 10A057S) from Guangdong NSF and Guangzhou Education Commission (to J.L.); Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (NO.LYM09111, to C.Z.); US NIH grants HL072166 and HL085629 (to X.W.).
References
Articles from American Journal of Cardiovascular Disease are provided here courtesy of e-Century Publishing Corporation
Citations & impact
Impact metrics
Citations of article over time
Article citations
Molecular targets of gambogic acid in cancer: recent trends and advancements.
Tumour Biol, 37(10):12915-12925, 22 Jul 2016
Cited by: 28 articles | PMID: 27448303
Review
Ubiquitin-specific protease 14 regulates cardiac hypertrophy progression by increasing GSK-3β phosphorylation.
Biochem Biophys Res Commun, 478(3):1236-1241, 18 Aug 2016
Cited by: 26 articles | PMID: 27545607
Calcium channel blocker verapamil accelerates gambogic acid-induced cytotoxicity via enhancing proteasome inhibition and ROS generation.
Toxicol In Vitro, 28(3):419-425, 27 Dec 2013
Cited by: 13 articles | PMID: 24373880
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Gambogic acid suppresses pressure overload cardiac hypertrophy in rats.
Am J Cardiovasc Dis, 3(4):227-238, 01 Nov 2013
Cited by: 9 articles | PMID: 24224134 | PMCID: PMC3819582
Chronic Normobaric Hypoxia Induces Pulmonary Hypertension in Rats: Role of NF-κB.
High Alt Med Biol, 17(1):43-49, 20 Jan 2016
Cited by: 12 articles | PMID: 26788753
Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264.7 cells.
Biomed Pharmacother, 109:555-562, 03 Nov 2018
Cited by: 35 articles | PMID: 30399591
Deubiquitinase Inhibitor Auranofin Attenuated Cardiac Hypertrophy by Blocking NF-κB Activation.
Cell Physiol Biochem, 45(6):2421-2430, 15 Mar 2018
Cited by: 14 articles | PMID: 29554646
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL072166
Grant ID: R01 HL085629


