Abstract
Free full text

Pathophysiology of Thoracic Aortic Aneurysm (TAA): Is it not one Uniform Aorta? Role of Embryologic Origin
Abstract
Thoracic aortic aneurysm (TAA) is a clinically silent and potentially fatal disease whose pathophysiology is poorly understood. Application of data derived from animal models and human tissue analysis of abdominal aortic aneurysms may prove misleading given current evidence of structural and biochemical aortic heterogeneity above and below the diaphragm. Genetic predisposition is more common in TAA and includes multi-faceted syndromes such as Marfan, Loeys-Dietz, and type IV Ehlers-Danlos as well as autosomal-dominant familial patterns of inheritance. Investigation into the consequences of these known mutations has provided insight into the cell signaling cascades leading to degenerative remodeling of the aortic medial extracellular matrix (ECM) with TGF-β playing a major role. Targeted research into modifying the upstream regulation or downstream effects of the TGF-β1 pathway may provide opportunities for intervention to attenuate TAA progression.
Introduction
Thoracic aortic aneurysm (TAA) disease is the 15th leading cause of death in patients greater than 65 years of age, a demographic which continues to expand rapidly, and is often clinically silent until fatal rupture.1 Increased utilization of radiologic cross-sectional imaging has improved diagnostic rates, affording patients the opportunity for elective TAA repair when threshold diameters are surpassed,2 but interventions to minimize the rate of growth are lacking. Stabilizing aortic diameter hinges on improved understanding of the pathophysiology of TAA development, but much of current theory has been applied from animal models and biomolecular research conducted on abdominal aortic aneurysms (AAA). Evidence is accumulating, however, that the aorta is a heterogeneous structure with varying structural, biochemical, and genetic influences above and below the diaphragm.3 This regional disparity in form and function begins during embryologic development and has significant impact on the synthetic and contractile behavior of vascular smooth muscle cells (VSMC) of the aortic media. Those cells respond to environmental factors and concurrent diseases such as cigarette smoking, chronic obstructive pulmonary disease, hypertension, atherosclerosis, and elevated body mass index.4 But the contribution of currently identified genetic syndromes in early formation of TAA further supports intrinsic abnormalities leading to thoracic aortic dilation as a consequence. In this discussion, thoracic aortic growth and development will be explored, focusing on the contributions of embryologic origin and genetic predisposition as contributing components in aneurysm development.
Aortic Heterogeneity
As a large elastic artery, the aorta's three layers have diverse and distinct roles in development, homeostasis, and pathologic degradation. The media, composed of concentric bands of elastin, collagen, and VSMCs called lamellar units, provides viscoelasticity and is the location of degradative remodeling responsible for aneurysm formation. During embryologic development, the ascending and arch aorta to the ligamentum arteriosum formed by cells from the neural crest,. and the media grows by assembling sequential lamellar units to reach a total of 55-60 by adulthood, always maintaining a constant ratio of aortic diameter to medial thickness.5 Interestingly, that ratio remains consistent during abdominal aortic growth as well, but in that region the precursor cells originate in the mesoderm and the number of lamellar units remains constant while the thickness of each unit is expanded during maturation.5 This disparity in aortic cellular origin and segmental growth patterns contributes to differences in and extracellular matrix (ECM) microfibril density and VSMC reactivity to vasoactive growth factors , with downstream effects on susceptibility to aneurysmal degeneration.6
VSMCs of neural crest origin have been studied in the presence of various cardiovascular cytokines and growth factors, and their unique responses may contribute to TAA growth and highlight pathways for potential therapeutic intervention. The sulfur containing amino acid homocysteine, for instance, has been known to have cardiovascular toxicity at high levels, specifically contributing to accelerated atheroslcerosis, elastolysis, disorganized collagen deposition, and reduced aortic compliance.7,8 Hyperhomocysteinemia appears to induce oxidative stress and activation of latent matrix metalloproteinases (MMPs), a class of proteinases instrumental in ECM remodeling and aneurysm formation.9 Interestingly, neural crest VSMCs respond to homocysteine with amplified proliferation and synthetic activity while those of mesodermal origin are unaffected.10 Murine models of TAA development have localized MMP production to native VSMCs that have undergone a phenotypic switch away from contractility toward increased synthetic activity,11 providing a potential pathway in degenerative aortic remodeling that may be preferential to the thoracic aorta.
AngiotensinII (AngII) is a vasoactive peptide associated with hypertensive vascular remodeling and atherosclerotic plaque deposition.12 Continuous infusion of AngII is an established method of inducing AAA development in hypercholesteremic mice, wherein histologic examination describes media rupture, gross aortic dilation, and infiltration of inflammatory cells such as macrophages and lymphocytes.13 Progressive AAA dilation with ECM fragmentation then ensues. Prolonged AngII infusion in these mice also initiated ascending aortic dilation, characterized by elastin fragmentation and thickening of the outer media, suggesting regional disparity in the reactions of the VSMCs in the thoracic and abdominal aorta.13
A third growth factor demonstrating regional heterogeneity in its effects on aortic medial VSMCs is transforming growth factor-beta (TGF-β), a family of homodimeric peptides with widespread contributions to vascular development via regulation of cellular growth and differentiation.14 When treated with TGF-β, neural crest VSMCs demonstrated increased DNA synthesis and collagen production, while mesodermal VSMCs failed to augment collagen output.15 Additionally, the contractile response of thoracic aortic VSMCs is blunted by exposure to TGF-β in ex-vivo collagen matrices,16 supporting a role for this growth factor in modifying the cell-matrix interaction in the thoracic aorta and a potential contribution to the aforementioned phenotypic switch seen in these cells during aneurysm formation. TGF-β has been credited with regulating thoracic aortic ECM homeostasis, with a hypothesis that external or intrinsic factors may lead to altered levels of TGF-β, producing dysregulated ECM turnover.17 Acknowledging the differential aortic cellular activity above and below the diaphragm has allowed divergence from the atherosclerotic/inflammatory aneurysm paradigm of the abdominal aorta. This has focused research into the pathophysiology of TAA to explore the upstream signaling pathways contributing to ECM degradation in this region.
TGF-β and the Extracellular Matrix
TGF-β is stored within the matrix and bound by ligands. Upon release, the peptide dimerizes, binds to cell surface receptors (TGFβ-R I or II), and initiates an intracellular signaling pathway that terminates in the nucleus with direct transcriptional regulation.18,19 Classically this signaling pathway utilizes the Smad protein complex and has been associated with increased production of collagen, elastin, and the tissue inhibitors of metalloproteinases (TIMPs) for a net effect of ECM synthesis or stabilization.20,21 Conversely, via an alternate pathway, (ERK1/2) TGF-β appears to play a major role in proteolysis and ECM destruction. In its role as homeostatic regulator of the thoracic aortic ECM, recent data suggests that TGF-β contributes to TAA formation through modulation of MMP release and amplified proteolytic activity,22 but this association had been obscured in earlier investigations. Contemporary inhibitor studies focusing on genetically modified mouse strains have reported TGF-β-induced activation of extracellular signal-regulated kinases (ERK1/2) with consequent aortic dilation.23
These data reporting conflicting outcomes of TGF-β signaling may be reconciled by the recognition of the superfamily of components - including 30 different ligands, 7 type I receptors, 5 type II receptors, and 8 intracellular signaling variants - thereby allowing a multitude of downstream effects.24,25 Specifically, TGF-β1 has been identified as a significant contributor to TAA formation.
In narrowing the discussion to those aspects related to TAA growth, the relationship of TGF-β1 with MMPs, fibrillin-1, and type III collagen will be highlighted. MMP's. Within the MMP family of proteases, MMP-2 and MMP-9 are classified as gelatinases based upon their substrate specificity for non-fibrillar collagens, elastin, and various components of ground substance.26 Their contribution to ECM degradation during aneurysmal dilation has been established in biochemical and histologic analysis of animal and human thoracic aortic tissue.11,27 MMP-2 and -9 are released into the ECM as inactive pro-peptides that may be activated through several pathways, a common one being tissue plasminogen activator (t-PA). TGF-β1 has been shown to propagate MMP proteolytic activity through production of MMP-2, MMP-9 and t-PA, each of these is a protease.22,28Fibrillin-. Fibrillin-1 is a stable microfibril that forms a network around elastin filaments to add strength to the aortic wall and plays a crucial role in the sequestration and upstream regulation of TGF-β1 activity.29,30 Fibrillin-1 also binds SMC integrin receptors and is suspected to transmit cell-matrix positioning signals.31Type III collagen. Type III collagen is an abundant component of the media and adventitia which provides tensile strength to the aortic wall.32 In particular circumstances of vascular wall injury, TGF-β1 mediated production of type III collagen has been reported, identifying yet another link between this growth factor and the regulation of the aortic ECM.33
Genetic Syndromes of the Thoracic Aorta
Marfan Syndrome
Marfan syndrome is inherited in an autosomal dominant pattern and involves pathology of the cardiovascular, skeletal, and ocular systems. Although the disease is attributed to mutation of the fibrillin-1 gene (FBN-1),34 the multitude of documented mutations has made genetic testing difficult and the diagnosis is based on an extensive list of clinical manifestations (Table 1).35 The majority of morbidity and mortality, however, is related to aortic defects including annulo-aortic ectasia, aneurysms, and dissections, justifying the extensive research into this pathology.36 Within the aortic wall, the defective fibrillin-1 production causes poor elastin filament alignment and disorganization of entire lamellar units with consequent instability of the aortic wall.37 Additionally, TGF-β1 sequestration in the ECM is compromised by dysfunctional fibrillin-1 microfibrils, allowing for increased TGF-β1 signaling activity.38,39 This intracellular synthetic state has also been reported in a murine model of Marfan syndrome studying AngII signaling through the AT1 receptor.40
Table 1
Gene defects and characteristics of TAA syndromes
| Syndrome | Gene | Protein | Characteristics |
|---|---|---|---|
| Marfan | FBN1 | Fibrillin 1 | Aortic dilation, ectopia lentis, pectus deformity, spontaneous pneumothorax, increased arm span, hypermobile joints, abdominal wall laxity, highly arched palates |
| Loeys-Dietz | TGFBR1 TGFBR2 | TGF-βR I TGF-βR II | Arterial aneurysms, craniosynostosis, cleft palate, congenital heart defects, mental retardation |
| Ehlers-Danlos type IV | COL3A1 | Type III collagen | Arterial fragility, translucent skin, facial dysmorphology. Fragile tissues make surgery dangerous. |
| Familial TAA | ACTA2 MYH11 TGFBR2 | Smooth muscle actin β myosin heavy chain TGF-βR II | TAA TAA, patent ductus arteriosus TAA |
Loeys-Dietz Syndrome
Although similar to the clinical manifestations of Marfan Syndrome, the disease described by Loeys and Dietz in 2005 carries more significant cardiovascular risk due to younger onset of aortic aneurysms and dissections (Table 1).41 The syndrome was linked to heterozygous mutations of the TGF-β receptors I & II (TGF-βR1 and TGF-βR2), initially suggesting a lack of TGF-β1 signaling; but subsequent biochemical analysis of aortic tissue from these patients was consistent with upregulated signaling as evidenced by collagen production and nuclear translocation of known intracellular signaling molecules.41 Discovery and investigation of this syndrome has been instrumental in defining the role of TGF-β1 in aortic pathology.
Ehlers-Danlos type IV
Vascular Ehlers-Danlos (type IV) has been characterized by an autosomal dominant pattern of inheritance, translucent skin, easy bruising, and arterial susceptibility to aneurysms, dissections, and spontaneous rupture (Table 1).42 Greater than 70 mutations of the COL3A1 gene have been identified and the dysfunctional type III collagen leads to aortic dilation with such severe vascular friability that surgical interventions are often unsuccessful.43 Significant phenotypic overlap with Loeys-Dietz syndrome has been documented in patients with type IV Ehlers-Danlos, and subsequent investigations into TGF-β1 receptors and signaling cascades in these patients discovered upregulation of this pathway.44
Familial TAA
In the absence of a multi-faceted syndrome, familial patterns of inheritance of TAA as an isolated disease manifestation have been identified. Approximately 20% of these non-syndromic TAA patients have an affected family member, and these aneurysms manifest earlier onset with more rapid growth than sporadic TAAs.45 Several ethiologic mutations have been identified for familial aortic aneurysm. The ACTA2 gene encodes smooth muscle α2 actin, leading to VSMCs with disorganized and aggregated actin filaments and consequent impairment of cellular adaptation to local mechanical stress.46 The link between this VSMC dysregulation and aortic medial degeneration has yet to be determined, but ACTA2 is the most common site of mutation in familial TAA grouping. The VSMC contractile peptide β myosin heavy chain is encoded by the gene MYH11 and mutations in this location can predispose to TAA as well as patent ductus arteriosus.47 Histologic examination has identified loss of VSMCs, elastin, and collagen in the aortic media, as well as inflammatory invasion of vasa vasorum from the adventitia.47,48 Interestingly, aortic tissue from patients with MYH11 mutations had elevated levels of AngII, demonstrating a potential link to TGF-β1 activity.49 Apart from the earlier discussion of Loeys-Dietz syndrome, a subset of mutations in TGFBR2 affecting the Arginine amino acid at site 460 have been categorized in the familial TAA subgroup since this peptide alteration does not produce additional phenotypic features.50 The conundrum remains, however, as to the mechanism by which TGFBR2 mutations lead to elevated TGF-β1 activity.
TGF-β1 and TAA Pathophysiology
Given the clinical association of these aberrant proteins with thoracic aortic dilation, a molecular hypothesis of TAA formation may be deduced from our knowledge of these syndromes, with TGF-β1 as a keystone component (Figure 1). The upregulated activity of TGF-β1 may be achieved through ineffective sequestration in the ECM by defective fibrillin-1 or augmented intracellular signaling originating in mutated receptors TGF-βR I or II.51 AngII activation of the AT1 receptor generates reactive oxygen species and activates p38 mitogen activated protein kinase to amplify TGF-β1 signaling as well.52-54 While the classical Smad signaling pathway activated by TGF-β typically produces a pro-fibrotic response but may cause production of dysfunctional collagen subtypes,the ERK1/2 pathway may be initiated to stimulate aortic medial VSMCs to abandon contractile activity and display enhanced synthesis of t-PA, MMP-2, MMP-9 and collagen propeptides, inducing a proteolytic response.17,23 An additional regulatory role for TGF-β1 as a downstream signal transducer from the AT1 receptor has been described with subsequent production of microRNA29, a small oligonucleotide credited with modulating MMP expression and VSMC apoposis.55-57 When released into the ECM, MMPs may be activated by the concurrent abundance of t-PA with subsequent degradative remodeling. Enhanced production of mutated type III collagen and formation of disorganized collagen fibers may also compromise ECM integrity and allow dilation of the aortic wall. Alternatively, inherent deformities in the VSMC contractile proteins, smooth muscle α2 actin and β myosin heavy chain, may provide significant susceptibility to decaying aortic wall tensile strength, but an additional relationship to TGF-β1 ought to be explored. Targeting TGF-β1 as a final common pathway for TAA formation and AngII as a contributing regulatory peptide may suggest fruitful research endeavors to better understand the pathophysiology of TAA in syndromic, inherited, and sporadic cases.
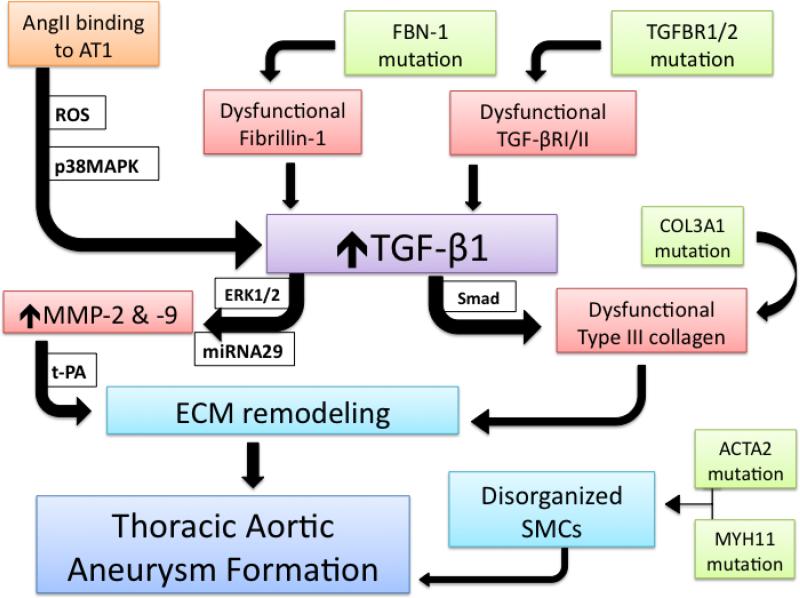
Centralized role of TGF-β1 in TAA formation. Upregulated TGF-β1 signaling in the aortic medial VSMCs may be initiated through ineffective sequestration by mutated fibrillin-1, by binding to dysfunctional receptors, or through downstream activity of AngII binding to its AT1 receptor. The generation of ROS and activation of p38MAPK can augment TGF-β1 activity. VSMC synthetic productivity is modulated by miRNA 29 and may produce MMP-2, MMP-9, and collagen. When MMPs are activated by t-PA, ECM remodeling with aneurysmal dilation ensues. Dysfunctional type III collagen and disorganization of VSMCs may further compromise the integrity of the aortic media and allow TAA formation.
Conclusions
As the utility and accuracy of three-dimensional imaging techniques continues to increase, the identification of asymptomatic TAA will continue to present a therapeutic dilemma. Surgical interventions carry significant risk and improved medical management must be developed to reliably and reproducibly reduce thoracic aortic aneurysm growth. Investigations into the pathophysiology of syndromic TAAs and identification of the pivotal role of the TGF-β1 cell signaling pathways will support further exploration to identify opportunities for directed therapy to attenuate degenerative aortic remodeling.
Acknowledgments
This study was supported by the following grants: NIH/NIA AG036954, NIH/NHLBI HL102121 and Veterans Affairs Merit Award 1 I01 BX000904-01
List of Abbreviations
| AngII | angiotensin II |
| AAA | abdominal aortic aneurysm |
| AT1 | angiotensin receptor 1 |
| ECM | extracellular matrix |
| ERK | extracellular signal-regulated kinase |
| MMP | matrix metalloproteinase |
| TAA | thoracic aortic aneurysm |
| TGF-β | transforming growth factor beta |
| TGF-βR1 & 2 | transforming growth factor beta receptor 1 & 2 |
| t-PA | tissue plasminogen activator |
| VSMC | vascular smooth muscle cell |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant conflicts of interest related to this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.pcad.2013.04.002
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3759819?pdf=render
Citations & impact
Impact metrics
Article citations
A porcine model of thoracic aortic aneurysms created with a retrievable drug infusion stent graft mirrors human aneurysm pathophysiology.
JVS Vasc Sci, 5:100212, 02 Jul 2024
Cited by: 0 articles | PMID: 39188992 | PMCID: PMC11345694
Divergent and Compensatory Effects of BMP2 and BMP4 on the VSMC Phenotype and BMP4's Role in Thoracic Aortic Aneurysm Development.
Cells, 13(9):735, 24 Apr 2024
Cited by: 0 articles | PMID: 38727271 | PMCID: PMC11083443
Use of iPSC-Derived Smooth Muscle Cells to Model Physiology and Pathology.
Arterioscler Thromb Vasc Biol, 44(7):1523-1536, 02 May 2024
Cited by: 0 articles | PMID: 38695171
Review
Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease.
Int J Mol Sci, 24(21):15640, 27 Oct 2023
Cited by: 2 articles | PMID: 37958625 | PMCID: PMC10650782
Review Free full text in Europe PMC
Abdominal Aortic Aneurysm: Natural History, Pathophysiology and Translational Perspectives.
Transl Med UniSa, 24(2):30-40, 27 Dec 2022
Cited by: 5 articles | PMID: 37476203 | PMCID: PMC10354862
Review Free full text in Europe PMC
Go to all (47) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis.
J Vasc Res, 46(2):119-137, 02 Sep 2008
Cited by: 107 articles | PMID: 18765947 | PMCID: PMC2645475
Review Free full text in Europe PMC
TGF-β (Transforming Growth Factor-β) Signaling Protects the Thoracic and Abdominal Aorta From Angiotensin II-Induced Pathology by Distinct Mechanisms.
Arterioscler Thromb Vasc Biol, 37(11):2102-2113, 20 Jul 2017
Cited by: 66 articles | PMID: 28729364 | PMCID: PMC5658248
Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms.
Cardiovasc Res, 89(2):446-456, 09 Sep 2010
Cited by: 63 articles | PMID: 20829218 | PMCID: PMC3020128
Age- and sex-specific biomechanics and extracellular matrix remodeling of the ascending aorta in a mouse model of severe Marfan syndrome.
Am J Physiol Heart Circ Physiol, 327(4):H1037-H1051, 30 Aug 2024
Cited by: 0 articles | PMID: 39212766
Funding
Funders who supported this work.
BLRD VA (1)
Grant ID: I01 BX000904
NHLBI NIH HHS (2)
Grant ID: HL102121
Grant ID: R01 HL102121
NIA NIH HHS (2)
Grant ID: AG036954
Grant ID: R01 AG036954





